Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5283
Revised: May 6, 2024
Accepted: June 17, 2024
Published online: August 16, 2024
Processing time: 126 Days and 16.6 Hours
A case report entitled “Primary gastroduodenal tuberculosis presenting as gastric outlet obstruction” recently published in the World Journal of Clinical Cases pre
Core Tip: Gastrointestinal tuberculosis is a rare pathology that can present in many atypical ways. This pathology is relatively rare and can be a significant challenge to diagnose. This editorial, in response to a recently published case report, aims to provide an update on diagnostic tests and approaches available.
- Citation: Brown L, Colwill M, Poullis A. Gastrointestinal tuberculosis: Diagnostic approaches for this uncommon pathology. World J Clin Cases 2024; 12(23): 5283-5287
- URL: https://www.wjgnet.com/2307-8960/full/v12/i23/5283.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i23.5283
The case report “Primary gastroduodenal tuberculosis presenting as gastric outlet obstruction” presented in this edition by Ali et al[1] highlights the difficulties in diagnosing gastrointestinal tuberculosis (TB). TB is an infectious disease caused by the bacteria Mycobacterium tuberculosis (M. tuberculosis) which affects an estimated 10.6 million people and causes over 1.3 million deaths each year. Though primarily a respiratory disease, extrapulmonary disease is found in around 20% of patients with TB and occurs both in isolation or conjunction with pulmonary TB[2]. Abdominal TB, affecting the gastrointestinal tract, visceral organs, peritoneum and/or lymph nodes is thought to represent between 3%-21% of cases of extrapulmonary TB[3]. Abdominal TB may develop through ingestion of infected lung secretions, direct invasion from adjacent infected tissues and through haematogenous or lymphatic spread[4]. Our previously published case series reported on all the cases of abdominal TB seen at St George’s Hospital, London between 2003 and 2013, 65 cases in total. Since the report of this series there have been a number of advances in TB diagnostics but it’s variable and non-specific clinical presentation mean that abdominal TB remains a challenge to diagnose (see Table 1)[5].
| Diagnostic tools | |
| Clinical signs | Vary according to site of infection (luminal, visceral, peritoneal) |
| Non-specific and may only be present in late stage of disease | |
| Imaging | Ultrasound is useful as a non-invasive imaging modality and can guide fine needle aspiration to obtain tissue for diagnosis of visceral TB, but has low sensitivity and specificity |
| Cross-sectional abdominal imaging is non-specific but recommended to assess extent of TB infection and identify complications | |
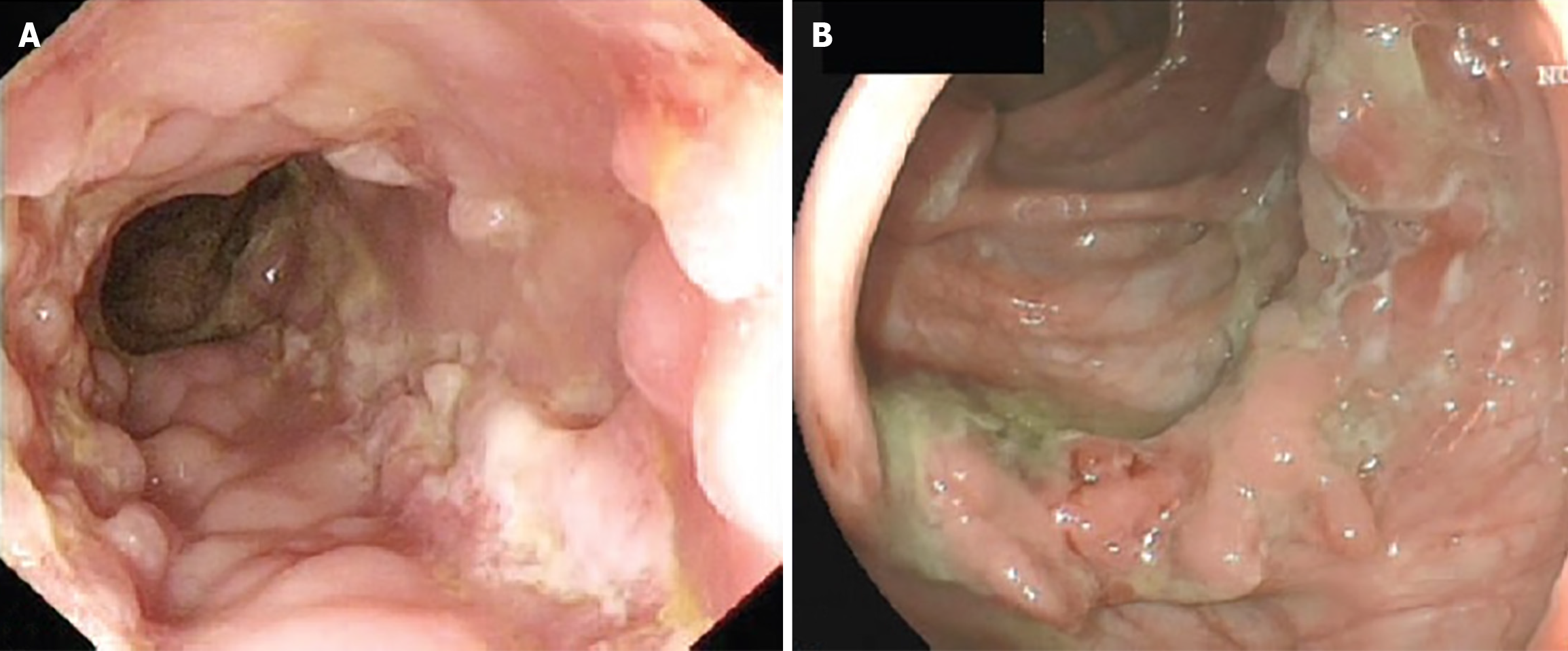
| Endoscopy | Gross endoscopic appearance may mimic other diseases such as Crohn’s (Figure 1) |
| Facilitates targeted tissue biopsy for histological and microbiological examination | |
| Histopathology | Characteristic tissue features include granulomas with caseating necrosis, Langerhans giant cells, chronic ulcers lined by conglomerate epithelioid histiocytes and disproportionate submucosal inflammation |
| Conventional microbiology | Ziehl Nelsen staining of acid-fast bacilli provides rapid diagnosis with high specificity |
| Both staining and culture have low sensitivity | |
| Due to slow growth rate of M. tuberculosis, culture may require 6-8 weeks | |
| Molecular | PCR provides very high specificity across all specimen types |
| PCR sensitivity is superior in tissue compared to ascitic fluid or paraffin-embedded specimens | |
| Stool PCR and cell-free DNA have potential as alternative, non-invasive tests but require further evaluation to confirm diagnostic performance | |
| Serology | T-cell based IGRA, including QuantiFERON® and T-SPOT® on blood or ascitic fluid cannot differentiate between latent and active infection but can complement other diagnostic modalities |
| Poor sensitivity in patients who are immunocompromised or who have disseminated disease | |
Abdominal TB often mimics other gastrointestinal conditions such as inflammatory bowel disease, peptic ulcer disease, lymphoma and other malignancies[6,7]. Delays in diagnosis contribute to the poor prognosis of abdominal TB and progression to severe and potentially life-threatening complications including acute gastrointestinal haemorrhage, obstruction or perforation[8,9].
Establishing a diagnosis of abdominal TB begins with clinical signs and symptoms. As in pulmonary TB, features of systemic infection including low-grade fever, night sweats and weight loss may be present[5,9]. Abdominal symptoms vary according to site of infection and may only become apparent late in the disease course[4]. Gastrointestinal (or luminal) TB typically presents with chronic, colicky abdominal pain, vomiting, loss of appetite, chronic diarrhoea and/or constipation. Weight loss, accompanied with iron deficiency anaemia, are often predominant features of gastrointestinal TB due to impaired absorption, decreased food intake and a chronic inflammatory process[8]. Complications of gastrointestinal TB include ulceration, haemorrhage, stricture and fistula formation, obstruction and perforation. In India, abdominal TB is the second most common cause of bowel perforation, after typhoid, usually proximal to a TB-related stricture[4]. Organ-specific signs (such as jaundice, elevated blood liver enzymes or amylase) may aid differential diagnosis of visceral TB, a less common type of abdominal TB which affects the liver, spleen, gallbladder, pancreas, biliary or genitourinary tract[10]. Peritoneal TB may present insidiously, with sub-acute abdominal pain, distension and eventually, peritonitis. Depending on the site of infection, examination may reveal an abdominal mass, ascites, organomegaly or lymphadenopathy[10].
Imaging should be performed in all patients with a suspicion of abdominal TB. Ultrasound is useful as an initial and non-invasive tool able to detect features of abdominal TB, without the use of ionising radiation, including ascites, peritoneal thickening, lymphadenopathy and hepatic or splenic lesions. However, in confirmed TB infection, ultrasound has a relatively low sensitivity of 63% and specificity of 68% for diagnosing abdominal disease[11]. Ultrasound-guided fine needle aspiration also offers the option to obtain tissue for histopathology and microbiological evaluation and is particularly useful for the diagnosis of visceral TB; whereas paracentesis or laparoscopy or may be required for definitive diagnosis of peritoneal disease[7,12,13]. Computed tomography (CT) findings are similarly non-specific but useful for evaluating the extent and type of abdominal TB and identifying any complications[7,14]. CT appearances of abdominal TB may mimic other infections, inflammatory disorders or malignancies including lymphoma[6,7].
In cases of gastrointestinal TB, alongside cross-sectional imaging, endoscopy is crucial to investigate the extent and severity of disease and provide an opportunity for tissue biopsy. The endoscopic findings in gastrointestinal TB are variable and may include a loss of vascular pattern, erythema, superficial to deep ulcerations, nodularity, polyps, inflammatory masses, strictures or colitis (see Figure 1)[5,15]. Endoscopic and histological appearance of gastrointestinal TB may be mistaken for Crohn’s, since both diseases have a predilection for the small bowel and cause chronic granulomatous inflammation[16].
Histopathological examination of biopsy samples may demonstrate typical (though non-specific) features of abdominal TB including granulomas with caseating necrosis, Langerhans giant cells, chronic ulcers lined by conglomerate epithelioid histiocytes and disproportionate submucosal inflammation[8]. Although the sensitivity of histopathology is relatively low, diagnostic accuracy can be improved through increasing the number, volume and depth of biopsy specimens[17]. Analysis of ascitic fluid obtained through paracentesis usually reveals leucocytosis, low glucose and a low serum ascites albumin gradient[18]. Raised adenosine deaminase levels in ascitic fluid is suggestive of peritoneal TB, but is not useful in HIV populations[19].
For many years, conventional microbiology techniques including Ziehl Nelsen staining of acid-fast bacilli (AFB) and culture were the mainstay of TB diagnostics. For the diagnosis of abdominal TB, culture and Ziehl Nelsen staining have very high specificity (reaching 100%) but their value is limited by very low sensitivity (17.3%-31.0% and 9.3%, respectively)[8]. Sensitivity is reduced further in peritoneal TB, where the bacterial load in ascitic fluid is typically very low[20]. Due to the slow growth rate of M. tuberculosis, it may require 6-8 weeks to obtain a culture result. As with histopathology, diagnostic yield of culture and AFB staining, also known as Ziehl-Neelsen staining, can be significantly improved by increasing the number of samples sent for microbiological examination[21]. Combining histopathology and culture findings increases diagnostic accuracy[8].
In recent years, molecular diagnostics such as PCR have emerged as promising new tools for the diagnosis of abdominal TB. The main advantage of PCR over conventional culture or microscopy is the rapid detection of even very low bacterial burdens directly from clinical specimens. Certain assays, such as Gene-XpertÒ, offer simultaneous detection of mutations conferring antimicrobial resistance, which can guide treatment regimens[8]. In a recent meta-analysis, the pooled sensitivity of PCR for the diagnosis of abdominal TB was low (58%, 95%CI 51%–64%) and specificity was high (99%, 95%CI 97%-99%) compared to a composite reference standard of clinical, radiological and histopathological evidence[22]. Though specificity remained consistently very high, there was significant variability in the sensitivity of PCR according to assay specifics. The sensitivity of multiplex PCR, which amplifies multiple DNA targets in a single reaction, was superior to simple PCR and Gene-XpertÒ (82%, vs 56% and 45%, respectively). PCR assays targeting the IS6110 gene were more sensitive than those targeting the rpoB gene (60% vs 45%). Other PCR assays including single-tube loop-mediated isothermal amplification and fluorescent quantitative PCR (qPCR) have been used for the diagnosis of abdominal PCR but data is limited. The sensitivity of PCR was superior in evaluation of tissue specimens over ascitic fluid (62% vs 51%), likely due to higher bacterial burdens found in the gastrointestinal tract. Sensitivity of PCR dropped significantly when using paraffin-embedded specimens, secondary to DNA degradation and introduction of PCR inhibitors (sensitivity 34% compared to 58% and 72% for fresh and frozen tissue specimens, respectively).
PCR analysis of stool samples provides a non-invasive diagnostic tool to aid in the diagnosis of gastrointestinal TB. Stool PCR has demonstrated a moderate sensitivity of 64%, though data is limited and larger, multicentre cohort studies are needed before widespread implementation is recommended[22]. Detection of circulating cell-free DNA (cfDNA) within easily-accessible blood, urine or ascitic fluid samples provides another potential non-invasive diagnostic test for abdominal TB[23]. A recent meta-analysis identified only a single study evaluating qPCR of cfDNA in ascitic fluid, with low sensitivity of 40% and specificity of 90% against a composite reference standard[24,25].
T-cell based interferon-gamma release assays (IGRA), including QuantiFERON® and T-SPOT® on blood or ascitic fluid, have largely replaced tuberculin skin test (TST) for diagnosis of active and latent TB[8]. Unlike TST, specificity of IGRA is not affected by prior BCG vaccination. For peritoneal TB, sensitivity and specificity of IRGA on peripheral blood was found to be high at 91% and 78%, respectively[26]. The utility of IGRA is limited by poor sensitivity in patients who are immunocompromised or have disseminated disease[8].
Abdominal TB continues to pose a major diagnostic challenge for clinicians. There exists no single test that has proved to be adequate in both sensitivity and specificity for the diagnosis of abdominal TB. In recent years, non-invasive diagnostic tools including stool or cfDNA PCR and serological tests have emerged with some promising results, though larger, prospective trials are needed before widespread implementation is recommended. Until then, combining results of multiple diagnostic modalities (including clinical, radiological, histopathological, microbiological, molecular and serological evidence) remains necessary in order to diagnose this clinical conundrum.
| 1. | Ali AM, Mohamed YG, Mohamud AA, Mohamed AN, Ahmed MR, Abdullahi IM, Saydam T. Primary gastroduodenal tuberculosis presenting as gastric outlet obstruction: A case report and review of literature. World J Clin Cases. 2024;12:1536-1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (4)] |
| 2. | World Health Organization. Global tuberculosis report 2023. Nov 7, 2023. [cited 4 May 2022]. Available from: https://www.who.int/publications/i/item/9789240083851. |
| 3. | Al-Zanbagi AB, Shariff MK. Gastrointestinal tuberculosis: A systematic review of epidemiology, presentation, diagnosis and treatment. Saudi J Gastroenterol. 2021;27:261-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White NJ, Garcia PJ. Manson's Tropical Diseases. 24th ed. Cham: Elsevier health sciences, 2023. |
| 5. | Nayagam JS, Mullender C, Cosgrove C, Poullis A. Abdominal tuberculosis: Diagnosis and demographics, a 10-year retrospective review from a single centre. World J Clin Cases. 2016;4:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Ladumor H, Al-Mohannadi S, Ameerudeen FS, Ladumor S, Fadl S. TB or not TB: A comprehensive review of imaging manifestations of abdominal tuberculosis and its mimics. Clin Imaging. 2021;76:130-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Abu-Zidan FM, Sheek-Hussein M. Diagnosis of abdominal tuberculosis: lessons learned over 30 years: pectoral assay. World J Emerg Surg. 2019;14:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Maulahela H, Simadibrata M, Nelwan EJ, Rahadiani N, Renesteen E, Suwarti SWT, Anggraini YW. Recent advances in the diagnosis of intestinal tuberculosis. BMC Gastroenterol. 2022;22:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Cho JK, Choi YM, Lee SS, Park HK, Cha RR, Kim WS, Kim JJ, Lee JM, Kim HJ, Ha CY, Kim HJ, Kim TH, Jung WT, Lee OJ. Clinical features and outcomes of abdominal tuberculosis in southeastern Korea: 12 years of experience. BMC Infect Dis. 2018;18:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Das CJ, Rednam N, Vora Z, Aggarwal A, Chandrashekhara SH, Kundra V. Abdominal visceral tuberculosis: a malignancy mimic. Abdom Radiol (NY). 2023;48:2705-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 11. | Van Hoving DJ, Griesel R, Meintjes G, Takwoingi Y, Maartens G, Ochodo EA. Abdominal ultrasound for diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV-positive individuals. Cochrane Database Syst Rev. 2019;9:CD012777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Islam J, Clarke D, Thomson SR, Wilson D, Dawood H. A prospective audit of the use of diagnostic laparoscopy to establish the diagnosis of abdominal tuberculosis. Surg Endosc. 2014;28:1895-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Handa U, Garg S, Mohan H. Fine needle aspiration cytology in the diagnosis of abdominal TB: a review of 92 cases. Trop Doct. 2009;39:30-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Lee WK, Van Tonder F, Tartaglia CJ, Dagia C, Cazzato RL, Duddalwar VA, Chang SD. CT appearances of abdominal tuberculosis. Clin Radiol. 2012;67:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Gan H, Mely M, Zhao J, Zhu L. An Analysis of the Clinical, Endoscopic, and Pathologic Features of Intestinal Tuberculosis. J Clin Gastroenterol. 2016;50:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Choudhury A, Dhillon J, Sekar A, Gupta P, Singh H, Sharma V. Differentiating gastrointestinal tuberculosis and Crohn's disease- a comprehensive review. BMC Gastroenterol. 2023;23:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Mehta V, Desai D, Abraham P, Rodrigues C. Making a Positive Diagnosis of Intestinal Tuberculosis with the Aid of New Biologic and Histologic Features: How Far Have We Reached? Inflamm Intest Dis. 2019;3:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Debi U, Ravisankar V, Prasad KK, Sinha SK, Sharma AK. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol. 2014;20:14831-14840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 231] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (4)] |
| 19. | Ntwari J, Dusabejambo V, Page C. Use of adenosine deaminase (ADA) to diagnose suspected peritoneal tuberculosis in Rwanda: a cross-sectional study. BMC Infect Dis. 2020;20:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Fitzgerald DW, Sterling TR, Haas DW. 251 - Mycobacterium tuberculosis. Mandell, Douglas, Bennett's Princ Pract Infect Dis. 2015;. [DOI] [Full Text] |
| 21. | Lowbridge C, Fadhil SAM, Krishnan GD, Schimann E, Karuppan RM, Sriram N, Rajahram GS, Menon J, Patel A, William T, Paul DC, Ralph AP. How can gastro-intestinal tuberculosis diagnosis be improved? A prospective cohort study. BMC Infect Dis. 2020;20:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Shen Y, Fang L, Ye B, Yu G. Meta-analysis of diagnostic accuracy of nucleic acid amplification tests for abdominal tuberculosis. PLoS One. 2023;18:e0289336. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 23. | Fernández-Carballo BL, Broger T, Wyss R, Banaei N, Denkinger CM. Toward the Development of a Circulating Free DNA-Based In Vitro Diagnostic Test for Infectious Diseases: a Review of Evidence for Tuberculosis. J Clin Microbiol. 2019;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Sharma P, Anthwal D, Kumari P, Gupta RK, Lavania S, Sharma N, Sharma LK, Rath D, Soraganvi PK, Sharma A, Gadpayle AK, Taneja RS, Tyagi JS, Haldar S. Utility of circulating cell-free Mycobacterium tuberculosis DNA for the improved diagnosis of abdominal tuberculosis. PLoS One. 2020;15:e0238119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Yu G, Shen Y, Ye B, Shi Y. Diagnostic accuracy of Mycobacterium tuberculosis cell-free DNA for tuberculosis: A systematic review and meta-analysis. PLoS One. 2021;16:e0253658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Luo Y, Xue Y, Mao L, Lin Q, Tang G, Song H, Wang F, Sun Z. Diagnostic Value of T-SPOT.TB Assay for Tuberculous Peritonitis: A Meta-Analysis. Front Med (Lausanne). 2020;7:585180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Ye Z, Lin Y, Cao Q, He Y, Xue L. Granulomas as the Most Useful Histopathological Feature in Distinguishing between Crohn's Disease and Intestinal Tuberculosis in Endoscopic Biopsy Specimens. Medicine (Baltimore). 2015;94:e2157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |