Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.5225
Revised: May 29, 2024
Accepted: June 19, 2024
Published online: August 6, 2024
Processing time: 81 Days and 24 Hours
Lidocaine/prilocaine (EMLA) cream is a local anesthetic that is applied to the skin or mucosa during painful therapeutic procedures with few reported side effects.
Here, we report the use of dermatoscopy to identify a case of erythema with pur
We conclude that erythema with purpura is caused by irritation and toxicity associated with EMLA, but the specific mechanism by which the toxic substance affects skin blood vessels is unclear. In response to this situation and for cosmetic needs, we recommend tranexamic acid, in addition to routine therapy, to prevent changes in pigmentation in patients with dermatitis.
Core Tip: We report erythema and purpura under dermatoscope induced by lidoca
- Citation: Lin X, Jiang JD, Guo XZ, Hu KK. Dermatoscope for the diagnosis of erythema with purpura induced by lidocaine/prilocaine cream: A case report. World J Clin Cases 2024; 12(22): 5225-5228
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/5225.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.5225
5% lidocaine/prilocaine (EMLA) is a 1:1 low eutectic compound mixture of lidocaine and prilocaine. EMLA is a local anesthetic that is applied to the skin or mucosa during painful therapeutic procedures, such as foreskin surgery, cryosurgery of verrucae, vaccinations, medical cosmetic treatment and so on. EMLA use has been proven to be safe, without interactions associated with laser treatments or other cosmetic procedures[1]. Thus far, some mild adverse reactions have been reported, including erythema, blister, urticaria, allergic contact dermatitis, irritating contact der
A 27-year-old female came to our department for facial cosmetic treatment. Large, scattered, edematous papules were found on the face, accompanied by burning and tingling sensations.
Our doctor planned to provide mesoderm therapy. Before the treatment, 5% EMLA (Tongfang Pharmaceutical Group Co., Ltd. Batch No. 210803) was evenly applied on the whole face (150 mg/cm2) and then covered with fresh-keeping film.
The patient did not have any obvious skin diseases, nor a history of chronic or infectious diseases, or drug allergies.
The patient had no history of allergy to EMLA cream and did not report previous use of any type of non-steroidal anti-inflammatory drugs (NSAIDs). She visited our department many times to receive cosmetic treatments and did not have any abnormalities under local anesthesia with 5% EMLA.
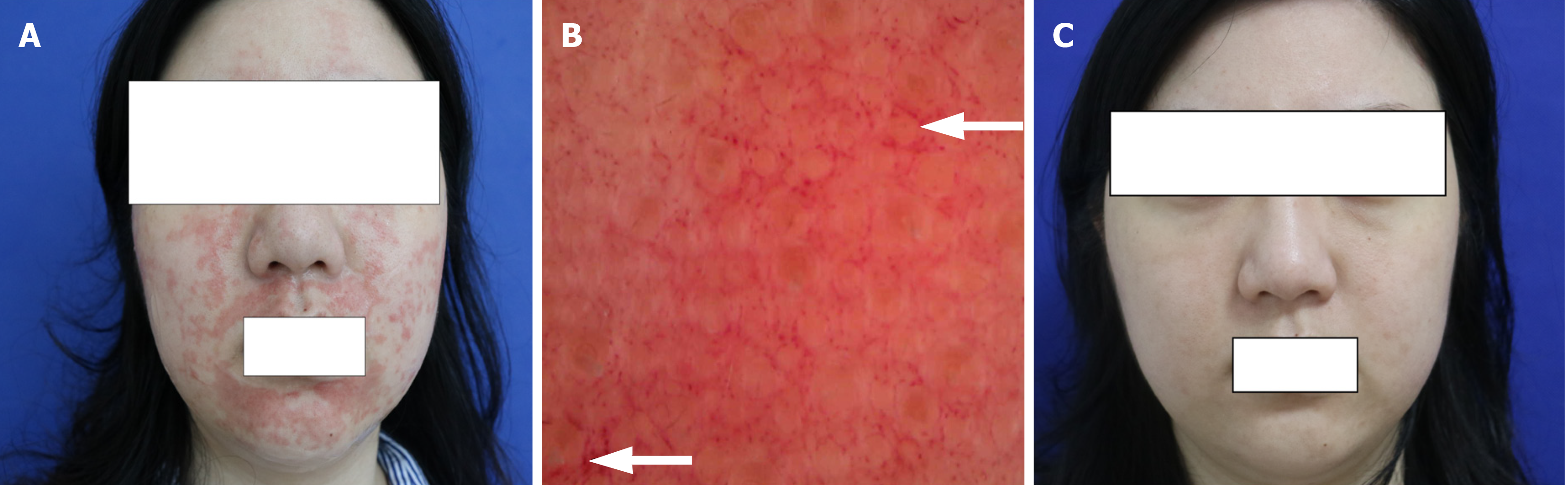
After 20 minutes, large, scattered, edematous papules were found on the face, accompanied by burning and tingling sensations. Although the symptoms disappeared, there were some scattered, erythematous purpuric eruptions that remained (Figure 1A).
The patient’s routine blood test result was normal.
Dermatoscope examination found that the skin lesion was filled with a purple background, vascular congestion was presented as a reticular structure, and dense purpuric globules were spreading (Figure 1B).
The patient received a diagnosis of dermatitis with purpura.
Our doctor immediately removed the EMLA cream and treated her with yellow light for 10 minutes, then the mask was refrigerated for 30 minutes. Medical treatments included prednisone 15 mg qd (every day), Cetirizine 10 mg qn (every night), Vitamin C tablets 0.1 tid (three times a day), and Ello soft paste for the local lesions.
After 10 days, erythematous purpura subsided, and light pigmentation remained locally (Figure 1C).
Only a few cases of erythema with purpura induced by 5% EMLA cream, a rare side effect, have been reported[4-7]. We concluded that most of the reported adverse events occurred in infants. Some authors considered that there was an increase in capillary fragility in young children, as they were prone to severe vomiting, coughing or crying during treatment[6]. Van der Spek et al[7] suspected that the skin lesion was a toxic drug reaction of drugs, to some extent, but they did not further elaborate the toxic process.
However, in contrast to previous reports, we reported a rare case of erythema with purpuric lesions after the app
EMLA (5%) is a 1:1 hypoeutectic compound mixture of lidocaine and prilocaine. In addition, an important excipient in the drug is castor oil, which is extracted from castor seeds and contains poisonous ricinine. We suspect that the castor oil excipient in the EMLA cream causes irritation and toxicity, and results in an erythematous purpuric rash. Although it has been documented that 5% EMLA has no damaging effect on skin microcirculation[7], we still believe that, according to dermoscopic manifestations, the toxic substance in EMLA acts directly on skin blood vessels. Although the exact me
The purpuric eruption often vanishes in approximately two weeks, but in this case, the purpura caused mild pig
In conclusion, we should be aware of the risk of purpura caused by 5% EMLA cream, especially in cosmetic treatments. We recommend the use of tranexamic acid for the prevention and treatment of changes in pigmentation in patients with dermatitis as a result of 5% EMLA cream.
Regarding the routine use of EMLA as a local anesthetic for cosmetic treatment, we should ask about the patient’s allergy history and focus on the dosage, area of application and time of use. Most importantly, we should focus on skin reactions during external application. Petechial and purpuric lesions are rare reactions, and the pathogenesis of these adverse drug reactions remains unclear, but the main cause of irritation and toxicity caused by EMLA cream may be the drug’s ability to act on blood vessels. We recommend tranexamic acid, in addition to routine therapy, to prevent changes in pig
| 1. | Yu W, Wang T, Zhu J, Qiu Y, Chen H, Jin Y, Yang X, Hu X, Chang L, Chen Y, Ma G, Lin X. EMLA cream does not influence efficacy and pain reduction during pulsed-dye laser treatment of port-wine stain: a prospective side-by-side comparison. Lasers Med Sci. 2018;33:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Wagner RF Jr, Flores CA, Argo LF. A double-blind placebo controlled study of a 5% lidocaine/prilocaine cream (EMLA) for topical anesthesia during thermolysis. J Dermatol Surg Oncol. 1994;20:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Kuiper-Prins E, Kerkhof GF, Reijnen CG, van Dijken PJ. A 12-Day-Old Boy with Methemoglobinemia After Circumcision with Local Anesthesia (Lidocaine/Prilocaine). Drug Saf Case Rep. 2016;3:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Neri I, Savoia F, Guareschi E, Medri M, Patrizi A. Purpura after application of EMLA cream in two children. Pediatr Dermatol. 2005;22:566-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Calobrisi SD, Drolet BA, Esterly NB. Petechial eruption after the application of EMLA cream. Pediatrics. 1998;101:471-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Neild GH, Kumar A, Butt I, Boyes S. Topical local anaesthetic cream causing persistent skin erythaema over haemodialysis graft. NDT Plus. 2011;4:215-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | de Waard-van der Spek FB, Oranje AP. Purpura caused by Emla is of toxic origin. Contact Dermatitis. 1997;36:11-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Wang JV, Valiga A, Geronemus RG. Real-World Experience With Oral Tranexamic Acid and Lasers for Pigmentary Disorders: A 5-Year Safety Review. Dermatol Surg. 2021;47:1303-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |