Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.5196
Revised: May 24, 2024
Accepted: June 12, 2024
Published online: August 6, 2024
Processing time: 86 Days and 21.3 Hours
Plasma cell myeloma (PCM) is characterized by hypercalcemia, renal impairment, anemia, and bone destruction. While pleural effusion, ascites, abdominal pain, and bloody stool are common manifestations of lung disease or gastrointestinal disorders, they are rarely observed in patients with PCM.
A 66-year-old woman presented with complaints of recurrent chest tightness, wheezing, and abdominal bloating accompanied by bloody stools. Computed tomography revealed pleural effusion and ascites. Pleural effusion tests showed inflammation, but the T-cell spot test and carcinoembryonic antigen were negative. Endoscopy showed colonic mucosal edema with ulcer formation and local intestinal lumen stenosis. Echocardiography revealed enlarged atria and reduced left ventricular systolic function. The diagnosis remained unclear. Further testing revealed elevated blood light chain lambda and urine immunoglobulin levels. Blood immunofixation electrophoresis was positive for immunoglobulin G lambda type. Smear cytology of the bone marrow showed a high proportion of plasma cells, accounting for about 4.5%. Histopathological examination of the bone marrow suggested PCM. Flow cytometry showed abnormal plasma cells with strong expression of CD38, CD138, cLambda, CD28, CD200, and CD117. Fluorescence in situ hybridization gene testing of the bone marrow suggested 1q21 gene amplification, but cytogenetic testing showed no clonal abnormalities. Colonic mucosa and bone marrow biopsy tissues were negative for Highman Congo red staining. The patient was finally diagnosed with PCM.
A diagnosis of PCM should be considered in older patients with pleural effusion, ascites, and multi-organ injury.
Core Tip: Plasma cell myeloma is a common hematologic disorder that typically presents with hypocalcemia, anemia, kidney damage, and bone destruction. However, some patients present it atypically, with symptoms from other systems as the main manifestation, making the diagnosis difficult. For patients with complex presentations, necessary tests should be gradually refined through a multidisciplinary approach to obtain an accurate diagnosis. Clinicians need to accumulate clinical experience to effectively diagnose such difficult cases.
- Citation: Yan MX. Pleural effusion, ascites, colon ulcers and hematochezia: What we can learn from the diagnostic process of a patient with plasma cell myeloma: A case report. World J Clin Cases 2024; 12(22): 5196-5207
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/5196.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.5196
Plasma cell myeloma (PCM) is a type of bone malignant tumor that includes solitary plasma cell tumors, extramedullary plasmacytomas, multiple myeloma (MM), and plasma cell leukemia. MM, which is manifested as a multi-site bone injury, often presents with hypercalcemia, renal impairment, anemia, and bone destruction. Literature shows an increasing incidence of MM, especially in developed industrialized countries[1,2]. An epidemiological study suggested that MM is the second most common hematologic malignancy in China, and the incidence is increasing with age[3]. Typical MM manifestations include calcium elevation, renal insufficiency, anemia, bone disease, and secondary amyloidosis, which are easy to diagnose clinically. However, the clinical manifestations of PCM alone are atypical or more diverse, making its early diagnosis challenging.
Abdominal pain, blood in the stool, and ascites are typical manifestations of gastrointestinal disorders. Although other non-digestive disorders or systemic diseases can also present with these manifestations, digestive disorders such as ischemic or inflammatory bowel disease, colonic malignancy, and liver cirrhosis are often considered initially. When non-digestive diseases cause the above manifestations, manifestations of the primary disease are often more obvious. Patients with ischemic bowel disease may have a history of cardiovascular and cerebrovascular diseases in addition to abdominal pain and bloody stool. Patients with malignant tumors often appear emaciated and chronically ill, while patients with ascites caused by liver cirrhosis often have a history of chronic liver disease or long-term alcoholism. Relevant laboratory or imaging studies can help confirm the initial diagnosis.
However, some primary disorders that cause these symptoms are insidious, making a definitive diagnosis difficult, especially for young physicians who lack clinical experience. Careful clinical thinking is important for diagnosing difficult diseases, making it important for clinicians to gradually cultivate logical thinking skills in clinical practice. This report reviews the clinical diagnostic process of a patient and shows the process of step-by-step selection of tests based on symptoms, gradually excluding certain diseases, and finally making a clear diagnosis. This approach aims to provide references for enhancing the clinical diagnostic experience.
The patient, a 66-year-old woman, was referred to our hospital because of recurrent chest tightness, wheezing, and abdominal bloating accompanied by bloody stools, and her treatment at a local hospital was not effective.
A computed tomography (CT) scan conducted at the local hospital revealed pleural effusion, ascites, mesenteric and omental thickening, and exudative changes. Antibacterial and enema treatments were ineffective. The patient passed dark red bloody stools 3-4 times a day, accompanied by abdominal discomfort.
A history of hypertension for more than 20 years.
Unremarkable.
On admission, the patient had a body temperature of 36.8 °C, a heart rate of 80 beats/min, a breathing rate of 20 times/min, a blood pressure of 90/60 mmHg, a height of 165 cm, and a weight of 65 kg. The patient’s nutritional status was average, with normal skin appearance and no jaundice. Auscultation of the lungs revealed low breath sounds in both lungs but no rales. There was no enlargement of the heart, the heartbeat rhythm was regular, and there was no obvious pathological murmur. The abdomen was slightly distended but soft, without obvious tenderness. The liver and spleen were not palpable, and Murphy’s sign was negative. There was no percussion pain in the liver and kidney area. Bowel sounds occurred approximately 5 times/min, and mobile dullness was negative. The dorsalis pedis artery pulses were normal in both lower extremities.
The main laboratory test results of blood, chest drainage fluid, and stool are shown in Table 1. The results indicated anemia, gastrointestinal bleeding, electrolyte imbalances, and significant hypoproteinemia. Pleural effusion tests revealed inflammatory markers, but the T-cell spot test and tumor marker carcinoembryonic antigen were negative.
| Blood test | Pleural effusion test | Stool test | |||
| WBC | 8.74 × 109/L | Color | Yellow | White blood cells | Small amount |
| Hb | 101 g/L↓ | Transparent | Turbid | Red blood cells | Small amount |
| K+ | 3.09 mmol/L↓ | Rivalta test | Positive | Pus cells | None |
| Na+ | 127 mmol/L↓ | Red blood cell count | 2017 × 106/L | Macrophages | None |
| Ca2+ | 1.87 mmol/L↓ | White blood cell count | 3000 × 106/L | Occult blood | Positive |
| CRP | 124.1 mg/L↑ | Granulocytes | 85% | Clostridioides difficile toxin A/B | Negative |
| PCT | 0.213 ng/mL↑ | Lymphocytes | 10% | Clostridioides difficile glutamate dehydrogenase antigen | Positive |
| Albumin | 25.1 g/L↓ | Mesothelial cells | 5% | Fecal calprotectin | 600 μg/g↑ |
| Creatinine | 32 μmol/L↓ | Protein | 21 g/L | ||
| BNP | 801.05 pg/mL↑ | Cholesterol | 0.66 mmol/L | ||
| T-SPOT | 0 | CEA | 1.03 ng/mL | ||
| CEA | 4.40 ng/mL | ||||
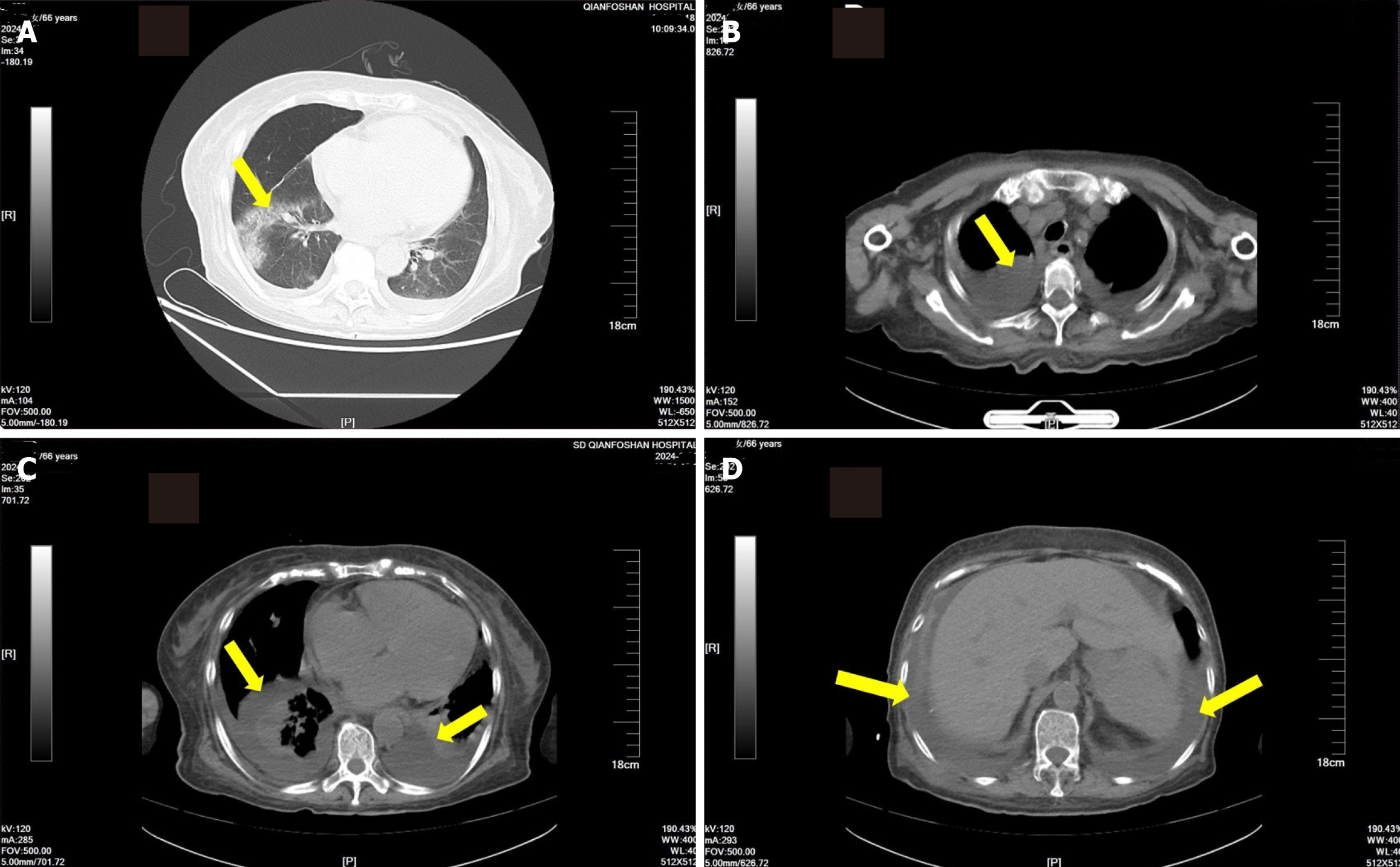
CT scan revealed that the patient had a lung infection with both pleural effusion and ascites. Pleural effusion was more evident (Figure 1).
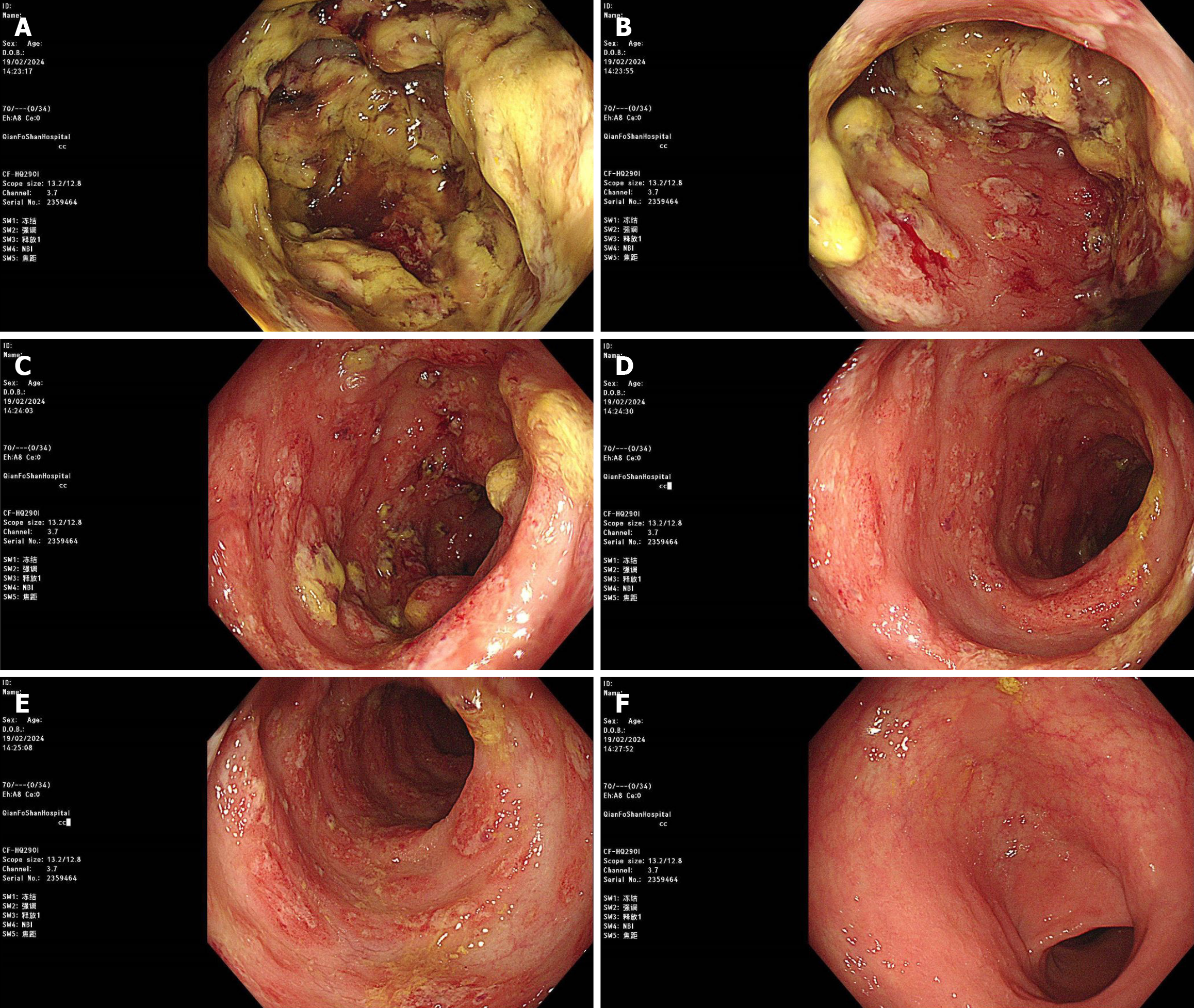
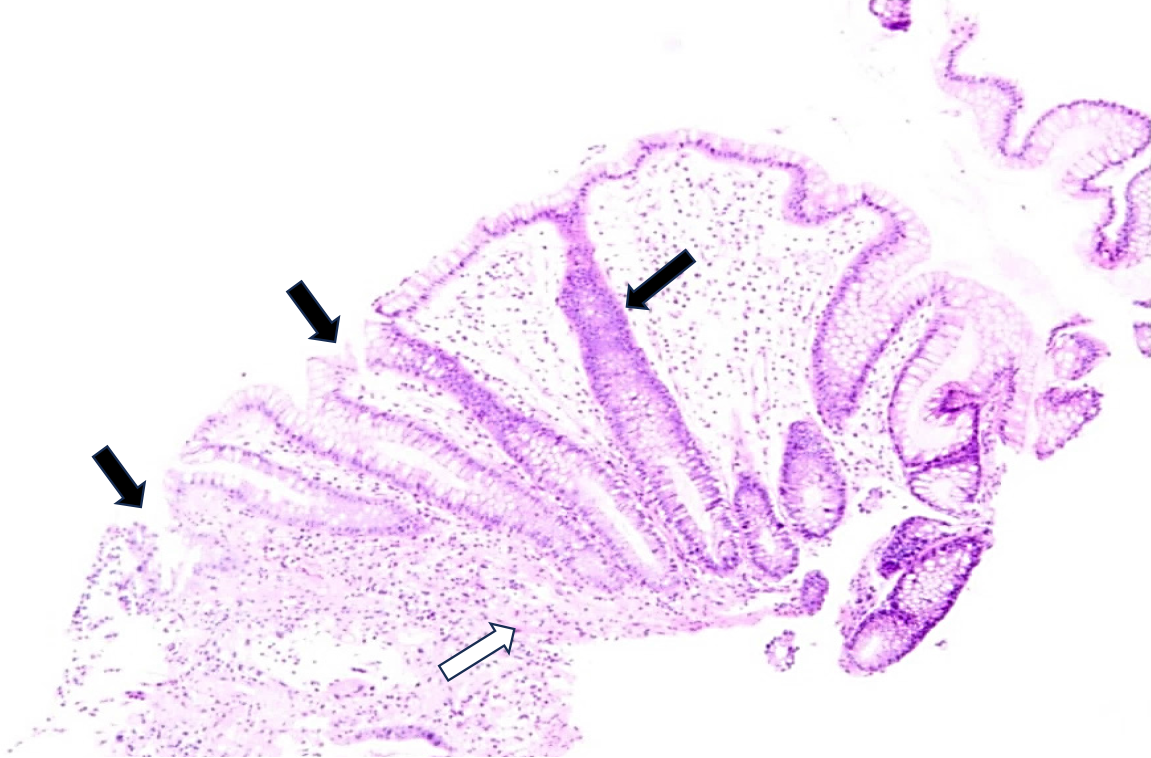
Because the patient had intermittent abdominal pain and bloody stool, a colonoscopy was performed, which showed colonic mucosal edema with ulcer formation and local intestinal lumen stenosis (Figure 2). A biopsy of the mucosal histopathology showed infiltration of inflammatory cells in the mucosa, local ulcer exudation, and fibrogranulomatous tissue hyperplasia (Figure 3).
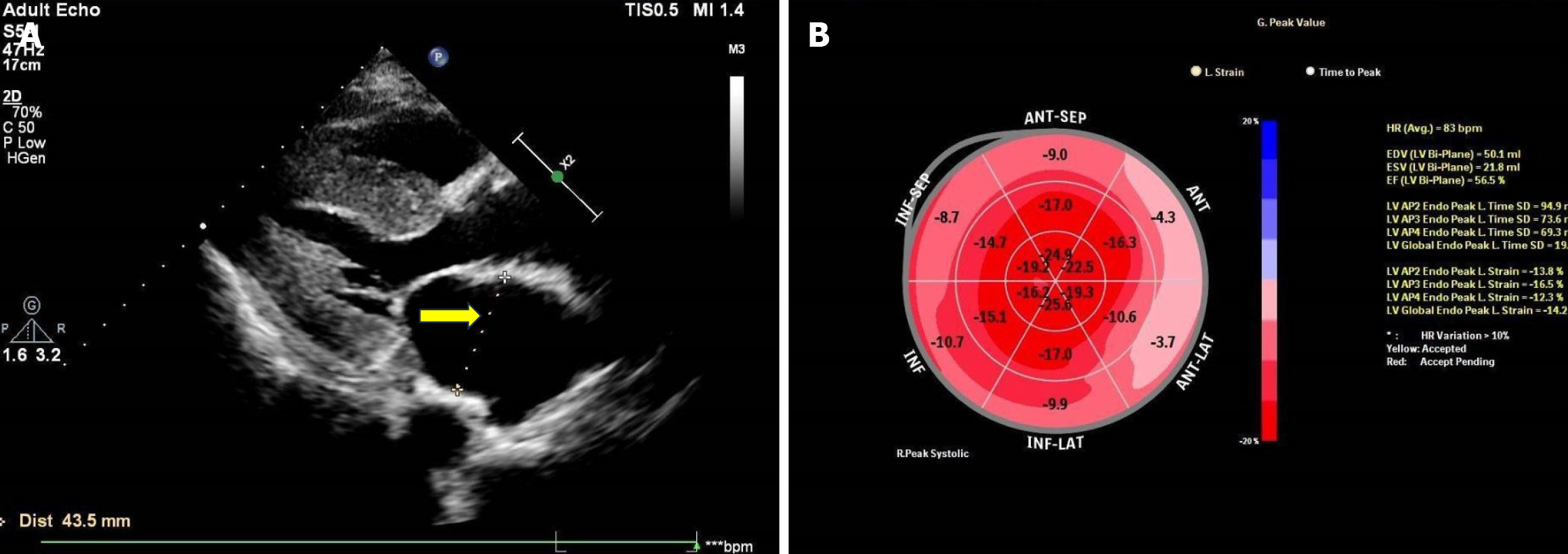
Echocardiography revealed bilateral atria enlargement (left 43.5 mm, right 60 mm); the ventricular septum was thickened (15 mm), and the myocardial echo was heterogeneous. The aortic valve was thickened, with enhanced echo. The left ventricular ejection fraction was 55%, but two-dimensional speckle tracking echocardiography showed that the global longitudinal strain (GLS) of the left ventricle was reduced at −14.2%, with apical preservation. The myocardial velocity of the mitral annulus during systole and diastole was reduced and less than 5 cm/s, and the apical GLS/basal segment GLS ratio of the ventricular septum was 2.8 (Figure 4).
The clinical manifestation of the patient was complex and the diagnosis remained unclear. Hence, experts from hematology, rheumatology and immunology, respiratory, and gastroenterology were involved in multidisciplinary consultation and discussion. Based on the results, it was decided that malignant pleural effusion and ascites due to primary lung or pleural tumor and abdominal malignancy could be excluded. Myeloma or secondary cardiac amyloidosis were considered potential diagnoses. Therefore, further laboratory tests, including bone marrow cytology and biopsy, were recommended.
Blood and urine light chain, blood immunofixation electrophoresis, β2 microglobulin and anti-β2 glycoprotein 1 antibody detection: The results showed that the light chain lambda (λ) levels in blood and urine were 2.61 g/L and 20.9 mg/L, respectively, which were higher than the normal values (0.9-2.1g/L and < 3.9 mg/L, respectively). The ratio of light chain kappa (κ) to λ (κ/λ) in blood was 0.370, which was lower than the normal range (1.35-2.65). Blood immunofixation electrophoresis showed a positive result for immunoglobulin G (IgG) lambda type. The urinary immunoglobulin kappa light chain level was 23.9 mg/L, which was also elevated compared to the normal reference value (< 7.1 mg/L). Serum β2 microglobulin was normal at 2.43 mg/L (range: 1.0-3.0 mg/L), but the anti-β2 glycoprotein 1 antibody level (253 RU/mL) was obviously higher than the normal reference value (0-20 RU/mL).
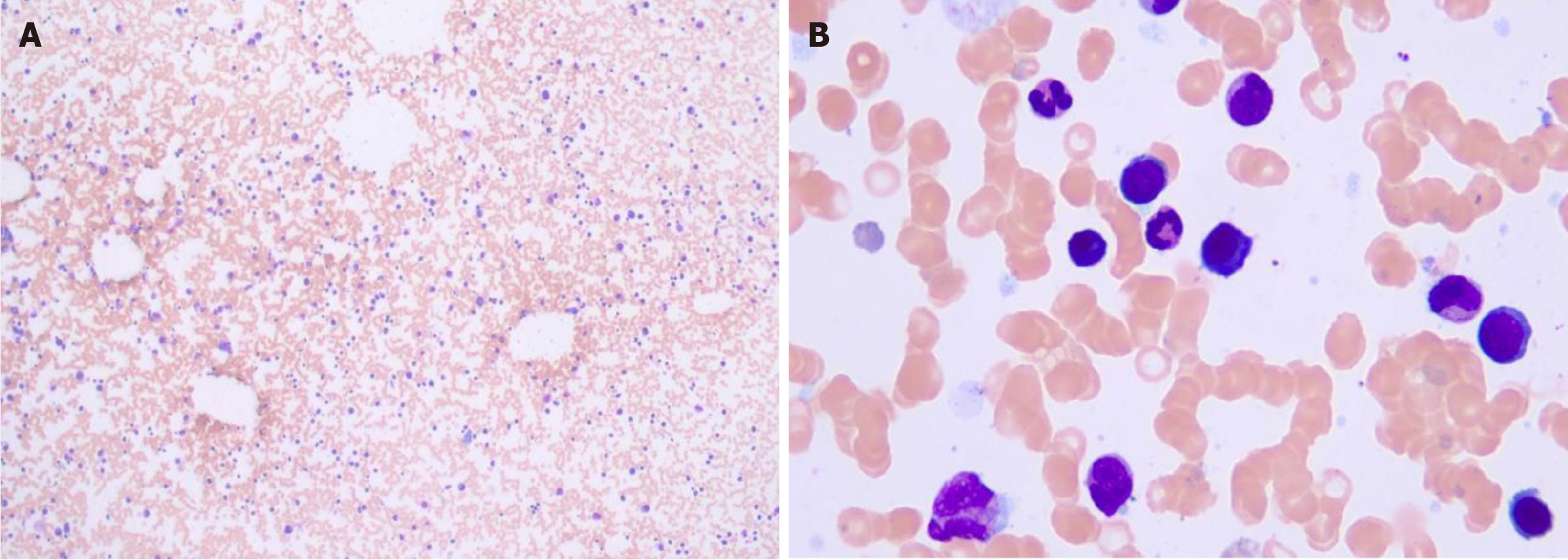
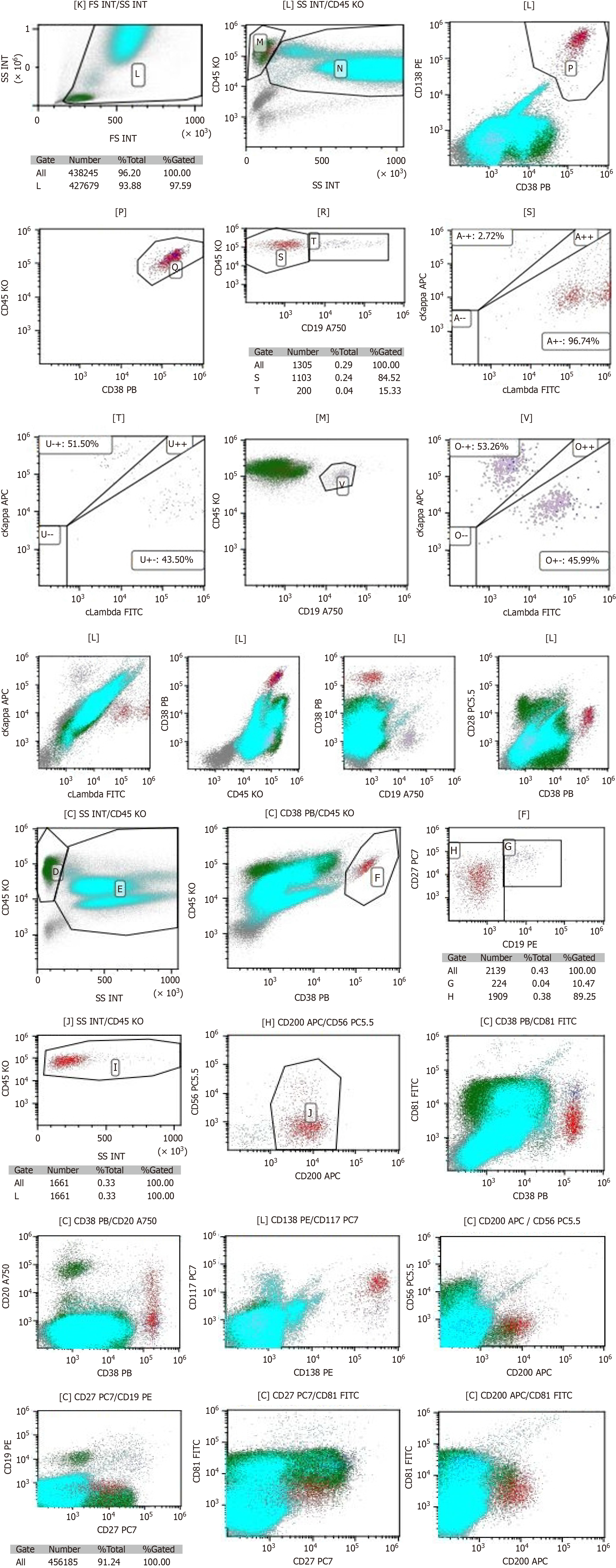
Smear cytology of the bone marrow aspirate fluid showed that the bone marrow hyperplasia was active, the proportion of erythroid cells was low, and the proportion of plasma cells was high, accounting for about 4.5% (Figure 5). Flow cytometry showed the presence of abnormal plasma cells in the specimen, accounting for about 0.26% of the nucleated cells. CD38, CD138, cLambda, CD28, CD200 and CD117 were strongly expressed. CD27 and CD20 were weakly expressed, while CD45, CD19, cKappa, CD56, and CD81 were not expressed (Figure 6).
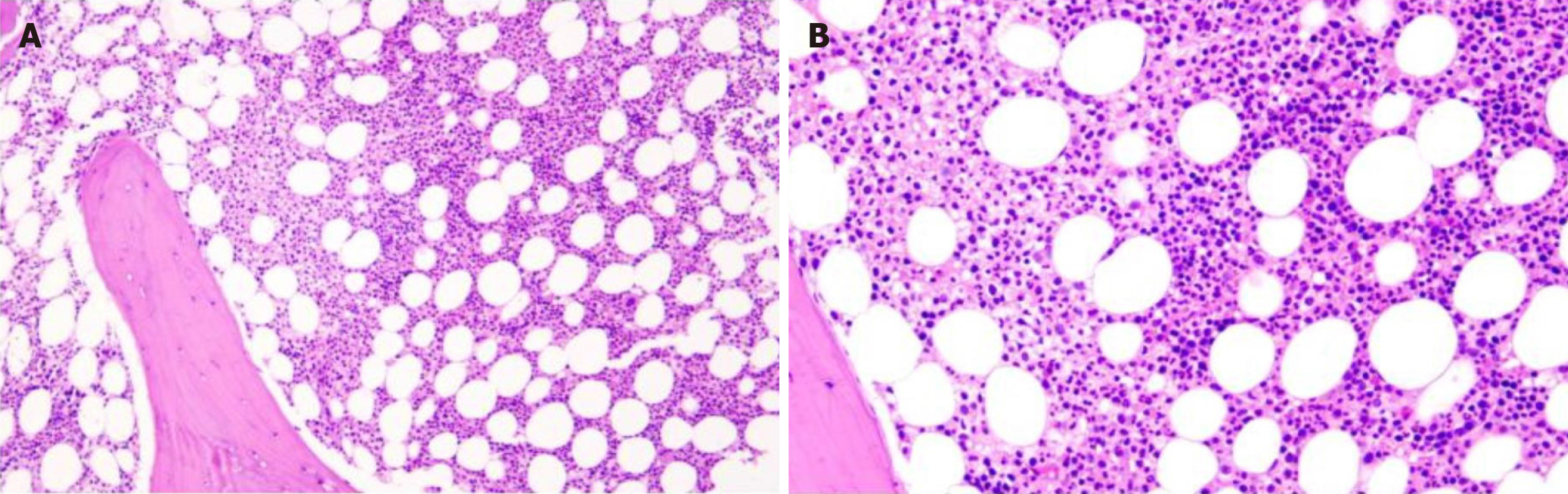
Bone marrow histopathology showed active bone marrow hyperplasia with an increased proportion of granulocyte erythrocytes, predominantly granulocytic hyperplasia. There was no obvious abnormality in granuloerythrocytes at all stages. Trabecular plasma cells were proliferative and scattered, with positive expressions for CD38 and CD138, Lambda, and kappa in individual cells. A small number of megakaryocytes were scattered, with no hemosiderosis and fibrous tissue hyperplasia. Histopathological features suggested PCM (Figure 7).
Twenty cells were analyzed separately for each probe type, and the results suggested 1q21 gene amplification (26%, 3 copies). There were no abnormalities in RB1, FGFR3/IGH, MAF/IGH, and TP53 genes.
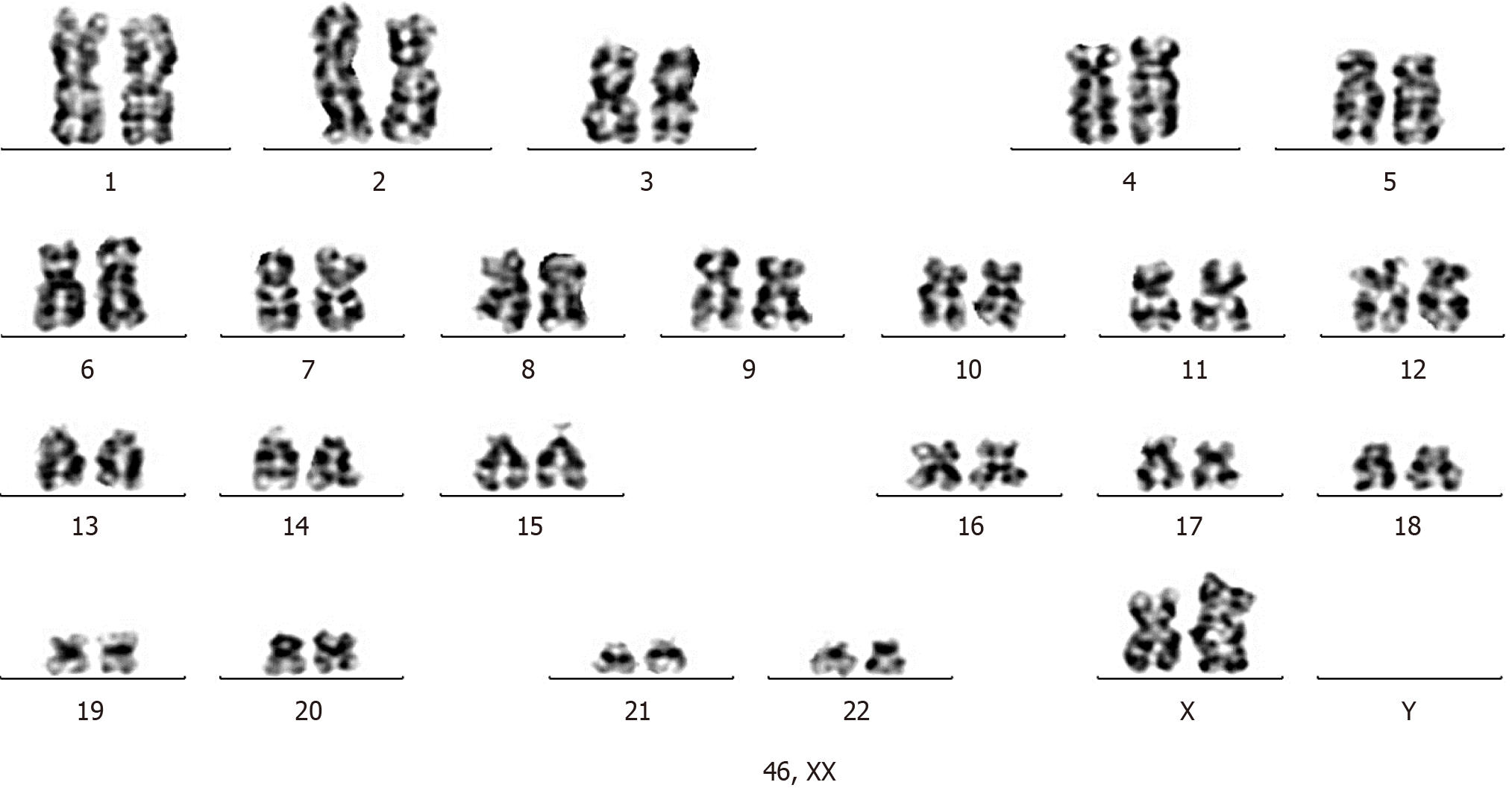
In total, 20 cells were counted, and 20 division images were analyzed. The results showed 46 XX karyotype with no clonal abnormalities (Figure 8).
To confirm the presence of amyloidosis, the colonic mucosa and bone marrow biopsy tissues were further stained with Highman Congo red staining. The results showed that the colonic mucosa and bone marrow tissues were both negative for amyloid deposits by Congo red staining.
The patient’s bone marrow aspirate pathology and cytology, including bone marrow flow cytometry, support the diagnosis of IgG-type PCM. The colonic mucosal and bone marrow tissues were negative for amyloidosis as indicated by Congo red staining. Fluorescence in situ hybridization suggested a poor prognosis. Therefore, the final diagnosis for this patient was PCM.
The patient was transferred to the hematology department for continued treatment, which included a combined approach of etiology-specific treatment, symptomatic treatment, and nutritional support.
The patient’s condition stabilized and her symptoms improved; however, the long-term effects of the treatment need to be further observed.
The main clinical manifestations of this patient were chest tightness, abdominal distension, and intermittent blood in the stool. The initial examination at the local hospital revealed that the chest and abdominal effusion were accompanied by CT findings of local edema of the colon; hence, the patient was first considered to have gastrointestinal disease. The pleural effusion was the main cause of chest tightness and wheezing after movement.
Inflammation and primary or secondary tumors of the lungs and pleura are common causes of pleural effusions, which can be identified through examinations of pleural fluid and chest CT[4]. In this case, the patient did not have hypoalbuminemia at the time of onset of symptoms, and although hypoalbuminemia was present in the later stages, it could not be considered a cause of pleural effusion. The patient did not have a fever or chest pain, and chest CT showed lung infection without pleural thickening. No tumor cells were found in the pleural effusion, excluding the possibility of a neoplastic nature or purulent infection of the effusion.
There are many causes of ascites, such as peritonitis, primary or secondary peritoneal tumors, and severe hypoproteinemia due to liver failure or massive protein loss. The patient did not initially have hypoalbuminemia, and CT did not reveal any abdominal or peritoneal mass lesions or liver damage. Severe acute pancreatitis can also cause ascites and pleural effusions, but pancreatitis was ruled out in this patient through CT and laboratory test results. CT showed local edema of the colon, but no signs of peritonitis. Therefore, the ascites could not be attributed to abdominal inflammation. Thus, the patient’s pleural effusion and ascites were not induced by tumors or severe inflammation, suggesting another cause.
The patient underwent a colonoscopy to determine the cause of hematochezia and anemia, which revealed colonic ulcers and narrowing of the colonic lumen. However, pathology excluded colon tumors. Inflammatory bowel diseases such as ulcerative colitis or Crohn’s disease have typical characteristic findings under colonoscopy. Some colonic ulcerative lesions, such as ischemic bowel disease, radiation enteritis, or intestinal tuberculosis infection, do not have specific characteristic findings on colonoscopy[5-7]. Enteric tuberculosis infection must be diagnosed with active tuberculosis tests, such as a positive T-cell spot test. Radiation enteritis requires a clear history of radiation therapy. The typical manifestation of ischemic bowel disease is recurrent abdominal pain accompanied by bloody diarrhea. This condition is common in patients with vascular dysfunction, such as those with cardiovascular or cerebrovascular arteriosclerosis, and causes ischemic mucosal damage and even ulcer formation, mainly on the left side of the colon[5]. In this patient, the presence of ischemic bowel disease could neither be excluded nor be confirmed.
Another question to consider is whether the patient had Clostridium difficile (C. difficile)-associated enteritis because her stool testing for C. difficile was found to be negative for C. difficile toxin A/B but positive for C. difficile glutamate dehydrogenase antigen. Patients with C. difficile-associated enteritis often have a history of long-term antibiotic or immunosuppressant therapy; however, this patient did not have such a history. C. difficile infection is diagnosed when the patient is positive for both fecal C. difficile toxin A/B and C. difficile glutamate dehydrogenase antigen. A positive result for glutamate dehydrogenase antigen alone is insufficient for diagnosis[8]. Thus, the result is not sufficient to diagnose C. difficile infection; however, it suggests that the patient may have intestinal microbiota abnormalities, which may be related to her intestinal lesions. It has been reported that changes in intestinal microbiota are related to the occurrence and development of MM[9]. Furthermore, the characteristics of intestinal microbiota in MM patients are changed[10-12]. In this case, the intestinal microbiota was not studied, but the lesions on the colon and the positive stool C. difficile glutamate dehydrogenase antigen indicated abnormalities in the intestinal microenvironment.
Thus, the presence of pleural effusion, ascites, hematochezia, and colonic ulcers in this patient cannot be explained by common clinical etiologies. In this case, immune or blood system disorders need to be considered. Diseases of solid organs can usually be identified through clinical manifestations and imaging tests, while diseases of the immune and blood system are sometimes insidious. For this patient, cardiac ultrasonography revealed abnormalities, indicating the possibility of cardiac amyloidosis[13]. The most common type of systemic amyloidosis is systemic light chain (AL) amyloidosis, a disease in which the monoclonal immunoglobulin light chain misfolds to form amyloid deposits in tissues and organs, including cardiac amyloidosis causing tissue structure destruction, organ dysfunction, and progressive deterioration, mainly related to abnormal proliferation of clonal plasma cells. AL amyloidosis is more common in older adults, with a median age at diagnosis of around 60 years and a median survival of less than 1 year for patients with severe cardiac involvement[14-16]. Although this patient had abnormal cardiac dysfunction, later pathological staining excluded systemic amyloidosis.
Thus, other immune and hematologic diseases should be investigated. The patient had no abnormalities in a variety of autoantibody tests, but the immunofixation electrophoresis and abnormal findings in the hematuria light chain suggested a high probability of myeloma. Therefore, bone marrow histology became necessary. The patient’s bone marrow cytology, cytometry, and histopathology all supported the presence of PCM, confirming the diagnosis. Bone marrow fluorescence in situ hybridization showed amplification of the 1q21 gene, indicating that the patient would have a poor prognosis[17]. CT showed no significant or multiple bone destruction, suggesting that her myeloma had not progressed to advanced stages.
Common manifestations of PCM, in particular MM, include bone pain, anemia, renal impairment, and increased serum calcium levels[18]. However, the clinical features of this patient were not typical. The patient experienced chest tightness and abdominal bloating, and the diagnostic process began with the discovery of pleural effusion and ascites. Although there has been a case report of an elderly patient with MM presenting with pleural effusion and ascites as the main clinical manifestations[19], this is not typical of PCM. It has been reported that pleural effusion in MM patients is due to the direct infiltration of myeloma cells adjacent to the chest wall or lymphatic obstruction, with IgG myeloma being the most common[20].
The intestinal lesions in this patient are speculated to be related to the occurrence and development of myeloma, which causes long-term immune dysfunction and intestinal flora imbalance. The microbiome may influence the pathogenesis of MM[9]. The diagnosis of PCM is based on histopathological testing of the bone marrow. A recent study found that the most prevalent age group for MM was 60-64 years, and cytogenetic abnormalities were found in 51.3% of patients[21]. No chromosomal abnormalities were found in this patient. Overall, this patient represents a case of PCM with an atypical clinical presentation.
The clinical manifestations of PCM are complex. The diagnosis of PCM should be considered in elderly patients with pleural effusion and ascites. Such patients may have multiple tissue and organ injuries, such as the colon. When the diagnosis is challenging, bone marrow cytology and histopathology should be performed.
| 1. | Cowan AJ, Allen C, Barac A, Basaleem H, Bensenor I, Curado MP, Foreman K, Gupta R, Harvey J, Hosgood HD, Jakovljevic M, Khader Y, Linn S, Lad D, Mantovani L, Nong VM, Mokdad A, Naghavi M, Postma M, Roshandel G, Shackelford K, Sisay M, Nguyen CT, Tran TT, Xuan BT, Ukwaja KN, Vollset SE, Weiderpass E, Libby EN, Fitzmaurice C. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018;4:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 2. | Padala SA, Barsouk A, Barsouk A, Rawla P, Vakiti A, Kolhe R, Kota V, Ajebo GH. Epidemiology, Staging, and Management of Multiple Myeloma. Med Sci (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 3. | Wang S, Xu L, Feng J, Liu Y, Liu L, Wang J, Liu J, Huang X, Gao P, Lu J, Zhan S. Prevalence and Incidence of Multiple Myeloma in Urban Area in China: A National Population-Based Analysis. Front Oncol. 2019;9:1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Pleural and Mediastinal Diseases Working Group (Preparatory) of Chinese Thoracic Society. [Chinese expert consensus on diagnosis of pleural effusion]. Zhonghua Jie He He Hu Xi Za Zhi. 2022;45:1080-1096. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Ahmed M. Ischemic bowel disease in 2021. World J Gastroenterol. 2021;27:4746-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (4)] |
| 6. | Hale MF. Radiation enteritis: from diagnosis to management. Curr Opin Gastroenterol. 2020;36:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Maulahela H, Simadibrata M, Nelwan EJ, Rahadiani N, Renesteen E, Suwarti SWT, Anggraini YW. Recent advances in the diagnosis of intestinal tuberculosis. BMC Gastroenterol. 2022;22:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Kelly CR, Fischer M, Allegretti JR, LaPlante K, Stewart DB, Limketkai BN, Stollman NH. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am J Gastroenterol. 2021;116:1124-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 316] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 9. | Ahmed N, Ghannoum M, Gallogly M, de Lima M, Malek E. Influence of gut microbiome on multiple myeloma: friend or foe? J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Antoine Pepeljugoski C, Morgan G, Braunstein M. Analysis of Intestinal Microbiome in Multiple Myeloma Reveals Progressive Dysbiosis Compared to MGUS and Healthy Individuals. Blood. 2019;134:3076-3076. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Jian X, Zhu Y, Ouyang J, Wang Y, Lei Q, Xia J, Guan Y, Zhang J, Guo J, He Y, Wang J, Li J, Lin J, Su M, Li G, Wu M, Qiu L, Xiang J, Xie L, Jia W, Zhou W. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome. 2020;8:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | Pianko MJ, Devlin SM, Littmann ER, Chansakul A, Mastey D, Salcedo M, Fontana E, Ling L, Tavitian E, Slingerland JB, Slingerland AE, Clurman A, Gomes ALC, Taur Y, Pamer EG, Peled JU, van den Brink MRM, Landgren O, Lesokhin AM. Minimal residual disease negativity in multiple myeloma is associated with intestinal microbiota composition. Blood Adv. 2019;3:2040-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6446] [Cited by in RCA: 9320] [Article Influence: 932.0] [Reference Citation Analysis (0)] |
| 14. | Kourelis TV, Kyle RA, Dingli D, Buadi FK, Kumar SK, Gertz MA, Lacy MQ, Kapoor P, Go RS, Gonsalves WI, Warsame R, Lust JA, Hayman SR, Rajkumar SV, Zeldenrust SR, Russell SJ, Lin Y, Leung N, Dispenzieri A. Presentation and Outcomes of Localized Immunoglobulin Light Chain Amyloidosis: The Mayo Clinic Experience. Mayo Clin Proc. 2017;92:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | An N, Li X, Shen M, Chen SL, Huang ZX. [Clinical analysis of multiple myeloma-associated amyloidosis]. Zhonghua Yi Xue Za Zhi. 2018;98:365-369. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Diao X, Li J, Ouyang J, Liu J, Huang B, Chen M, Gu J. Flow cytometry-based immunophenotypic analysis of primary systemic light chain amyloidosis. Oncol Lett. 2017;13:2691-2697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Xu J, Xu B, Li P, Yang Y, Wang W, Xu T, Maihemaiti A, Lan T, Wang P, Ren L, Zhou C, Aihemaiti X, Liu P. The prognostic role of 1q21 gain/amplification in newly diagnosed multiple myeloma: The faster, the worse. Cancer. 2023;129:1005-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Chinese Hematology Association; Chinese Society of Hematology. [Guidelines for the diagnosis and management of multiple myeloma in China (2022 revision)]. Zhonghua Nei Ke Za Zhi. 2022;61:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Xue BY, Shi QL, Xu RT, Tang QY, Zhu GQ. One case of elderly multiple myeloma with multiple serous effusion as the first manifestation. Shiyong Laonian Yixue. 2021;35:1107-1108. [DOI] [Full Text] |
| 20. | Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 21. | Shokripour M, Hosseini SE, Omidifar N, Mokhtari M, Safaei A. Cytogenetic, Clinical, Hematologic, Demographic, Immunohistochemical, and Flow Cytometry Characteristics of Patients with Plasma Cell Neoplasm in Five Years: A First Report from Iran. Iran J Med Sci. 2024;49:77-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |