Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.5159
Revised: May 30, 2024
Accepted: June 18, 2024
Published online: August 6, 2024
Processing time: 118 Days and 14.1 Hours
Lower extremity lymphedema is a common complication following treatment for gynecological malignancies. Its incidence rate can reach up to 70%, affecting ~20 million people worldwide. However, specialized treatment centers are scarce, and there is a lack of consensus on treatment approaches. Furthermore, there are even fewer reports on the systematic and effective treatment of severe lymphedema with malformations. Effective management of this condition remains a significant challenge for clinicians.
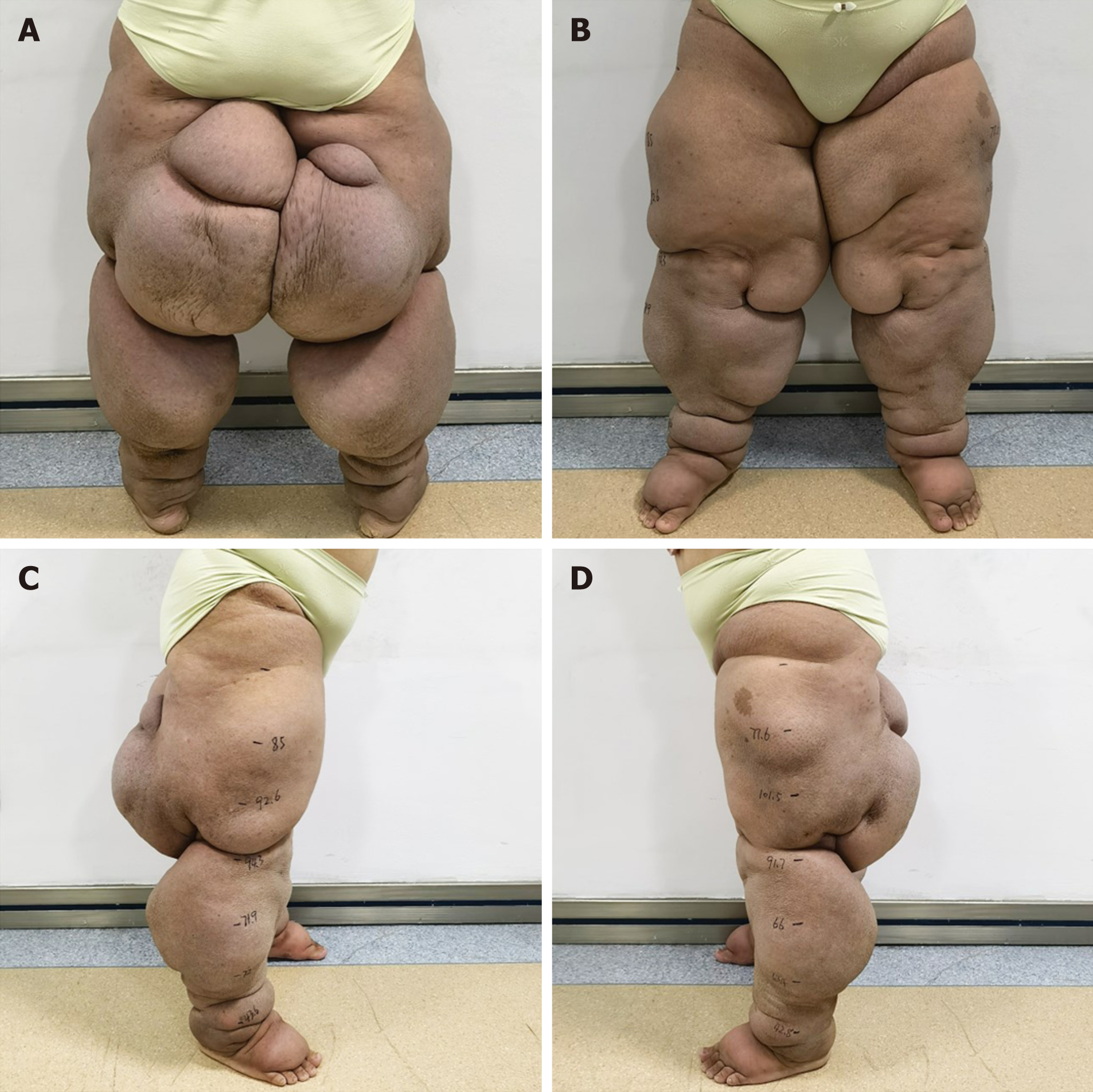
A 40-year-old woman developed bilateral leg swelling 6 years after receiving treatment for endometrial cancer. Since August 2018, she experienced > 30 episodes of lymphangitis. Upon presentation, she exhibited bilateral leg swelling and deformation, with four large swellings in the posterior thigh that impeded movement, and pain in the limbs. Skin manifestations included lichenoid lesions and features of deep sclerosis. Radionuclide lymphoscintigraphy confirmed the diagnosis of lower limb lymphedema. After 6 mo of complex decongestive the
The combined application of CDT and LVA therapy demonstrates significant positive effects in the treatment of severe, deformed stage III lymphedema.
Core Tip: We report a 40-year-old woman who developed severe lower extremity lymphedema following treatment for endometrial cancer. She underwent a combination of complex decongestive therapy (CDT) and lymphaticovenous anastomosis (LVA). This significantly improved lower limb motor function, with the near-complete resolution of discomfort and a substantial return to normal limb appearance. This case suggests that combined application of CDT and LVA offers a promising therapeutic approach for severe, deformed lymphedema. This minimally invasive strategy, with its associated benefits of minimal surgical trauma and good postoperative aesthetics, has the potential to equip future clinicians with improved management strategies for severely deformed lower extremity lymphedema.
- Citation: Wang HJ, He QQ, Liu CR, Wang YY, Liu XW. Lymphovenous anastomosis and complex decongestive therapy for severe deformed lymphedema with recurrent infection: A case report. World J Clin Cases 2024; 12(22): 5159-5167
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/5159.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.5159
Lymphedema is a chronic circulatory disorder primarily caused by abnormalities in the lymphatic system. It can also be triggered by infections, radiotherapy, chemotherapy, and surgical procedures[1]. This condition arises when impaired lymphatic fluid drainage leads to localized fluid accumulation, resulting in edema. Secondary lower limb lymphedema is a frequent consequence of treatment for gynecological tumors. It can significantly affect patients' physical function and appearance. Over time, it may contribute to varying degrees of impairment in social interactions, quality of life, and mental health[1,2]. The reported incidence of lymphedema in patients treated for gynecological tumors ranges from 25% to 70%[3]. Notably, the incidence of lower limb lymphedema following surgery for endometrial cancer exhibits significant variation, with reported rates between 1.2% and 47%[4].
Currently, there are various treatment approaches for lymphedema, including direct excision, radical reduction with perforator preservation (RRPP), lymphaticovenous anastomosis (LVA), vascularized lymph node transfer, suction-assisted lipectomy (SAL), and complex decongestive therapy (CDT)[5]. Istranov et al[6] reported successful combination of CDT and reconstructive surgery for a giant scrotal lymphedema. Lobato et al[7] described a case of massive lym
We present a case of severe lower limb lymphedema with recurrent infection treated with a combination of CDT and LVA surgery.
Bilateral lower extremity swelling for 6 years.
A 40-year-old woman underwent endometrial cancer surgery at a hospital in Henan Province in December 2017. This was followed by chemotherapy and radiotherapy. Since August 2018, the patient experienced over 30 episodes of lymphadenitis. By October 2018, edema appeared on the right thigh, spreading to the left thigh 6 mo later. Subsequently, the swelling extended to both lower limbs and the perineum. Despite visiting multiple hospitals, the patient did not receive any treatment until presenting to our hospital on June 26, 2023.
Hypertension, tubal obstruction, and severe anemia.
Family history of hypertension, no personal history.
She exhibited swelling and deformation in both lower limbs. Four large swellings on the posterior thighs hindered her mobility, and she reported limb pain. Cutaneous manifestations included lichenoid lesions and deep sclerotic features, consistent with an initial assessment of ISL stage III (Figure 1).
We conducted a series of tests on the patient, including gynecological tumor markers, infectious diseases, biochemistry, and routine blood, urine and stool examination. The only abnormalities found were elevated triglyceride levels and decreased levels of high-density lipoprotein cholesterol.
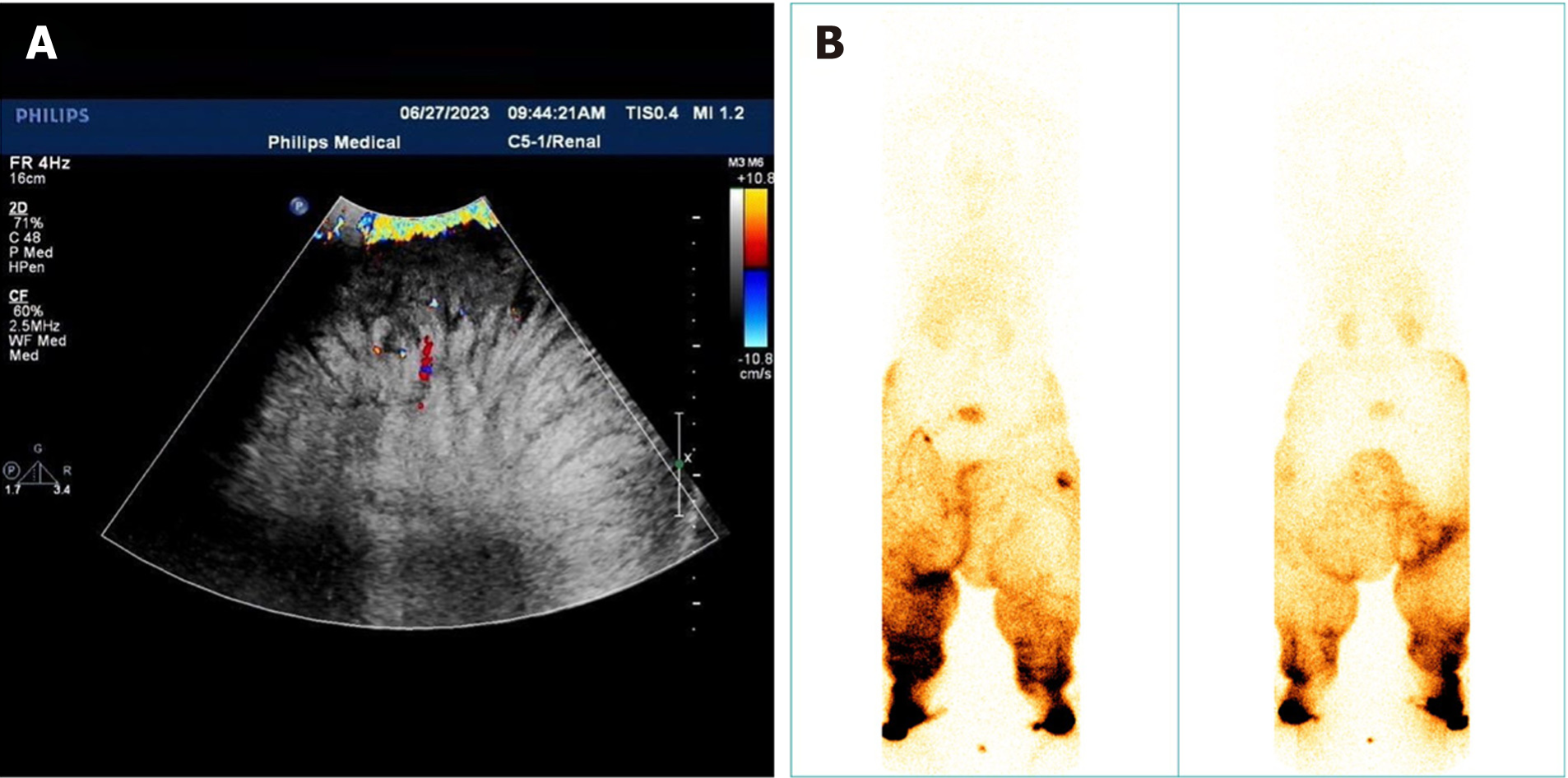
Initially, we suspected a tumor on the inner thigh. However, ultrasound imaging (Figure 2A) revealed that the swollen area consisted of adipose tissue, eliminating the possibility of a tumor. Subsequently, lymphoscintigraphy (Figure 2B) was conducted, confirming the diagnosis of lymphedema.
Considering the clinical presentation and investigative findings, the final diagnosis was lymphedema, ISL stage III.
Indocyanine green (ICG) lymphangiography revealed the presence of only a few functional lymphatic vessels within the mass. Surgical interventions such as direct excision, RRPP, and SAL would likely remove these well-functioning vessels, potentially compromising long-term treatment efficacy and leading to poor cosmetic outcomes. CDT is currently recognized as a relatively effective treatment method worldwide. This therapy incorporates several components: Skin care, manual lymphatic drainage (MLD), pressure therapy, and functional exercise. While CDT requires a prolonged treatment course, its therapeutic effect, safety profile, and long-term prognosis are well established. In fact, a prospective study demonstrated that CDT can achieve a limb volume reduction of ~60% in patients with moderate to severe lymphedema[8].
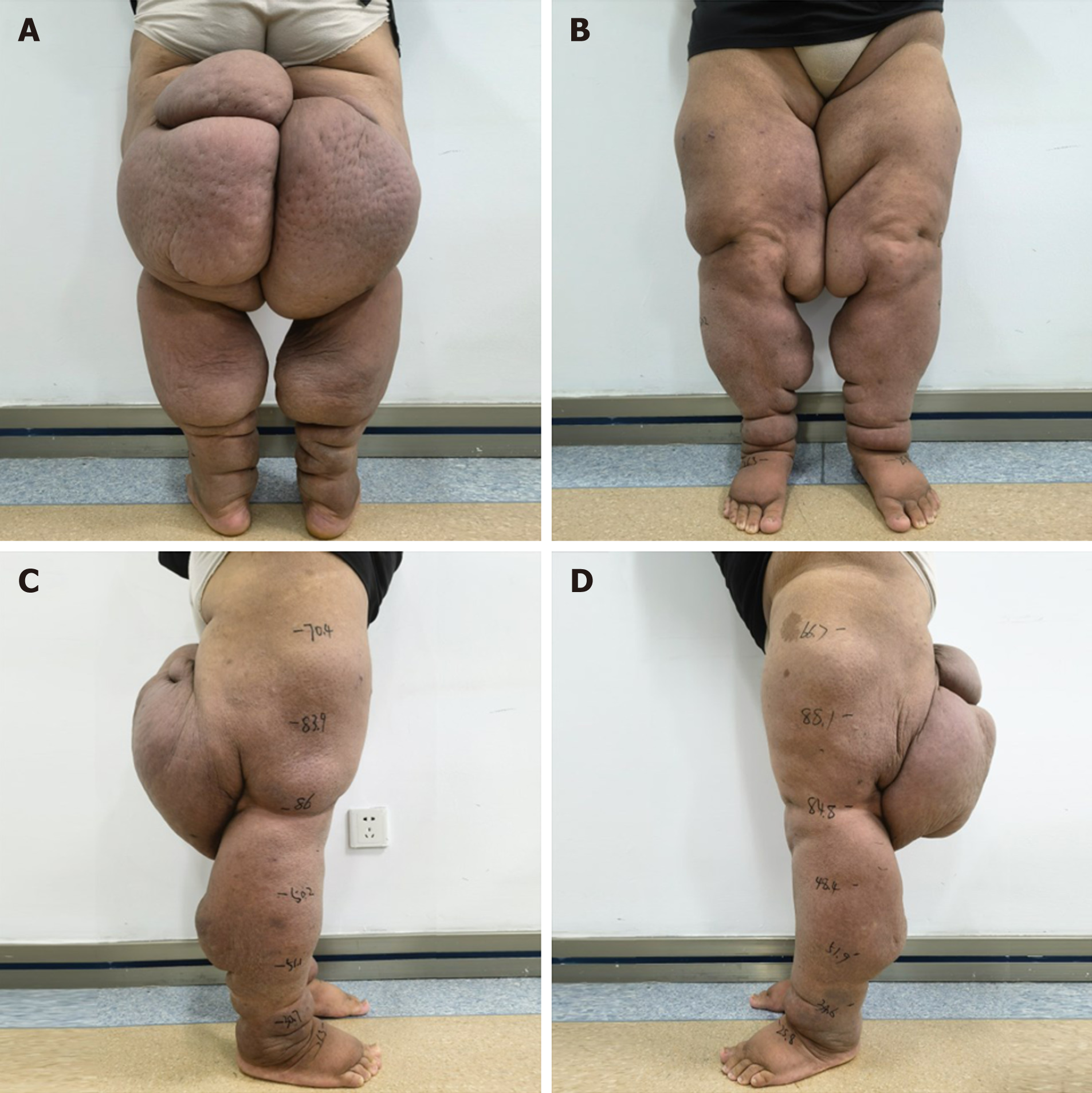
Early in the course of CDT, intermittent pneumatic compression was not feasible due to the patient's significant limb circumference and severe malformation. Elastic bandage fixation proved ineffective, and pressure therapy could not be consistently applied to the thigh area because of frequent dislodgment. However, as the treatment progressed, limb deformity improved, allowing for gradual increase in pressure therapy duration and coverage area. Despite the initial limitations with pressure therapy, the patient's limb circumference has shown continuous reduction since treatment initiation. After 2 mo of CDT, the patient experienced a weight loss of 37 kg. Edema showed signs of alleviation, and limb circumference significantly decreased. Notably, calf circumference nearly returned to normal (Table 1). Skin softness, elasticity, and luster all improved. However, swelling on the inner thighs persisted, limiting mobility (Figure 3).
| Back of the foot | 10 (cm) | 20 (cm) | 30 (cm) | 40 (cm) | 50 (cm) | 60 (cm) | |
| Before treatment | 31.0 | 43.6 | 72.0 | 71.9 | 94.3 | 92.6 | 85.0 |
| After 2 mo of CDT | 26.3 | 30.7 | 51.1 | 50.2 | 86.0 | 83.9 | 70.4 |
| After 1st LVA | 24.6 | 28.0 | 44.2 | 48.3 | 79.0 | 85.8 | 72.0 |
| After 2nd LVA | 25.1 | 25.5 | 38.5 | 43.4 | 69.0 | 68.0 | 70.0 |
| After 3rd LVA | 24.6 | 24.3 | 34.5 | 40.9 | 63.7 | 65.5 | 65.3 |
While CDT resulted in overall limb circumference reduction, the persistent mass on the inner thigh significantly affected the patient's walking and appearance. To achieve further therapeutic improvement, we opted for surgical intervention with continued CDT following each surgery. Following the initial 2 mo of CDT, ICG angiography was repeated, revealing the emergence of several new functional lymphatic vessels in both the calves and thighs. Currently, for severe lymphedema cases with substantial fat accumulation, surgical approaches such as liposuction and direct resection are commonly used. However, liposuction necessitates lifelong pressure therapy and carries the risk of lymphatic vessel damage. Resection surgery comes with a high risk of intraoperative blood loss, significant trauma, and poor cosmetic outcomes[9]. Additionally, both procedures simply remove excess tissue without addressing the under
Given the severity of the patient's lymphedema and the complexity of LVA surgery, we opted for a staged surgical approach to prioritize patient safety and optimize treatment effectiveness. The selection of surgical incisions was guided by the anatomical location of functional lymphatic vessels. Initially, we prioritized the inner aspect of the limb for incisions, believing that addressing distal lymphatic reflux would naturally improve drainage in the proximal area. Following the first surgery on the left lower limb, which included five anastomoses in the calf and two in the thigh, all located on the inner side, postoperative measurements revealed a significantly greater reduction in calf circumference compared to the thigh. Notably, there was no significant improvement in swelling on the outer aspect of the limb. These findings prompted us to re-evaluate our approach. Wolfs et al[12] suggested a positive correlation between the number of successful anastomoses and improvement in clinical symptoms.
Based on these findings, we modified our approach for the second surgery. We increased the number of anastomoses performed on the right thigh and included incisions beyond the inner aspect of the limb, resulting in a total of 23 anastomoses. As anticipated, the right thigh demonstrated a significantly greater reduction in swelling compared to the left thigh following the second operation.
During the third surgery, we performed 11 LVA targeting the large mass on the inner thigh, achieving a satisfactory reduction in postoperative swelling.
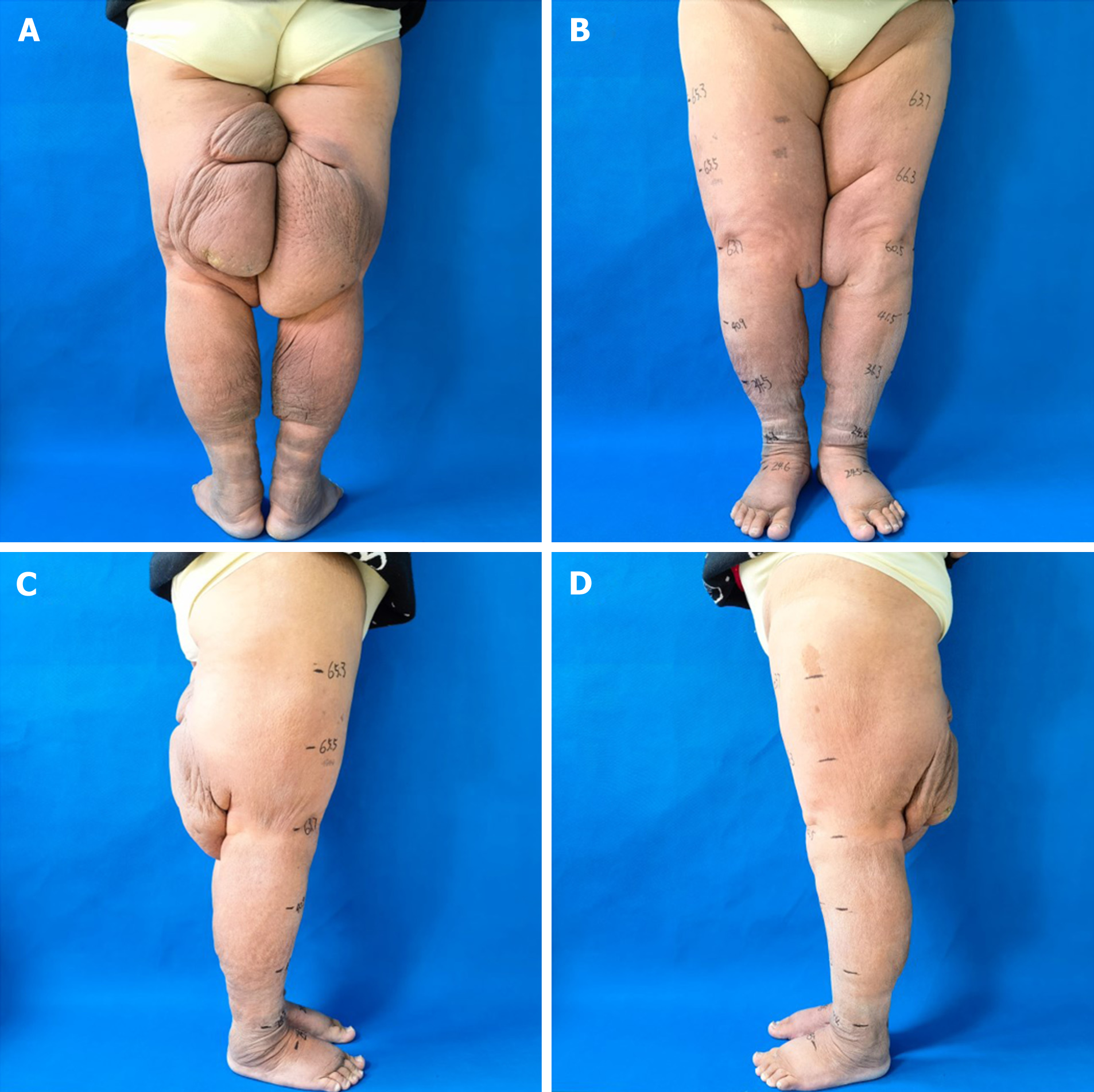
Following 6 mo of comprehensive treatment, the patient achieved significant improvements. Her weight decreased by 49 kg, with a maximum reduction in limb circumference of 35.2 cm in the left lower limb and 37.5 cm in the right lower limb (Table 1, Figure 4). Her body mass index (BMI) improved from 57.02 to 36.63. The patient subjectively reported a substantial reduction in swelling and limb heaviness. Additionally, her pain and difficulty walking completely resolved. Notably, her appearance after wearing loose-fitting undergarments closely resembled that of a healthy individual. Importantly, she has not experienced any episodes of lymphangitis since treatment initiation.
Current treatment approaches for severe lymphedema often prioritize the removal of abnormal tissues. While these methods can achieve a rapid reduction in limb volume and demonstrate noticeable short-term effects, they often involve invasive and destructive procedures on the subcutaneous and skin tissues, resulting in poor cosmetic outcomes[13,14]. In contrast, LVA offers a minimally invasive treatment option for severe lymphedema patients, aiming to achieve therapeutic benefits while minimizing the impact on skin aesthetics. LVA boasts several advantages, including minimal invasiveness, rapid recovery time, and high patient satisfaction regarding post-surgical appearance, making it a promising approach for this patient population. While surgery is generally considered for patients with higher than ISL stage II who have failed conservative treatment, there is no definitive consensus among experts[15]. Some experts have suggested that LVA is best suited for patients without significant fat edema or fibrosis[16]. A review of LVA literature from 1985 to 2019 by Forte et al[17] found that all reported studies demonstrated improvements in symptoms, signs, or lymphatic function. Thirteen studies suggested a significant reduction in infection rates following LVA. Demirtas et al[18] suggested that LVA offers better outcomes in the early stages, whereas Mihara et al[19] proposed that LVA may be even more effective in the later stages of lymphedema. Regardless of the stage, it is evident that LVA can be beneficial for a wide range of lymphedema patients. The optimal number of anastomoses per patient remains a topic of debate. Forte et al[17] suggested that a greater number of anastomoses leads to better outcomes, while Chen[20] proposed that 7–12 are sufficient. Conversely, Koshima et al[21] suggested that as few as two or three anastomoses can achieve significant results. Further research is needed to establish a definitive consensus on the ideal number of anastomoses for LVA procedures.
Effective management of lymphedema requires a comprehensive evaluation that considers the underlying cause, contributing factors, and the patient's specific clinical presentation. This personalized approach allows for the development of a tailored treatment plan to optimize outcomes. Research has established a positive correlation between high BMI and the incidence of lymphedema[22,23]. Animal studies have supported this link, demonstrating how increased fat accumulation can impair lymphatic vessel function[24]. This can lead to an overburdened lymphatic system, hindered drainage, and ultimately, worsening swelling and deformity. Prior to developing lymphedema, the patient's BMI of 37.46 fell within the obese category. Lower limb lymphedema often compromises a patient's ability to exercise, leading to a gradual decrease in exercise frequency and duration. This, in turn, can exacerbate obesity and contribute to worsening swelling. In the past 2 years, the patient's severe deformity has significantly limited her ability to exercise. Based on this information, it is reasonable to infer that the patient's obesity may be a significant factor contributing to the development and progression of her lymphedema.
A meta-analysis study suggests a significant correlation between nonsuppurative cellulitis and lymphedema, highlighting the potential link between the patient's recurrent skin infections and the severity of her lymphatic damage[25]. With > 30 skin infections documented in the past 5 years, it is plausible that these episodes contributed to the compromised lymphatic system.
Therefore, our treatment approach extends beyond interventions specifically targeting lymphedema. We will also address the patient's obesity and skin health to minimize the burden on the lymphatic system, enhance lymphatic drainage, and ultimately promote overall improvement. We recommend weight management strategies including dietary adjustments and increased physical activity, along with daily skin care and cleansing routines. To optimize treatment effectiveness, it is crucial for the patient to maintain a daily record of weight, dietary intake and output volume, and observe any changes in skin condition. Consistent communication with the medical team will allow for timely adju
Research by Thompson et al[26] has demonstrated the efficacy of MLD in improving lymphatic drainage and reducing edema in lymphedema patients. While a typical MLD session lasts for 30 min, the patient's severe condition necessitates a more intensive approach. Therefore, we have implemented MLD therapy five times per week, with each session lasting 1 h. As shown in Table 2, the patient's calf circumference had nearly normalized after 2 mo of CDT. Additionally, the circumference of the thigh also exhibited gradual improvement. Furthermore, ICG angiography revealed the presence of newly formed functional lymphatic vessels. These findings suggest that MLD played a pivotal role in the treatment process, laying a strong foundation for the subsequent LVA surgery.
| Back of the foot | 10 (cm) | 20 (cm) | 30 (cm) | 40 (cm) | 50 (cm) | 60 (cm) | |
| Before treatment | 28.7 | 42.8 | 63.4 | 66.0 | 91.7 | 101.5 | 77.6 |
| After 2 mo of CDTs | 25.8 | 34.6 | 51.9 | 48.4 | 84.8 | 88.1 | 66.7 |
| After 1st LVA | 25.4 | 27.8 | 42.2 | 46.7 | 76.8 | 79.4 | 68.8 |
| After 2nd LVA | 25.0 | 26.2 | 37.7 | 43.5 | 66.1 | 76.6 | 65.7 |
| After 3rd LVA | 24.5 | 24.4 | 36.3 | 41.5 | 60.5 | 66.3 | 63.7 |
Following the first LVA surgery, the patient experienced a significant reduction in swelling near the anastomosis sites. To optimize the surgical outcome, the second surgery aimed to increase the number of anastomoses. As expected, this approach resulted in demonstrably improved clinical results. The third surgery served to refine and supplement the initial intervention, ultimately achieving successful outcomes in both lower limbs. Notably, the patient has not experienced any skin infections since the surgical interventions.
This case demonstrates the feasibility of pursuing LVA following a period of CDT for severe lymphedema. Effective and continuous CDT has the potential to improve lymphatic function and facilitate the reconstruction of lymphatic vessels, thereby promoting successful outcomes for subsequent LVAs. For patients with severe and deformed lymphedema, a thorough preoperative assessment is essential to tailor a personalized and comprehensive treatment plan that addresses their unique condition. Throughout the treatment regimen, maintaining active communication with the patient is crucial. This allows for close monitoring of disease progression and the flexibility to adjust treatment strategies as needed to ensure optimal outcomes. MLD, recognized as the cornerstone of lymphedema treatment, plays a pivotal role. It promotes the recovery and reconstruction of lymphatic function, thereby enhancing the overall effectiveness of the treatment plan. Early initiation of complete pressure therapy, alongside surgical intervention, is paramount. Surgical procedures may be staged, with each operation aiming to anastomose as many functional lymphatic vessels as possible in the edematous region to maximize lymphatic drainage. Following surgery, continued CDT is essential to reinforce surgical outcomes and achieve satisfactory clinical results.
We would like to express our gratitude to our patient and their family for allowing us to publish this case report.
| 1. | Carter J, Huang HQ, Armer J, Carlson JW, Lockwood S, Nolte S, Kauderer J, Hutson A, Walker JL, Fleury AC, Bonebrake A, Soper JT, Mathews C, Zivanovic O, Richards WE, Tan A, Alberts DS, Barakat RR, Wenzel LB. GOG 244 - The Lymphedema and Gynecologic cancer (LeG) study: The impact of lower-extremity lymphedema on quality of life, psychological adjustment, physical disability, and function. Gynecol Oncol. 2021;160:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Watson CH, Lopez-Acevedo M, Broadwater G, Kim AH, Ehrisman J, Davidson BA, Lee PS, Valea F, Berchuck A, Havrilesky LJ. A pilot study of lower extremity lymphedema, lower extremity function, and quality of life in women after minimally invasive endometrial cancer staging surgery. Gynecol Oncol. 2019;153:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116:5138-5149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 348] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 4. | Dessources K, Aviki E, Leitao MM Jr. Lower extremity lymphedema in patients with gynecologic malignancies. Int J Gynecol Cancer. 2020;30:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Ramachandran S, Chew KY, Tan BK, Kuo YR. Current operative management and therapeutic algorithm of lymphedema in the lower extremities. Asian J Surg. 2021;44:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Istranov AL, Makarov IG, Makarova NV, Tulina I, Ulasov IV, Isakova YI. Combination of conservative and surgical methods in the treatment of giant lymphedema of the scrotum: A case report. Front Surg. 2023;10:1048159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Lobato RC, Zatz RF, Cintra Junior W, Modolin MLA, Chi A, Van Dunem Filipe de Almeida YK, Gemperli R. Surgical treatment of a penoscrotal massive localized lymphedema: Case report. Int J Surg Case Rep. 2019;59:84-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Shaitelman SF, Cromwell KD, Rasmussen JC, Stout NL, Armer JM, Lasinski BB, Cormier JN. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J Clin. 2015;65:55-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Schaverien MV, Coroneos CJ. Surgical Treatment of Lymphedema. Plast Reconstr Surg. 2019;144:738-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 10. | Lee M, Perry L, Granzow J. Suction Assisted Protein Lipectomy (SAPL) Even for the Treatment of Chronic Fibrotic and Scarified Lower Extremity Lymphedema. Lymphology. 2016;49:36-41. [PubMed] |
| 11. | Rosian K, Stanak M. Efficacy and safety assessment of lymphovenous anastomosis in patients with primary and secondary lymphoedema: A systematic review of prospective evidence. Microsurgery. 2019;39:763-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Wolfs JAGN, de Joode LGEH, van der Hulst RRWJ, Qiu SS. Correlation between patency and clinical improvement after lymphaticovenous anastomosis (LVA) in breast cancer-related lymphedema: 12-month follow-up. Breast Cancer Res Treat. 2020;179:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Garza R 3rd, Skoracki R, Hock K, Povoski SP. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer. 2017;17:468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Boyages J, Kastanias K, Koelmeyer LA, Winch CJ, Lam TC, Sherman KA, Munnoch DA, Brorson H, Ngo QD, Heydon-White A, Magnussen JS, Mackie H. Liposuction for Advanced Lymphedema: A Multidisciplinary Approach for Complete Reduction of Arm and Leg Swelling. Ann Surg Oncol. 2015;22 Suppl 3:S1263-S1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Lurie F, Malgor RD, Carman T, Dean SM, Iafrati MD, Khilnani NM, Labropoulos N, Maldonado TS, Mortimer P, O'Donnell TF Jr, Raffetto JD, Rockson SG, Gasparis AP. The American Venous Forum, American Vein and Lymphatic Society and the Society for Vascular Medicine expert opinion consensus on lymphedema diagnosis and treatment. Phlebology. 2022;37:252-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Bittar S, Simman R, Lurie F. Lymphedema: A Practical Approach and Clinical Update. Wounds. 2020;32:86-92. [PubMed] |
| 17. | Forte AJ, Khan N, Huayllani MT, Boczar D, Saleem HY, Lu X, Manrique OJ, Ciudad P, McLaughlin SA. Lymphaticovenous Anastomosis for Lower Extremity Lymphedema: A Systematic Review. Indian J Plast Surg. 2020;53:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Demirtas Y, Ozturk N, Yapici O, Topalan M. Supermicrosurgical lymphaticovenular anastomosis and lymphaticovenous implantation for treatment of unilateral lower extremity lymphedema. Microsurgery. 2009;29:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Mihara M, Hara H, Tange S, Zhou HP, Kawahara M, Shimizu Y, Murai N. Multisite Lymphaticovenular Bypass Using Supermicrosurgery Technique for Lymphedema Management in Lower Lymphedema Cases. Plast Reconstr Surg. 2016;138:262-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Chen WF. How to Get Started Performing Supermicrosurgical Lymphaticovenular Anastomosis to Treat Lymphedema. Ann Plast Surg. 2018;81:S15-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Koshima I, Nanba Y, Tsutsui T, Takahashi Y, Itoh S, Fujitsu M. Minimal invasive lymphaticovenular anastomosis under local anesthesia for leg lymphedema: is it effective for stage III and IV? Ann Plast Surg. 2004;53:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Leitao MM Jr, Zhou QC, Gomez-Hidalgo NR, Iasonos A, Baser R, Mezzancello M, Chang K, Ward J, Chi DS, Long Roche K, Sonoda Y, Brown CL, Mueller JJ, Gardner GJ, Jewell EL, Broach V, Zivanovic O, Dowdy SC, Mariani A, Abu-Rustum NR. Patient-reported outcomes after surgery for endometrial carcinoma: Prevalence of lower-extremity lymphedema after sentinel lymph node mapping versus lymphadenectomy. Gynecol Oncol. 2020;156:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Sudduth CL, Greene AK. Lymphedema and Obesity. Cold Spring Harb Perspect Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C, Detmar M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One. 2014;9:e94713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Muluk SC, Hirsch AT, Taffe EC. Pneumatic compression device treatment of lower extremity lymphedema elicits improved limb volume and patient-reported outcomes. Eur J Vasc Endovasc Surg. 2013;46:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Thompson B, Gaitatzis K, Janse de Jonge X, Blackwell R, Koelmeyer LA. Manual lymphatic drainage treatment for lymphedema: a systematic review of the literature. J Cancer Surviv. 2021;15:244-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |