Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.4965
Revised: May 16, 2024
Accepted: June 5, 2024
Published online: August 6, 2024
Processing time: 84 Days and 19.4 Hours
There is still some room for optimizing ambulatory pediatric surgical procedures, and the preoperative and postoperative management quality for pediatric patients needs to be improved.
To discuss the safety and feasibility of the enhanced recovery after surgery (ERAS)-based management model for ambulatory pediatric surgical procedures.
We selected 320 pediatric patients undergoing ambulatory surgery from June 2023 to January 2024 at The First People’s Hospital of Liangshan Yi Autonomous Prefecture. Of these, 220 received ERAS-based management (research group) and 100 received routine management (control group). General information, post
The general information of the research group (sex, age, disease type, single pa
The ERAS-based management model was safe and feasible in ambulatory pediatric surgical procedures and worthy of clinical promotion.
Core Tip: Ambulatory surgical procedures have the advantages of high efficiency, high-quality services, low medical costs, and short hospital stays while providing sufficient guarantees in terms of medical quality, safety, and rapidity. However, there is some room for improvement. This study proposed a management model based on the concept of enhanced recovery after surgery, which was safe and feasible in ambulatory pediatric surgeries, accelerating postoperative ambulation, relieving postoperative pain, reducing the incidence of postoperative complications, and improving family satisfaction. This model provided a better management option for ambulatory pediatric surgical procedures.
- Citation: Fan GQ, Zhang XD, He YK, Lu XG, Zhong JY, Pang ZY, Gan XY. Safety and feasibility of enhanced recovery after surgery-based management model for ambulatory pediatric surgical procedures. World J Clin Cases 2024; 12(22): 4965-4972
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/4965.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.4965
Ambulatory surgical procedures involve specialties, such as pediatric surgery, general surgery, orthopedics, obstetrics and gynecology, and ophthalmology, which are mainly performed through day surgery centers equipped with higher clinical qualifications, anesthesia spare parts, and medical equipment[1,2]. The hospital establishes dedicated day ope
Enhanced recovery after surgery (ERAS) is a global surgical quality improvement program that is now closely inte
There is limited research on the safety and feasibility of the ERAS-based management model for ambulatory pediatric surgical procedures. This study aimed to fill this research gap.
In total, 320 pediatric patients undergoing ambulatory surgical procedures between June 2023 and January 2024 at The First People’s Hospital of Liangshan Yi Autonomous Prefecture were selected as research participants. The types of diseases in children were hernia in 236 cases, superficial mass in 30 cases, ganglion cyst in 2 cases, tendonitis stenosans in 2 cases, polydactyly in 24 cases, umbilical hernia in 11 cases, varicocele in 5 cases, and hydrocele in 10 cases. Among them, 220 cases (research group) were treated with ERAS-based management, and 100 cases (control group) received routine management.
Inclusion criteria: The children, aged over 1-year-old, were diagnosed by the outpatient department and could be cared for by parents after discharge, with no contraindications to surgery and complete case records.
Exclusion criteria: Children with severe cardiopulmonary disease, strangulation and necrosis of the hernial contents, blood and immune system diseases, or surgery requiring only local infiltration anesthesia.
The research group received ERAS-based management including: (1) Health education. After diagnosis, the nursing staff guided the families of the children to the hospital preparation center for registration and appointment, strengthened the health education of the family members of the child, and guided the family members to read about the disease; (2) Preoperative nursing. The child’s clinical symptoms were closely observed, and the examination results were analyzed 3 d before surgery. Once there were abnormalities, the child’s family was instructed to review or were reminded to seek medical treatment in time. The child and their family were informed to prepare for admission 1 d before surgery. One day before the procedure, the nurse strengthened communication with the child and their family to understand the child’s psychological status, introduced the surgical procedure, relevant precautions, and anesthesia methods, and explained surgical safety and successfully treated cases to avoid the negative emotions of the child and enable them to maintain an optimistic attitude. The child was told to abstain from food and drink 3-6 h before surgery but was allowed to supplement glucose as needed; (3) Intraoperative nursing. During the procedure, operating room temperature and humidity were optimally adjusted. Insulation measures for children were strengthened, such as covering the nonsurgical parts of children with thermal blankets or heated mattresses and heating the liquid infused to approximately 36 °C. On the day of operation, a personalized fasting plan was initiated, and the admission procedures went through according to the order of appointment, ensuring that the time from admission to operation was < 3 h. At the same time, the evaluation of the child was strengthened. The surgeon and the anesthesiologist conducted a preoperative interview with the child to evaluate the child’s condition. During the operation, regional block anesthesia was used as much as possible, and the dosage of anesthetic drugs was accurately calculated. Before the end of the operation, ropivacaine was used for local infiltration; (4) Postoperative care. The child was given lollipops to stimulate gastrointestinal motility 2 h after post
The control group adopted a conventional management model. Before the operation, the nurse strengthened the health education for the child’s families, cleaned and disinfected the operation site, and asked the child to fast and refrain from drinking for 8-12 h. Before the surgery, the nurse guided the child to empty the bladder, assisted the child in correctly placing the surgical position, and prepared drugs and instruments. Insulation measures for the child were strengthened during the operation. The child’s oral secretions were promptly cleaned up after the operation to keep the respiratory tract unobstructed. Vital signs were closely observed after surgery. The child was advised to partake liquid foods after they woke up for 6 h. The child’s oxygenation function and complications were observed, and the related activities were guided from 1 d after surgery.
Patient general information, postoperative ambulation activities, surgical outcomes, postoperative pain, postoperative complications, and satisfaction of family members were observed and recorded in both groups. After surgery, children were encouraged to turn over in bed and ambulate as early as possible, and patient ambulation was recorded within 2 h, 4 h, and 6 h after surgery. The surgical outcomes of the two groups were compared by recording operation time, post
Measurement data were statistically described as the mean ± standard deviation, and between-group comparisons were made using independent sample t-tests. Counting data were expressed as the ratio (percentage), and between-group comparisons were performed using χ2 tests. SPSS 21.0 (IBM Corp., Armonk, NY, United States) was used for data analysis. P value < 0.05 denoted statistical significance.
The research and control groups did not differ markedly in general patient data (sex, age, disease type, single parent, and family history) (P > 0.05) (Table 1).
| Indicators | Research group, n = 220 | Control group, n = 100 | χ2/t | P value |
| Sex | 0.316 | 0.574 | ||
| Male | 150 (68.18) | 65 (65.00) | ||
| Female | 70 (31.82) | 35 (35.00) | ||
| Age in yr | 7.84 ± 4.14 | 7.53 ± 3.47 | 0.652 | 0.515 |
| Disease type | 9.913 | 0.194 | ||
| Hernia | 167 (75.91) | 69 (69.00) | ||
| Polydactyly | 12 (5.45) | 12 (12.00) | ||
| Umbilical hernia | 5 (2.27) | 6 (6.00) | ||
| Hydrocele of tunica vaginalis | 6 (2.73) | 4 (4.00) | ||
| Varicocele | 4 (1.82) | 1 (1.00) | ||
| Superficial mass | 22 (10.00) | 8 (8.00) | ||
| Ganglion cyst | 2 (0.91) | 0 (0.00) | ||
| Tendonitis stenosans | 2 (0.91) | 0 (0.00) | ||
| Single parent | 0.217 | 0.641 | ||
| Yes | 35 (15.91) | 18 (18.00) | ||
| No | 185 (84.09) | 82 (82.00) | ||
| Family medical history | 1.108 | 0.293 | ||
| With | 11 (5.00) | 8 (8.00) | ||
| Without | 209 (95.00) | 92 (92.00) |
The rates of ambulation activities at 2 h, 4 h, and 6 h after surgery were markedly higher in the research group than the control group (P < 0.05) (Table 2).
| Indicators | Research group, n = 220 | Control group, n = 100 | χ2 | P value |
| Ambulation activities within 2 h after surgery | 66 (30.00) | 12 (12.00) | 12.084 | < 0.001 |
| Ambulation activities within 4 h after surgery | 59 (26.82) | 15 (15.00) | 5.401 | 0.020 |
| Ambulation activities within 6 h after surgery | 95 (43.18) | 24 (24.00) | 10.830 | 0.001 |
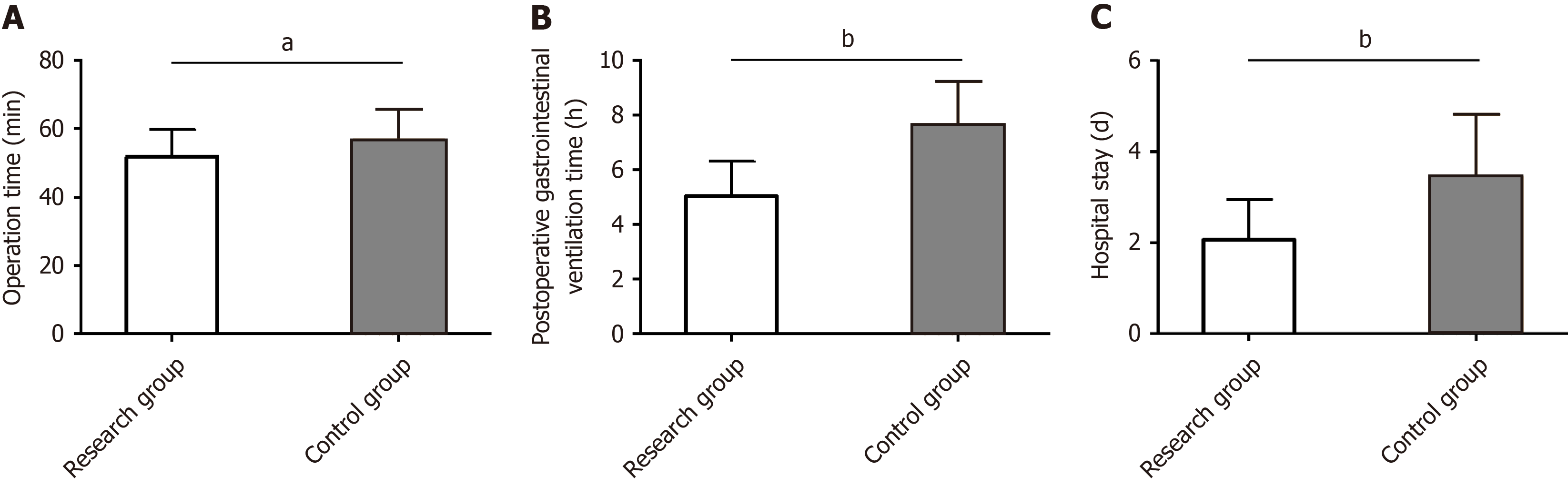
We evaluated the influence of the two management models on the surgical outcomes of pediatric patients undergoing ambulatory surgery by detecting operation time, postoperative gastrointestinal ventilation time, and hospital stay. The data revealed statistically shorter operation time, postoperative gastrointestinal ventilation time, and hospital stay in the research group than the control group (P < 0.05) (Figure 1).
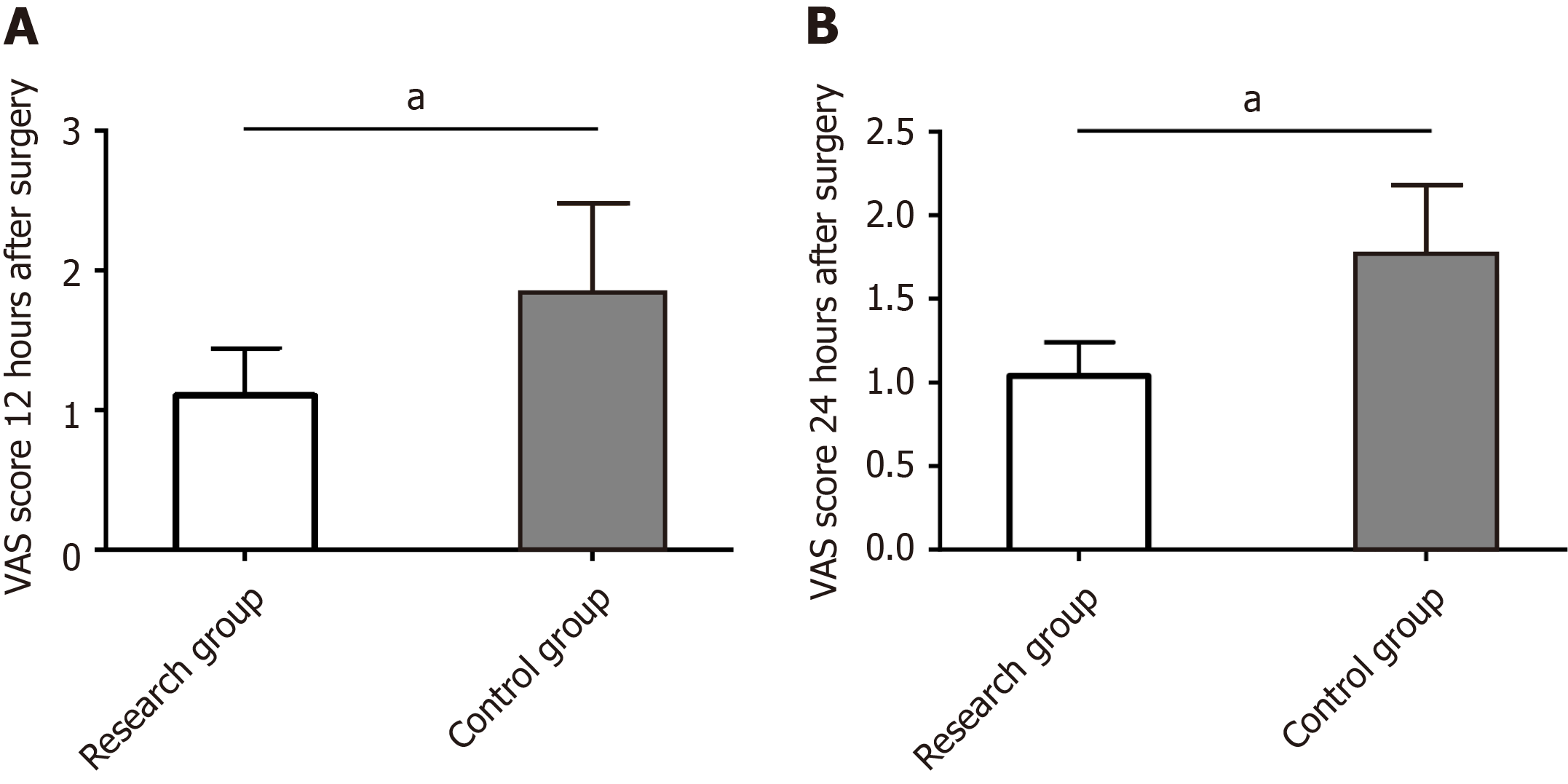
We used VAS to evaluate the impact of the two management models on postoperative pain in pediatric patients undergoing ambulatory surgery. According to the evaluation data, VAS scores at 12 h and 24 h after surgery were significantly lower in the research group than the control group (P < 0.05) (Figure 2).
The incidence of incision infection, abdominal distension, fever, and nausea and vomiting were counted to evaluate the influence of the two management models on postoperative complications in children undergoing ambulatory surgery. The total incidence of adverse events in the research group was 6.82%, which was markedly lower than 19.00% in the control group (P < 0.05) (Table 3).
| Indicators | Research group, n = 220 | Control group, n = 100 | χ2 | P value |
| Incision infection | 0 (0.00) | 4 (4.00) | ||
| Abdominal distension | 4 (1.82) | 5 (5.00) | ||
| Fever | 4 (1.82) | 4 (4.00) | ||
| Nausea and vomiting | 7 (3.18) | 6 (6.00) | ||
| Total | 15 (6.82) | 19 (19.00) | 10.744 | 0.001 |
Statistical analysis of the satisfaction of family members in the two groups showed that total satisfaction was 98.18% in the family members of the research group. This was significantly higher than 92.00% in the control group (P < 0.05) (Table 4).
| Indicators | Research group, n = 220 | Control group, n = 100 | χ2 | P value |
| Very satisfied | 155 (70.45) | 45 (45.00) | ||
| Satisfied | 61 (27.73) | 47 (47.00) | ||
| Dissatisfied | 4 (1.82) | 8 (8.00) | ||
| Overall satisfaction | 216 (98.18) | 92 (92.00) | 7.279 | 0.007 |
This study included 320 pediatric patients undergoing ambulatory surgical procedures, with the control group receiving conventional management and the research group receiving ERAS-based management. We compared and analyzed the clinical advantages of the two management models in 320 pediatric patients undergoing ambulatory surgery to provide a better clinical management model for patients.
First, the rate of postoperative ambulation activities was significantly higher in the research group than the control group at 2 h, 4 h, and 6 h after surgery, suggesting that the management model based on the ERAS concept can promote postoperative ambulatory activities in pediatric patients undergoing ambulatory surgery and help them ambulate early. The research group showed a significantly shorter operation time, postoperative gastrointestinal ventilation time, and hospital stay than the control group, indicating that ERAS-based management can not only help shorten surgical duration but also significantly promote postoperative rehabilitation in pediatric patients undergoing ambulatory surgery.
Modrzyk et al[16] reported that application of the ERAS-based management model to children undergoing reverse stoma surgery helped shorten hospital stay, time to oral fluid intake, time to regular diet, and total parenteral nutrition time while relieving metabolic stress, with favorable safety and therapeutic effects. Pearson et al[17] reported that the ERAS-based management model significantly shortened hospitalization time, oral feeding time, and defecation time, suggesting that it is beneficial for postoperative rehabilitation, similar to our research results. This may be attributed to the use of ERAS-based management, which provides health education to children and their families to strengthen their understanding of the disease.
Second, preoperative care was given to the children to help them face the operation with a good attitude. Professional comfort care and anesthesia care were provided during the operation. The children were given meticulous and comprehensive postoperative care from the aspects of diet and psychology[18,19].
Pain assessment data showed that VAS scores were lower in the research group than the control group at 12 h and 24 h after surgery, indicating that the ERAS-based management model used in the research group is more conducive to postoperative pain relief. According to Han et al[20], the ERAS-based management model applied to pediatric urological reconstruction surgery provided some relief for postoperative pain while exerting a positive effect on other anesthesia results, which supports our findings. George et al[21] reported that average VAS scores of children undergoing colorectal surgery after receiving ERAS-based management decreased significantly both 1 d after surgery and during the hospital stay, which is in line with our observations. In this study, the pain-relieving effect of the ERAS-based management model may lie in timely and effective pain management as well as timely pain assessment and appropriate drug intervention according to the degree of pain and age of the children[22,23].
The incidence of incision infection, abdominal distension, fever, and nausea and vomiting was statistically analyzed. The total incidence of the adverse events was notably lower in the research group than the control group (6.82% vs 19.00%), indicating that the management model based on the ERAS concept accepted by the research group was a sig
Finally, the survey on family satisfaction identified obviously higher total satisfaction in family members in the research group than the control group, indicating that the management model based on the ERAS concept is more popular and recognized by the children’s family members, similar to the research results of Heis et al[25].
In summary, the ERAS-based management model can guarantee the safety and feasibility of pediatric ambulatory surgical procedures, which can accelerate postoperative ambulation, relieve postoperative pain, reduce postoperative complications, and improve the satisfaction of family members, providing a better management choice for ambulatory pediatric surgical procedures.
| 1. | Quercioli C, Cevenini G, Messina G, Carta GA, Becattini G, Sancasciani S. Reducing waiting times of elective surgical procedures: effectiveness evaluation of a multi-interventions approach. Ann Ig. 2022;34:635-649. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Nordin AB, Shah SR, Kenney BD. Ambulatory pediatric surgery. Semin Pediatr Surg. 2018;27:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Yoon SZ, Lee SI, Lee HW, Lim HJ, Yoon SM, Chang SH. The effect of increasing operating room capacity on day-of-surgery cancellation. Anaesth Intensive Care. 2009;37:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Agozzino E, Naddei M, Schiavone B. Day surgery: the role and training needs of nurses. Ig Sanita Pubbl. 2014;70:81-91. [PubMed] |
| 5. | Smith I, Cooke T, Jackson I, Fitzpatrick R. Rising to the challenges of achieving day surgery targets. Anaesthesia. 2006;61:1191-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Vaughan J, Gurusamy KS, Davidson BR. Day-surgery versus overnight stay surgery for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2013;CD006798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Nunes JS, Gomes R, Povo A, Alves EC. Quality Indicators in Ambulatory Surgery: A Literature Review Comparing Portuguese and International Systems. Acta Med Port. 2018;31:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Palumbo P, Perotti B, Amatucci C, Pangrazi MP, Leuzzi B, Vietri F, Illuminati G. Perceived quality in Day Surgery Units Proposal of an enquiry postoperative questionnaire. Ann Ital Chir. 2016;87:172-176. [PubMed] |
| 9. | Ljungqvist O, de Boer HD, Balfour A, Fawcett WJ, Lobo DN, Nelson G, Scott MJ, Wainwright TW, Demartines N. Opportunities and Challenges for the Next Phase of Enhanced Recovery After Surgery: A Review. JAMA Surg. 2021;156:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 10. | Steenhagen E. Enhanced Recovery After Surgery: It's Time to Change Practice! Nutr Clin Pract. 2016;31:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Neville A, Lee L, Antonescu I, Mayo NE, Vassiliou MC, Fried GM, Feldman LS. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg. 2014;101:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Beverly A, Kaye AD, Ljungqvist O, Urman RD. Essential Elements of Multimodal Analgesia in Enhanced Recovery After Surgery (ERAS) Guidelines. Anesthesiol Clin. 2017;35:e115-e143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (1)] |
| 13. | Kaye AD, Urman RD, Cornett EM, Hart BM, Chami A, Gayle JA, Fox CJ. Enhanced recovery pathways in orthopedic surgery. J Anaesthesiol Clin Pharmacol. 2019;35:S35-S39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 14. | Lau CS, Chamberlain RS. Enhanced Recovery After Surgery Programs Improve Patient Outcomes and Recovery: A Meta-analysis. World J Surg. 2017;41:899-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Purcell LN, Marulanda K, Egberg M, Mangat S, McCauley C, Chaumont N, Sadiq TS, Lupa C, McNaull P, McLean SE, Hayes-Jordan A, Phillips MR. An enhanced recovery after surgery pathway in pediatric colorectal surgery improves patient outcomes. J Pediatr Surg. 2021;56:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Modrzyk A, Pasierbek MJ, Korlacki W, Grabowski A. Introducing enhanced recovery after surgery protocol in pediatric surgery. Adv Clin Exp Med. 2020;29:937-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Pearson KL, Hall NJ. What is the role of enhanced recovery after surgery in children? A scoping review. Pediatr Surg Int. 2017;33:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Short V, Atkinson C, Ness AR, Thomas S, Burden S, Sutton E. Patient experiences of perioperative nutrition within an Enhanced Recovery After Surgery programme for colorectal surgery: a qualitative study. Colorectal Dis. 2016;18:O74-O80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Short HL, Taylor N, Thakore M, Piper K, Baxter K, Heiss KF, Raval MV. A survey of pediatric surgeons' practices with enhanced recovery after children's surgery. J Pediatr Surg. 2018;53:418-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Han DS, Brockel MA, Boxley PJ, Dönmez Mİ, Saltzman AF, Wilcox DT, Rove KO. Enhanced recovery after surgery and anesthetic outcomes in pediatric reconstructive urologic surgery. Pediatr Surg Int. 2021;37:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | George JA, Salazar AJG, Irfan A, Prichett L, Nasr IW, Garcia AV, Boss EF, Jelin EB. Effect of implementing an enhanced recovery protocol for pediatric colorectal surgery on complication rate, length of stay, and opioid use in children. J Pediatr Surg. 2022;57:1349-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Walter CM, Abbasian N, Olbrecht VA. Trends in Pediatric Pain: Thinking Beyond Opioids. Anesthesiol Clin. 2020;38:663-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Flowers T, Winters R. Postoperative pain management in pediatric cleft lip and palate repair. Curr Opin Otolaryngol Head Neck Surg. 2021;29:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Rove KO, Brockel MA, Saltzman AF, Dönmez MI, Brodie KE, Chalmers DJ, Caldwell BT, Vemulakonda VM, Wilcox DT. Prospective study of enhanced recovery after surgery protocol in children undergoing reconstructive operations. J Pediatr Urol. 2018;14:252.e1-252.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Heiss KF, Raval MV. Patient engagement to enhance recovery for children undergoing surgery. Semin Pediatr Surg. 2018;27:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |