Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.4932
Revised: June 5, 2024
Accepted: June 17, 2024
Published online: August 6, 2024
Processing time: 110 Days and 6.5 Hours
Collision tumor are neoplasms, including two histologically distinct tumors that coexist in the same mass without histological admixture. The incidence of col
To investigate ultrasound images and application of ovarian-adnexal reporting and data system (O-RADS) to evaluate the risk and pathological characteristics of ovarian collision tumor.
This study retrospectively analyzed 17 cases of ovarian collision tumor diagnosed pathologically from January 2020 to December 2023. All clinical features, ultrasound images and histopathological features were collected and analyzed. The O-RADS score was used for classification. The O-RADS score was determined by two senior doctors in the gynecological ultrasound group. Lesions with O-RADS score of 1-3 were classified as benign tumors, and lesions with O-RADS score of 4 or 5 were classified as malignant tumors.
There were 17 collision tumors detected in 16 of 6274 patients who underwent gynecological surgery. The average age of 17 women with ovarian collision tumor was 36.7 years (range 20-68 years), in whom, one occurred bilaterally and the rest occurred unilaterally. The average tumor diameter was 10 cm, of which three were 2-5 cm, 11 were 5-10 cm, and three were > 10 cm. Five (29.4%) tumors with O-RADS score 3 were endometriotic cysts with fib
The ultrasound images of ovarian collision tumor have certain specificity, but diagnosis by preoperative ultra
Core Tip: The ultrasound image characteristics of ovarian collision tumor have certain specificity, which can be divided into three types, but preoperative ultrasound is still difficult to make a clear diagnosis; The combination of epithelial cell tumor and mesenchymal cell tumor is the most common type of ovarian collision tumor; The o-rads system can sensitively detect malignant tumors when the o-rads score of ovarian collision tumors is mostly 4 or above.
- Citation: Yin C, Wang Y, Fei ZH, Sun LH, Zhou WA, Li H. Ovarian-adnexal reporting and data system ultrasound evaluation and pathological characteristics of ovarian collision tumor. World J Clin Cases 2024; 12(22): 4932-4939
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/4932.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.4932
Collision tumors are neoplasms including two histologically distinct tumors that coexist in the same mass without histological admixture. The incidence of collision tumor is low and is rare clinically. Because there is no direct transition between the two tumor cells, they are viewed as separate primary neoplasms[1-3]. Ovarian collision tumors are not common, and lack specific ultrasound diagnostic features, which have mostly been reported previously in case reports. Retrospective studies with clear pathological diagnosis have found that ovarian collision tumors are mostly multilocular cystic tumors, which may contain parenchymal components, or papillary bulges, and their ultrasonic signs are different. This is a challenge for preoperative ultrasound diagnosis of ovarian collision tumors.
At present, there are three hypotheses about the pathogenesis of collision tumor. First, two tumors are accidentally concurrent, and two different types of tumors meet at the same site or the same organ by chance[4]. Second, the first tumor changes the microenvironment of surrounding tissues and then other different types of tumors occur[5,6]. Third, due to the heterogeneity of genetic phenotypes, clonal cells with homogeneous genetic genes will differentiate into two different histological types of tumor cells[7]. However, the mechanism of ovarian collision tumor is still unclear.
There have been few studies on ovarian-adnexal reporting and data system (O-RADS) ultrasound of ovarian collision tumor. O-RADS is a classification system proposed in recent years, which is applied to the management of ovarian lesions. It recommends six categories (O-RADS 0-5) that range from normal to high risk of malignancy. It comprehensively evaluates the risk of ovarian masses through accurate homogeneous classification management, and provides diagnosis and treatment guidance for clinicians. This study evaluated the malignant risk of ovarian expansive tumor using O-RADS, to determine the ultrasonic phenotypic characteristics of ovarian collision tumor combined with the histological pathology and clinical characteristics, so as to provide the basis for clinical diagnosis and treatment.
This was a retrospective analysis of 17 ovarian collision tumors from 6274 female patients who underwent gynecological surgery in Changde region between January 2020 and December 2023. All clinical records, ultrasound images, biochemical examination and pathological findings were collected. The data collection was approved by Hunan Changde maternal and child health hospital and the ethics committee of Hunan University of Arts and Sciences (No. JSDX2022-00). Informed consent was waived. No private information from any patient was revealed.
The inclusion criteria were: (1) The coexistence of two histologically distinct tumors in one ovarian mass proven by histological findings; (2) Intact clinical, ultrasonography, and surgical records; and (3) An interval between ultrasonography and surgery of < 3 months.
All cases were examined by intravaginal ultrasound or vaginal ultrasound combined with abdominal ultrasound. The O-RADS risk classification was carried out according to the ultrasonic characteristics of the mass, including the shape and size, cystic and solid nature, internal structure, and vascularization. The O-RADS score and lexicon descriptors were determined by two senior doctors who were engaged in ovarian tumor ultrasonic imaging diagnosis for > 5 years. To improve the accuracy of O-RADS classification, we selected 200 cases of ovarian tumors and trained four doctors in the gynecological ultrasound group on the homogenization of O-RADS tumor classification to improve their experience.
We used the standard of O-RADS tumor scoring guidelines for gynecological oncology of the Chinese Medical Association: O-RADS score of 2, < 1% likelihood of malignancy; O-RADS score of 3, malignancy risk of 1%-10%; O-RADS score of 4, malignancy risk of 10%-50%; O-RADS score of 5, malignancy risk of > 50%. Lesions with O-RADS score of 1-3 were classified as benign tumors, and score of 4 or 5 were classified as malignant tumors.
PHILIPS7W, AFFINIT79, MINDRAY-I9, GE-E8, GE-E10. The probe frequency was 2-9 MHz, 1-6 MH. The examination method was mainly transvaginal ultrasound, combined with abdominal ultrasound.
This was a retrospective analysis of 17 ovarian collision tumors, and their ultrasound images, O-RADS score, and postoperative histology and pathology were analyzed.
We detected 17 collision tumors in 16 of 6274 patients who underwent gynecological surgery. The incidence was approxi
| No. | Age (year) | O-RADS | Size (cm) | Biochemical indicators, CA125 (kU/L), CA19-9 normal | Site of occurrence | Operation | Preoperative ultrasound | Pathology |
| 1 | 32 | 3 | 5.4 × 5.5 | CA125: 31.5 | Left | Laparoscope | Chocolate cyst; Fibroma | Endometriotic cyst with fibroma |
| 2 | 22 | 3 | 5.2 × 5.0 | CA125: 26.6 | Left | Laparoscope | Chocolate cyst | Endometriotic cyst with serous cystadenoma |
| 3 | 32 | 3 | 5.4 × 4.3 | CA125: 29.5 | Left | Laparoscope | Chocolate cyst | Endometriotic cyst with fibroma |
| 4 | 39 | 3 | 2.7 × 2.0 | CA125: 19.7 | Left | Laparoscope | Chocolate cyst | Mucinous cystadenoma with chocolate cyst |
| 5 | 22 | 3 | 2.8 × 2.4 | CA125: 17.3 | Left | Laparoscope | Serous cystadenoma | Endometriotic cyst with serous cystadenoma |
| 6 | 27 | 4a | 22.1 × 18.0 | - | Right | Laparoscope | Serous cystadenoma | Serous cystadenoma with fibroma |
| 7 | 27 | 4a | 19.2 × 14.6 | - | Right | Laparoscope | Serous cystadenoma | Serous cystadenoma with cystic fibroma |
| 8 | 22 | 4b | 37.1 × 30.6 | CA125: 33.6 | Left | Laparoscope | Cystadenoma | Mucinous cystadenoma with chocolate cyst |
| 9 | 20 | 4b | 6.4 × 4.9 | CA125: 47.8 | Right | Laparoscope | Teratoma | Serous cystadenoma with goiter |
| 10 | 60 | 4b | 12.0 × 9.5 | CA125: 42 | Left | Laparoscope | Serous cystadenoma | Serous cystadenoma with ovarian goiter |
| 11 | 46 | 4b | 7.4 × 7.3 | CA125: 38.8 | Right | Laparoscope | Cystadenoma | Mucinous cystadenoma with teratoma |
| 12 | 27 | 4b | 3.2 × 2.0 | CA125: 65.6 | Right | Laparoscope | Teratoma | Serous cystadenoma with fibroma |
| 13 | 42 | 4b | 7.4 × 4.9 | CA125: 119 | Right | Laparoscope | Serous cystadenoma | Serous cystadenoma with fibroma |
| 14 | 58 | 4c | 8.5 × 4.0 | CA125: 147.2 | Right | Laparotomy | Cystadenoma | Borderline mucinous cystadenoma with epidermoid cyst |
| 15 | 43 | 4c | 9.0 × 8.0 | CA125: 156.1; CA19-9: 442.3 | Bilateral (left) | Laparotomy | Serous cystadenoma | Serous cystadenoma with fibroma; Focal junctional |
| 16 | 43 | 4c | 8.0 × 7.0 | CA125: 156.1; CA19-9: 442.3 | Bilateral (right) | Laparotomy | Serous cystadenoma | Serous cystadenoma with fibroma; Focal junctional |
| 17 | 68 | 5 | 6.2 × 4.1 | CA125: 275; CA19-9: 523.5 | Right | Laparotomy | Ovarian carcinoma | High-grade serous carcinoma with cystic mature teratoma |
There were 17 collision tumors detected in 16 of 6274 patients, with one occurring on both sides, 15 occurring on one side, seven on the left and eight on the right. The average age of the 16 patients with ovarian collision tumors was 36.7 years (range: 20-68 years). For seven patients (43.8%), the lesion was found during physical examination, and these patients did not present with significant symptoms. Two patients (12.5%) had prolonged menstruation. Two patients (12.5%) had pain in the groin area and distension in the lower abdomen. Four patients (25.0%) had increased abdominal circumference, and one (6.2%) had vaginal bleeding. Serological test results showed that nine patients (56.3%) had elevated serum CA125, which was classified as O-RADS score ≥ 4, and two patients (12.5%) had elevated serum CA19-9, including one with as O-RADS 4 and one with O-RADS 5.
For surgical management, 13 (81.3%) patients underwent laparoscopic ovarian tumor dissection, two (12.5%) underwent open ovariectomy (one of which was a bilateral collision tumor), and one (6.2%) underwent radical surgery for ovarian carcinoma.
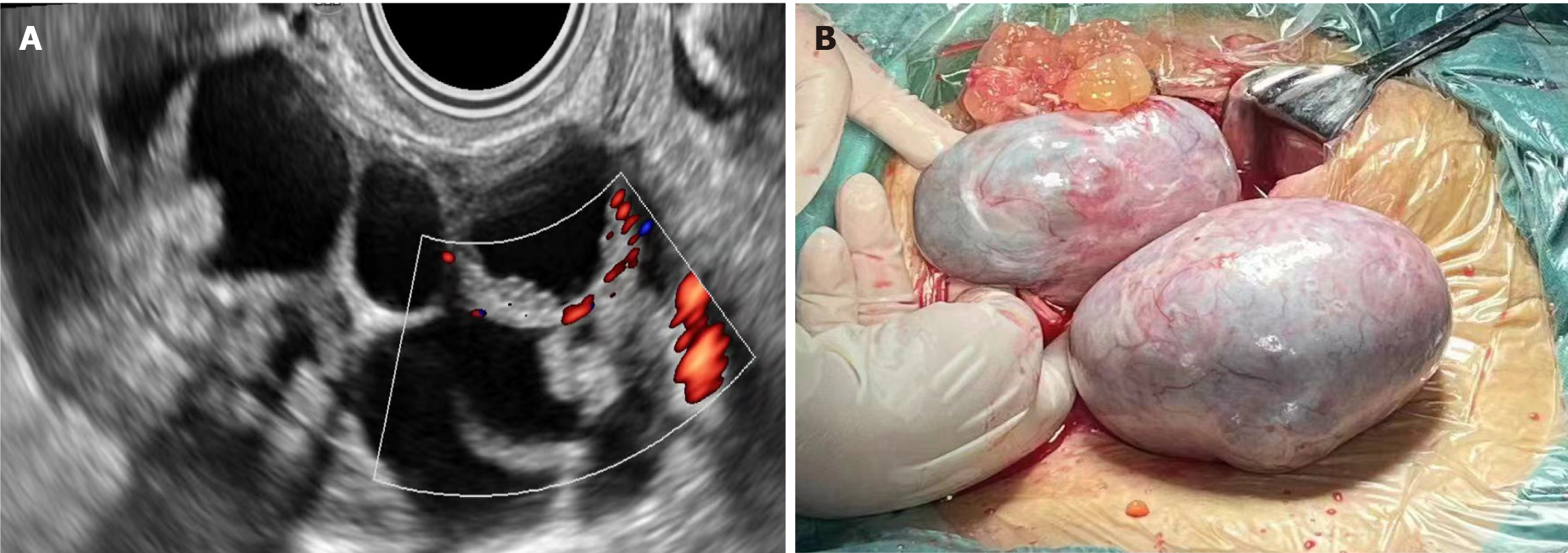
There were 15 epithelial-cell-derived combined with germ-cell- or mesenchymal-cell-derived tumors among the 17 ovarian collision tumors. Ten had combination of serous cystadenoma and (cystic) fibroma/mature cystic teratoma/ovarian goiter/chocolate cyst; four had mucinous cystadenoma combined with teratoma/epidermoid cyst; one had high-grade serous cystadenocarcinoma combined with cystic mature teratoma with cancer tissue invasion; and two had endometriotic cysts combined with fibroma. There were one patient with bilateral tumor and one with unilateral tumor as borderline or focal borderline collision tumor. Exophytic "cauliflower" was seen in the left tumor in patients with bilateral tumors (Figure 1). The histological and pathological results suggested that both sides were ovarian cystadenoma with fibroma. The pathological manifestations of ovarian cysts were serous epithelium, papillary processes on the inner wall, and fibrous stromal components. Tumor cells in some areas proliferated actively, and had focal borderline changes.
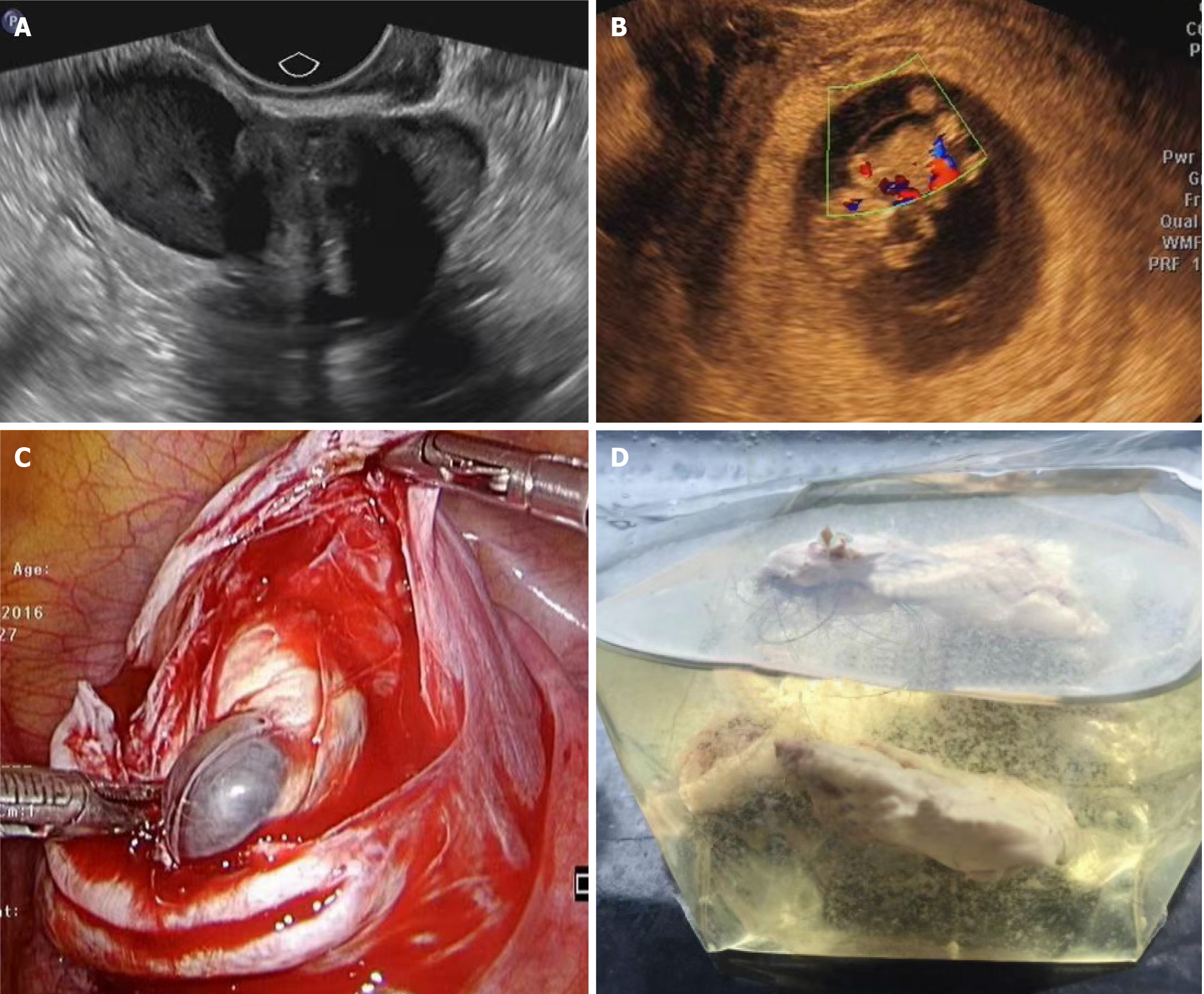
Five (29.4%) tumors had an O-RADS score of 3, and the maximum diameter was 4.1 cm. Two of them were endometriotic cysts combined with fibromas. The ultrasound phenotype was a combination of two types of tumors and the boundary was clear. They had the sonographic characteristics of chocolate cysts and fibromas, respectively (Figure 2A). We classified them as the first type of ultrasound phenotype: Two types of tumors combined into a tumor with clear boundaries, showing a "back-to-back" relationship[7]. Preoperative ultrasound suggested two independent masses and could not make a diagnosis. Two cases were chocolate cysts with serous cystadenoma, and one was chocolate cyst with mucinous cystadenoma. These three preoperative ultrasound images showed the characteristics of fine dot echo of chocolate cysts, showing several separations, suggesting chocolate cysts. Eleven (64.7%) tumors had O-RADS score of 4, and the maximum diameter was 12.7 cm. There were two in category 4A, six in 4B and three in 4C. The O-RADS score of 4 was multilocular cystic tumor. The higher the classification level of 4A-4C, the thicker and more uneven the ultrasound image separation, and the cyst wall was irregular or did not contain solid components. The higher the number and volume of nipple components, the higher the blood flow score.
Collision tumors with an O-RADS score of 4 also showed two sonographic phenotypic characteristics: One of which we summarized as a type 2 sonographic phenotype: Tumor in capsule, large capsule with small capsule (Figure 2B). Its histological pathology was serous cystadenoma and goiter. The ultrasonic image showed that the lateral large cyst contained a liquid dark area with sparse dot like echo. The small cyst was located in the large cyst, and the internal structure was complex. It showed multiple round solid echoes with a clear boundary, smooth outline and different size. Multiple blood flow signals were seen on color Doppler flow imaging (CDFI). According to the surgical results, there was a 7-cm tumor in the right ovary. The capsule was about 2 cm thick and contained clear mucus. After the cyst wall was completely stripped, there was a small cystic mass of about 4 cm × 3 cm × 3 cm (Figure 2C). The interior of the small cyst contained yellow lipid and hair-like tissue (Figure 2D).
The other ultrasound phenotype in collision tumors with O-RADS score of 4 was classified as category 3. The two tumors fused to form a tumor without obvious demarcation. The ultrasound phenotype had typical features of one tumor, but the echo was more complex. Some features of another tumor were seen inside or on one side of the tumor (Figure 1A). It is difficult to diagnose this category of tumor with preoperative ultrasound. There was one (7.1%) tumor with the O-RADS score of 5, which was a malignant tumor. The ultrasound image comprised multiple hypoechoic solid structures of different sizes and irregular shapes. Each solid structure could be seen with a septum, a small amount of ascites could be detected, and CDFI showed abundant blood flow signals. There were five ultrasound phenotypic features in Category 1, 2 in Category 2, and 10 in Category 3 in 17 collision tumors of these 16 patients.
Ovaries are composed of epithelial, germ and mesenchymal cells, which can randomly form a variety of ovarian collision tumors; the most common of which is combination of epithelial and germ cell tumors, and the incidence of other types is low[6]. This study found that the combination of epithelial and mesenchymal cell tumors accounted for the highest proportion of ovarian collision tumors; serous cystadenoma combined with fibroma was the most common, followed by combination of epithelial and germ cell tumors. This is different from previous studies[1,6].
By studying the ultrasound image characteristics of 17 cases of collision tumor, we found that it can be summarized into three categories. The first category is that two tumors are combined rather than fused into a large tumor, and the boundaries of the two tumors are clear and have their own ultrasound imaging characteristics. The second type is the tumor in the capsule or the large capsule with small capsule. Both the outer large capsule and inner small capsule have their own ultrasonic imaging characteristics. The third category is a single mass, which has particular ultrasound imaging characteristics, but the image is more complex and cannot be summarized by a single ultrasound imaging feature.
The first type of ultrasound phenotype in this study is usually misdiagnosed as two separate tumors in daily life. In fact, the two tumors coexist in one organ (ovary), the edges are attached to each other, and there is no obvious tissue mixing[6]. The second type of ultrasound phenotype accounted for the smallest proportion. There were only two cases of collision tumors in 17 cases; one of which was a small capsule located in the center of the large capsule, and the small capsule was thickened. The other one was a small capsule located on one side of the large capsule, and the capsule was thin. Preoperative examination suggested that this type of tumor could not be clearly diagnosed. The third ultrasound phenotype accounted for the largest proportion, which indicates that the ultrasound imaging characteristics of ovarian collision tumors depend on the tumor composition of the collision tumors, and the composition proportion mode of the collision tumors. Therefore, ovarian collision tumors with the same histology and pathology can have significantly different ultrasound imaging characteristics. O-RADS can sensitively evaluate the benign and malignant of ovarian collision tumors, and provide accurate diagnostic imaging information.
The mechanism of ovarian collision tumor is still unclear, and there are few comprehensive studies on use of O-RADS for classification of ovarian collision tumor. Because of its nonspecific ultrasound images and clinical manifestations, and complex histopathological composition, the diagnosis rate of ovarian collision tumors is still low, even with preoperative needle biopsy, which is a major difficulty in preoperative diagnosis[7]. Based on the retrospective analysis of the pathological follow-up results, we found that the low incidence of ovarian collision tumor was the main reason for the difficulty with preoperative ultrasound diagnosis. It is also possible that our pathological diagnosis experts paid insufficient attention to rare ovarian collision tumors. By studying the ultrasound images and pathological characteristics of ovarian collision tumors in the past 3 years, we successfully diagnosed two cases of ovarian collision tumors by preoperative ultrasound, which were confirmed as cystadenoma and teratoma by histology and pathology.
The clinical symptoms of patients with ovarian collision tumor are different. Patients with tumor diameter > 5 cm have increased abdominal circumference, and lower abdominal or groin pain. There was one chocolate cyst with cystadenoma. The diameter of ovarian collision tumor was < 3 cm, and the clinical manifestations also included lower abdominal pain. One patient with malignant tumor with goiter had abnormal menstruation or bleeding, which was related to the size and pathology of the tumor.
CA125 is currently the most widely used biomarker for the diagnosis of epithelial ovarian cancer, and can also be used for disease severity monitoring[8]. The serum CA125 level in patients with ovarian collision tumor was significantly higher than the normal level, and CA199 increased in two cases; one of which was high-grade serous cystadenocarcinoma with cystic mature teratoma with cancer tissue invasion. Serum CA125 Level can reflect the progression of serous ovarian cancer, and can be used as an evaluation standard for tumor lesion grade, stage and lymph node metastasis.
The main limitation of this study was that ultrasound doctors and pathological diagnosis experts paid insufficient attention to rare ovarian collision tumors. It is also possible that the sample size was too small to analyze ovarian collision tumors comprehensively and objectively.
The ultrasound imaging characteristics of ovarian collision tumor have some specificity, which can be divided into three types, but it is still difficult to make a definitive diagnosis by preoperative ultrasound. The combination of epithelial and mesenchymal cell tumor is the most common type of ovarian collision tumor. O-RADS can sensitively detect malignant tumors when the score is ≥ 4.
We thank Professor Zhou QC from the Second Xiangya Hospital, Central South University for the guidance on this project, Dr. Li JC for providing clinical samples, Dr. Wang RS and Dr. Zhang WY from Hunan University of Arts and Sciences for proofreading the manuscript.
| 1. | Guo Y, Zhou S, Zhao B, Wen L, Liu M. Ultrasound Findings and O-RADS Malignancy Risk Stratification of Ovarian Collision Tumors. J Ultrasound Med. 2022;41:2325-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Kim SH, Kim YJ, Park BK, Cho JY, Kim BH, Byun JY. Collision tumors of the ovary associated with teratoma: clues to the correct preoperative diagnosis. J Comput Assist Tomogr. 1999;23:929-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Coca-Pelaz A, Triantafyllou A, Devaney KO, Rinaldo A, Takes RP, Ferlito A. Collision tumors of the larynx: A critical review. Am J Otolaryngol. 2016;37:365-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Bige O, Demir A, Koyuncuoglu M, Secil M, Ulukus C, Saygili U. Collision tumor: serous cystadenocarcinoma and dermoid cyst in the same ovary. Arch Gynecol Obstet. 2009;279:767-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Weigel J, Neher M, Schrey M, Wünsch PH, Steiner HH. Collision Tumor Composed of Meningioma and Cavernoma. J Korean Neurosurg Soc. 2017;60:102-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Peng Y, Lin J, Guan J, Chen L, Zhang X, Li S, Wang H, Liu M, Guo Y. Ovarian collision tumors: imaging findings, pathological characteristics, diagnosis, and differential diagnosis. Abdom Radiol (NY). 2018;43:2156-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Zhu Y, Serpooshan V, Wu S, Demirci U, Chen P, Güven S. Tissue Engineering of 3D Organotypic Microtissues by Acoustic Assembly. Methods Mol Biol. 2019;1576:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Zhang R, Siu MKY, Ngan HYS, Chan KKL. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 117] [Reference Citation Analysis (0)] |