Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.4924
Revised: May 13, 2024
Accepted: June 3, 2024
Published online: August 6, 2024
Processing time: 117 Days and 0.6 Hours
Traditional methods cannot clearly visualize esophageal cancer (EC) tumor co
To investigate the efficacy of the da Vinci robot in combination with nanocarbon lymph node tracers in radical surgery of EC.
In total, 104 patients with early-stage EC who were admitted to Liuzhou worker's Hospital from January 2020 to June 2023 were enrolled. The patients were assi
Compared with the control group, the observation group had significantly lower postoperative CRP, cortisol, and E levels (P < 0.05) with a milder inflammatory response, as indicated by lower IL-6, IL-10, and TNF-α levels (P < 0.05). Patients who underwent RAMIE had less intraoperative blood loss and shorter operation times and hospital stays than those who underwent traditional surgery. The ave
The treatment of EC using the da Vinci robot combined with nanocarbon lymph node tracers can achieve good surgical outcomes and demonstrates promising clinical applications.
Core Tip: Traditional surgical visualization for esophageal cancer (EC) cannot clearly delineate tumor contours and me
- Citation: Qi FQ, Sun Y. Efficacy and prognostic analysis of carbon nanotracers combined with the da Vinci robot in the treatment of esophageal cancer. World J Clin Cases 2024; 12(22): 4924-4931
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/4924.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.4924
In China, approximately 180000 people die of esophageal cancer (EC) every year[1]. Because the esophagus is surrounded by complex tissues, conventional open surgery can cause significant trauma, loss of healthy tissue, and poor quality of life postoperatively[2]. The development of medical technology has made minimally invasive procedures possible, which avoid patient trauma and preserve healthy tissues. The da Vinci robotic arm can perform finer and more accurate te
Da Vinci robot-assisted minimally invasive esophagectomy (RAMIE) can implant and preserve tumor margin tissue to the maximum extent, enabling complete resection. Radical resection of EC using RAMIE can improve surgical accuracy and efficiency while minimizing complications[4]. In 2003, da Vinci roboticists announced the first patient in the world to undergo da Vinci robot-assisted EC surgery[5]. However, conventional visualization methods cannot clearly display tumor outlines and metastases, which limits the accuracy of da Vinci robotic surgery.
Nanocarbon tracers comprise a new type of nanomaterial consisting of carbon-based materials, such as carbon na
This study included 104 patients with early-stage EC admitted to Liuzhou worker's Hospital from January 2020 to June 2023. The patients were divided into an observation group (n = 52), which underwent RAMIE with the intraoperative use of nanocarbon tracers, and a control group (n = 52), which underwent traditional surgery. The two groups did not differ significantly in sex, age, pathological type, lesion diameter, or body mass index (P > 0.05).
The control group underwent traditional surgery. The patient was assisted by single-lumen endotracheal intubation in both lungs, and a 15–20-cm incision was made in the fourth intercostal space on the anterolateral side of the right chest. Through this incision, the surgical area was examined in detail to identify the location, size, and shape of the tumor. During the procedure, the thoracic esophagus was mobilized, and the chest lymph nodes were dissected. Another arc-shaped incision, approximately 5 cm long, was created at the sternum, below the neck, and an anastomosis was per
The observation group underwent radical surgery with the da Vinci robot as the auxiliary system. Anesthesia was induced by intravenous inhalation, and endotracheal intubation was established using a single-lumen tube combined with a bronchus or double-lumen tube capable of collapsing a fully joined artificial pneumothorax. The procedure consisted of three parts and was performed as described previously[10]. A corresponding change in body position was required for each part of the operation: (1) When the thoracic esophagus was being mobilized by the robot, the patient was placed in the left decubitus position to facilitate lymphatic cleaning of the corresponding region; (2) When the robot was being used to free the stomach, the patient was placed in a supine posture with the head in a high position, the feet in a low position, and the body tilted 15° to the right; and (3) When the neck was being anastomosed mechanically or at the end of surgery, the pillow was removed from under the patient’s head so the patient can lie down completely.
A 1-mL nanocarbon suspension injection was prepared using 0.1 mL of nanocarbon suspension (specification: 1 mL, 50 mg; Laimei Pharmaceutical, Chongqing, China; national drug approval: H20041829) and 0.9 mL of 0.9% normal saline (total nanocarbon content: 5 mg). The suspension was injected 2 h preoperatively after confirming the location of the tumor through a gastroscope, with the patient in the lateral position. In total, 4–6 points that were approximately 1 cm away from the tumor were chosen. A fine needle was inserted approximately 5 mm into the tissue, and 0.1-0.3 mL of the tracer was slowly injected at each point.
Stress response: Anticoagulant test tubes were used to collect fasting venous blood from all patients preoperatively and postoperatively. The collected blood samples were centrifuged at 3000 rpm for 15 min, after which the serum was collected off the top into sterile test tubes. The test tubes were frozen at -70 °C until use. Enzyme-linked immunosorbent assay (ELISA) kits (Bohu Bio, Shanghai, China) were used to determine C-reactive protein (CRP), cortisol, and epinephrine (E) levels following the manufacturer’s instructions.
Inflammatory response: Blood samples were collected and processed in the same manner as above to evaluate serum levels of interleukin (IL)-6, IL-10, and tumor necrosis factor α (TNF-α) by ELISA.
Observation of relevant clinical indicators: Surgery-related indicators were recorded, including the total and average numbers of dissected lymph nodes and the average duration of lymph node dissection.
Complication rate: The safety of the procedure was evaluated based on the incidence of complications, such as gastro
Medium and long-term curative effect: Telephone follow-ups of all patients were conducted for 2 years to evaluate the disease recurrence rate, transfer rate, and mortality rate.
SPSS version 19 was used to analyze the data of this study. Measurements (expressed as mean ± SD) and count data (expressed as number and percentage) were compared between the observation and control groups using the inde
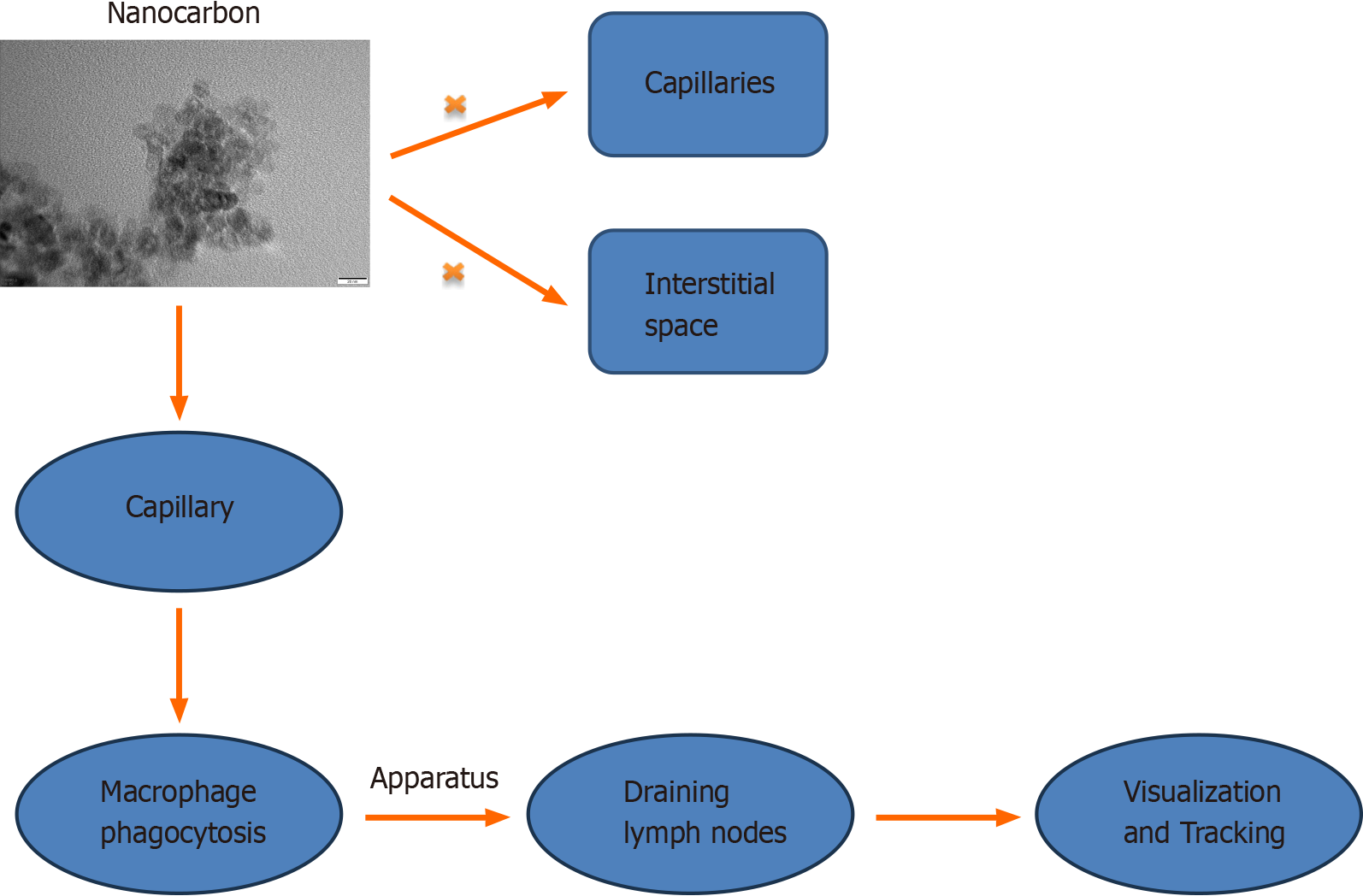
Nanocarbon materials have a small particle size and a large specific surface area, enabling them to enter lymphatic vessels and flow to the lymph nodes. However, nanocarbon materials cannot enter the capillaries and interstitial systems. In lymph nodes, carbon nanomaterials can be taken up by phagocytes or be otherwise retained. Therefore, after the nanocarbon material is injected into the body, it is quickly taken up by the lymphatic system and spread along the lym
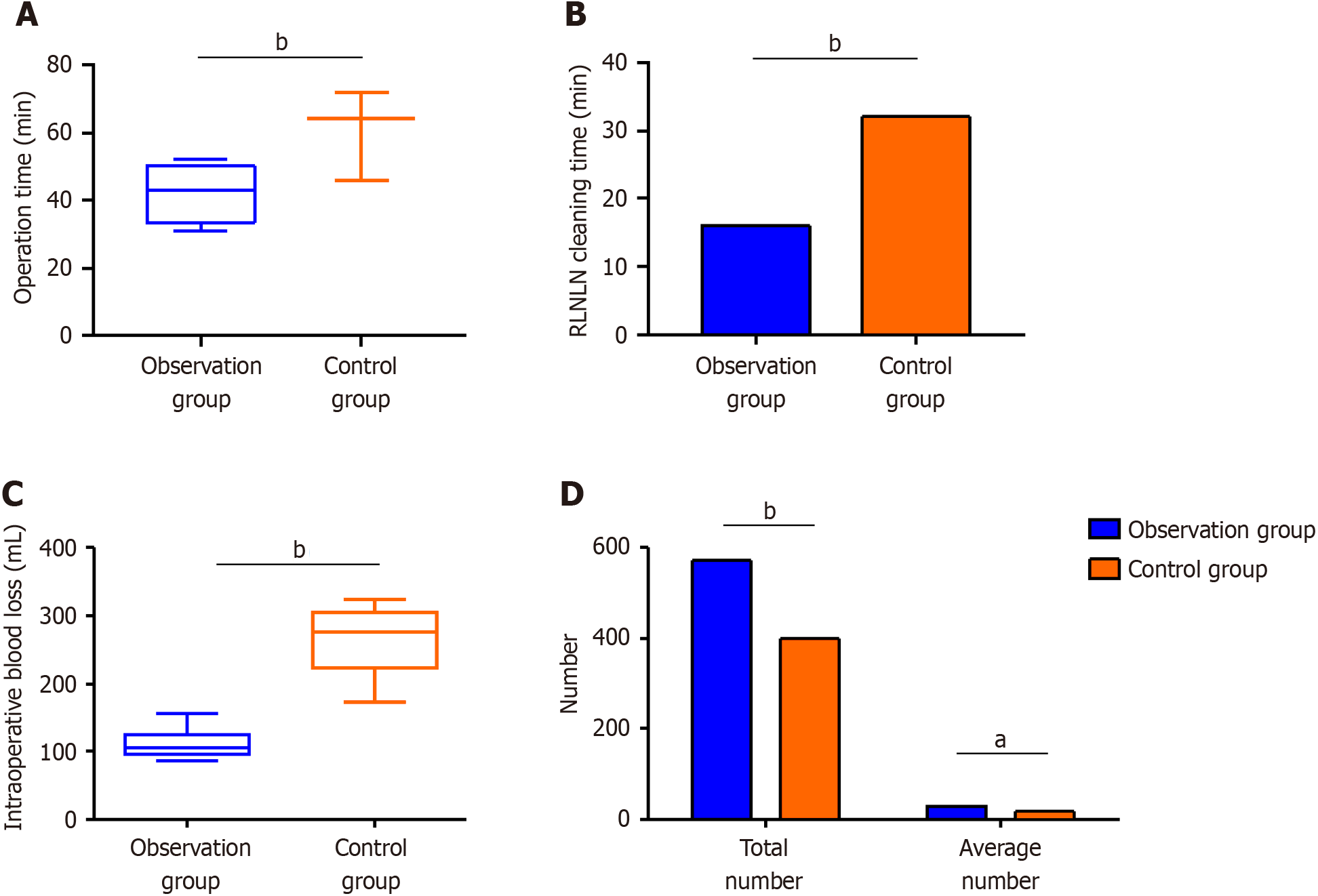
The observation group had shorter operation times and less intraoperative blood loss than the control group (Figure 2A). The observation group also showed a shorter recurrent laryngeal nerve lymph node (RLNLN) dissection time, smaller mean number of minimal lymph nodes, and greater total and average numbers of left and right RLNLNs dissected than the control group (Figure 2B–D).
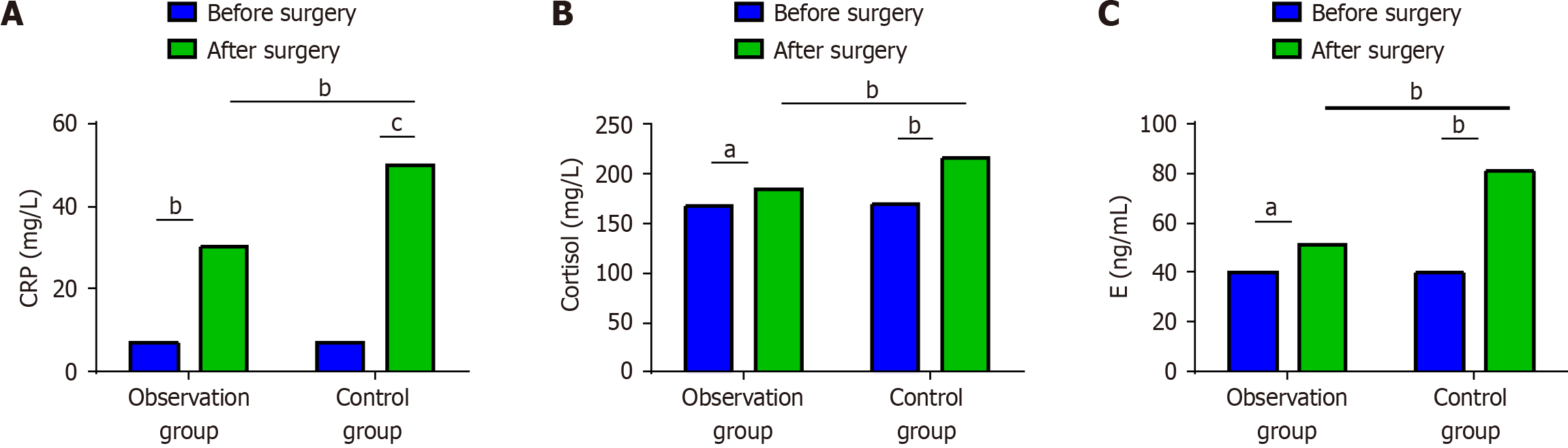
Both groups showed elevated postoperative CRP, cortisol, and E levels, with those in the observation group being lower than the control group (P < 0.05) (Figure 3).
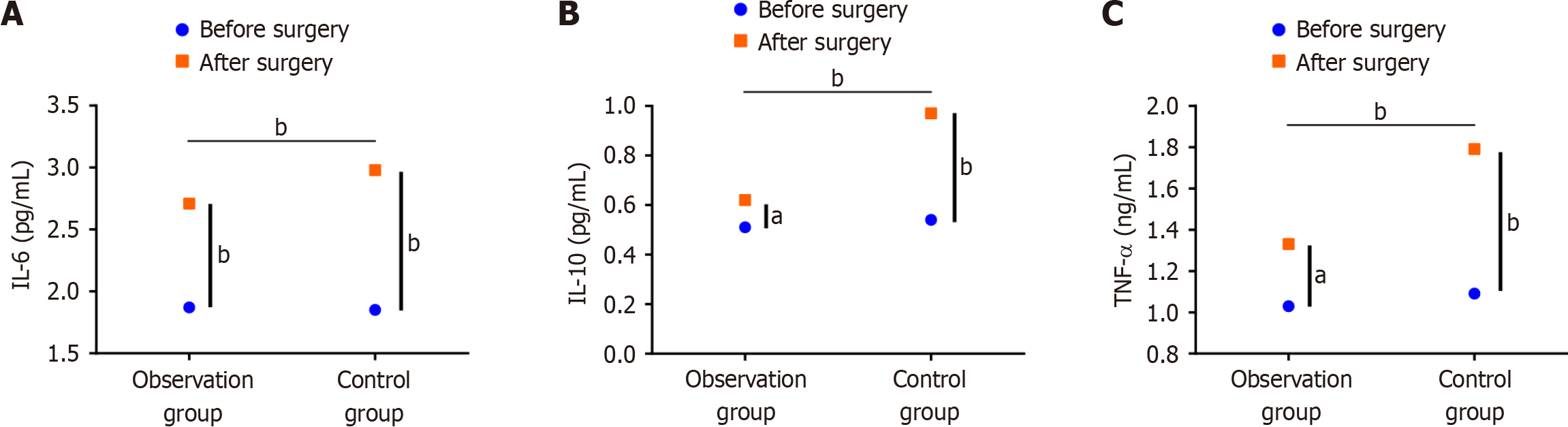
After radical surgery for EC, the relevant inflammatory indexes increased in both groups, with the most significant increase observed in TNF-α levels. The control group showed higher postoperative IL-6, IL-10, and TNF-α levels than the observation group (Figure 4).
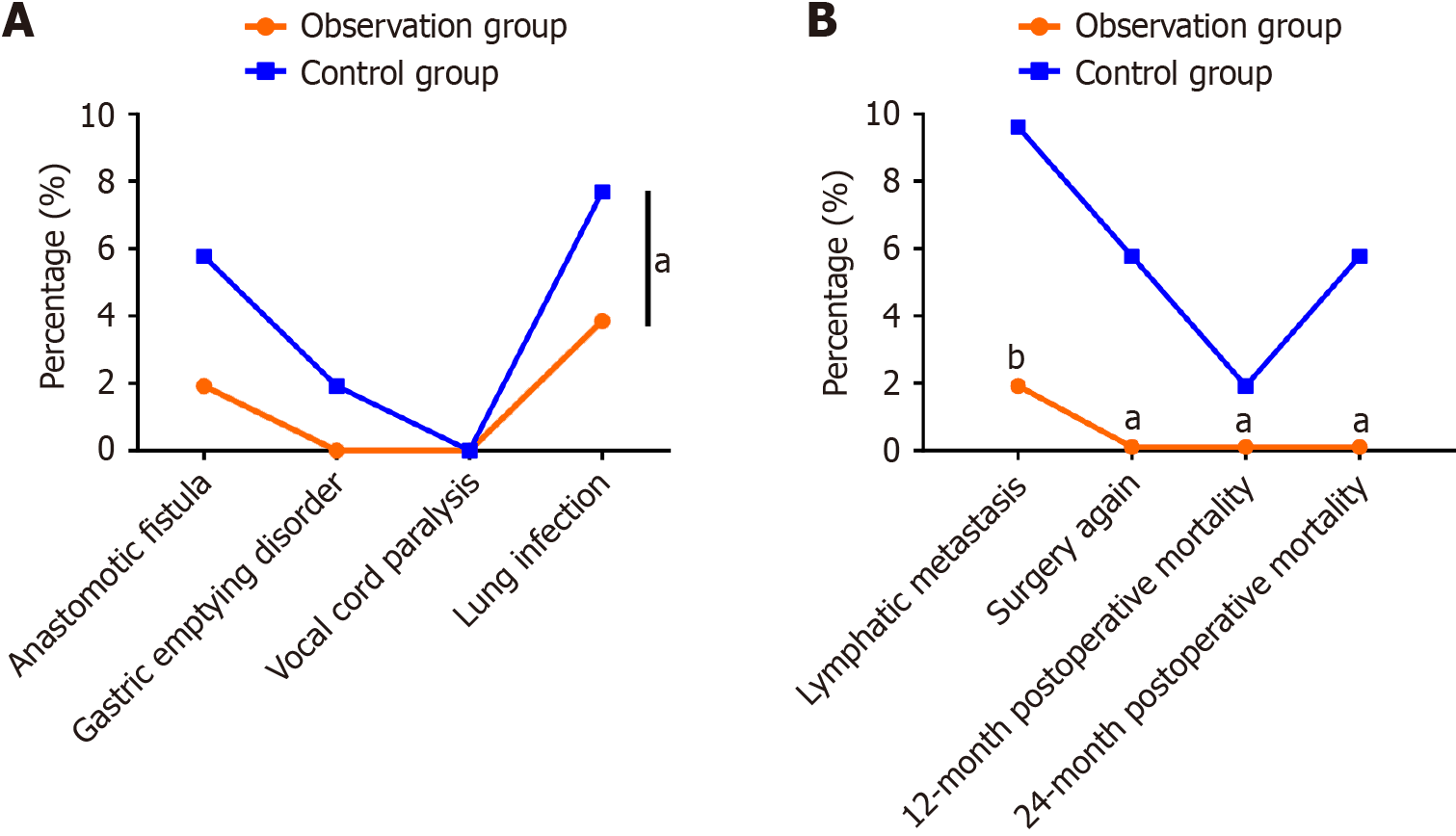
The incidence of adverse events, such as postoperative gastric emptying disorder, anastomotic leakage, and pulmonary infection, was 5.77% in the observation group, which was significantly lower than the 15.38% observed in the control group (Figure 5A). The lymphatic metastasis and reoperation rates and the 12 and 24-mo cumulative mortality rates in the observation group were 2.5%, 0%, 0%, and 2.5%, respectively, all of which were lower compared with those in the control group (Figure 5B).
EC is a clinical gastrointestinal malignancy that can directly endanger the physical and mental health of patients. It can be treated by diverse surgical methods. Traditional open radical resection of EC involves right thoracotomy and laparotomy; however, this approach is relatively harmful to the patient[11], with high postoperative morbidity and mortality. Fur
The use of nanocarbon tracers in the diagnosis and staging of EC has great potential. Carbon nanoparticles have high biocompatibility and stability and are the ideal tracers[16]. Compared with conventional methods, such as capsule endoscopy, nanocarbon tracers can provide higher resolution and contrast, thereby facilitating the early detection and accurate staging of EC[17]. In addition, nanocarbon tracers can be combined with other imaging techniques, such as fluorescence imaging, to further improve diagnostic and staging accuracy[18]. After it is injected into the tissues surrounding the tumor, the nanocarbon tracer is engulfed by macrophages, allowing the nanocarbon group to quickly penetrate the capillary lymphatic vessels and accumulate in the lymph nodes, giving them the indicative black stain that facilitates lymph node tracing[19]. Our study demonstrates the significant advantages of carbon nanotracers in com
CRP is a common acute response protein that can indicate the degree of injury. Cortisol and E are also indicators of the body’s stress response, as well as the extent of damage to the body’s immunologic function and overall status. Thoracoscopic treatment of EC has previously been reported to reduce traumatic stress responses in the patient[21]. Similar conclusions were drawn in this study. After radical esophagectomy using the da Vinci robot, significantly lower CRP, cortisol, and E levels were noted in the observation group than in the control group. Analysis of relevant inflammatory indicators before and after surgery also revealed that postoperative IL-6, IL-10, and TNF-α levels increased in both groups but were significantly lower in the observation group than in the control group. This may be because RAMIE requires smaller surgical incisions, which can effectively reduce the degree of stretching of various tissues and organs, thereby minimizing trauma and stress response by mitigating the secretion of inflammatory factors[22].
Although the carbon nanotracers combined with the da Vinci robotic surgical system have obvious advantages in treating EC, there are still some limitations. First, the high cost of production and use of carbon nanotracers may limit their widespread clinical application. Second, the high cost of the da Vinci robotic surgical system may limit its adoption in low-income settings. With regards the limitations of the present study, the retrospective design may have introduced selection and information biases.
This study demonstrated that, compared with traditional surgical methods, carbon nanotracers combined with da Vinci robotic surgery have significant curative effects and a good prognosis for EC. The advantages of the two technologies should be explored further in a multicenter prospective study with a larger dataset to screen suitable patient groups. Such studies would help optimize comprehensive treatment plans and improve therapeutic efficacy and quality of life in patients with EC.
| 1. | Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg. 2018;41:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 535] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 2. | Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg. 2013;61:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Banks KC, Hsu DS, Velotta JB. Outcomes of Minimally Invasive and Robot-Assisted Esophagectomy for Esophageal Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | de Groot EM, Goense L, Kingma BF, van den Berg JW, Ruurda JP, van Hillegersberg R. Implementation of the robotic abdominal phase during robot-assisted minimally invasive esophagectomy (RAMIE): results from a high-volume center. Surg Endosc. 2023;37:1357-1365. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Singh I. Robotics in urological surgery: review of current status and maneuverability, and comparison of robot-assisted and traditional laparoscopy. Comput Aided Surg. 2011;16:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Zhou NX, Chen JZ, Liu Q, Zhang X, Wang Z, Ren S, Chen XF. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot. 2011;7:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Mathew SA, Praveena P, Dhanavel S, Manikandan R, Senthilkumar S, Stephen A. Luminescent chitosan/carbon dots as an effective nano-drug carrier for neurodegenerative diseases. RSC Adv. 2020;10:24386-24396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Wang T, Yang H, Liang D. Effect of Tracer Staining Technology Based on Nano-Carbon Suspension on Patients with Oral Squamous Cell Carcinoma. J Nanosci Nanotechnol. 2021;21:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Liu P, Tan J, Tan Q, Xu L, He T, Lv Q. Application of Carbon Nanoparticles in Tracing Lymph Nodes and Locating Tumors in Colorectal Cancer: A Concise Review. Int J Nanomedicine. 2020;15:9671-9681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Ishikawa N, Kawaguchi M, Inaki N, Moriyama H, Shimada M, Watanabe G. Robot-assisted thoracoscopic hybrid esophagectomy in the semi-prone position under pneumothorax. Artif Organs. 2013;37:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 12. | Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1143] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 13. | van der Sluis PC, Ruurda JP, van der Horst S, Verhage RJ, Besselink MG, Prins MJ, Haverkamp L, Schippers C, Rinkes IH, Joore HC, Ten Kate FJ, Koffijberg H, Kroese CC, van Leeuwen MS, Lolkema MP, Reerink O, Schipper ME, Steenhagen E, Vleggaar FP, Voest EE, Siersema PD, van Hillegersberg R. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials. 2012;13:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Yang Y, Zhang X, Li B, Li Z, Sun Y, Mao T, Hua R, Yang Y, Guo X, He Y, Li H, Chen H, Tan L. Robot-assisted esophagectomy (RAE) versus conventional minimally invasive esophagectomy (MIE) for resectable esophageal squamous cell carcinoma: protocol for a multicenter prospective randomized controlled trial (RAMIE trial, robot-assisted minimally invasive Esophagectomy). BMC Cancer. 2019;19:608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Boone J, Schipper ME, Moojen WA, Borel Rinkes IH, Cromheecke GJ, van Hillegersberg R. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg. 2009;96:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130:10876-10877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3295] [Cited by in RCA: 2284] [Article Influence: 134.4] [Reference Citation Analysis (0)] |
| 17. | Wang Z, Yu J, Gui R, Jin H, Xia Y. Carbon nanomaterials-based electrochemical aptasensors. Biosens Bioelectron. 2016;79:136-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D, Dai H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat Nanotechnol. 2009;4:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 810] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 19. | Tian W, Jiang Y, Gao B, Zhang X, Zhang S, Zhao J, He Y, Luo D. Application of nano-carbon in lymph node dissection for thyroid cancer and protection of parathyroid glands. Med Sci Monit. 2014;20:1925-1930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Pitsinis V, Wishart GC. Comparison of Indocyanine Green Fluorescence and Blue Dye Methods in Detection of Sentinel Lymph Nodes in Early-Stage Breast Cancer. Ann Surg Oncol. 2017;24:581-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Chao YK, Wen YW. Cost-effectiveness analysis of thoracoscopic versus open esophagectomy for esophageal cancer: a population-based study. Dis Esophagus. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Tagkalos E, Goense L, Hoppe-Lotichius M, Ruurda JP, Babic B, Hadzijusufovic E, Kneist W, van der Sluis PC, Lang H, van Hillegersberg R, Grimminger PP. Robot-assisted minimally invasive esophagectomy (RAMIE) compared to conventional minimally invasive esophagectomy (MIE) for esophageal cancer: a propensity-matched analysis. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |