Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.4881
Revised: June 13, 2024
Accepted: June 17, 2024
Published online: August 6, 2024
Processing time: 52 Days and 20 Hours
Patients with deep venous thrombosis (DVT) residing at high altitudes can only rely on anticoagulation therapy, missing the optimal window for surgery or thro
To apply the nomogram model in the evaluation of complications in patients with HAPC and DVT who underwent anticoagulation therapy.
A total of 123 patients with HAPC complicated by lower-extremity DVT were followed up for 6-12 months and divided into recurrence and non-recurrence groups according to whether they experienced recurrence of lower-extremity DVT. Clinical data and laboratory indices were compared between the groups to determine the influencing factors of thrombosis recurrence in patients with lower-extremity DVT and HAPC. This study aimed to establish and verify the value of a nomogram model for predicting the risk of thrombus recurrence.
Logistic regression analysis showed that age, immobilization during follow-up, medication compliance, compliance with wearing elastic stockings, and peri
The column chart model for the personalized prediction of thrombotic recurrence risk has good application value in predicting thrombotic recurrence in patients with lower-limb DVT combined with HAPC after discharge.
Core Tip: This study found that age, immobilization during follow-up, medication compliance, compliance with wearing elastic stockings, and peripheral blood D-dimer and fibrin degradation product levels are independent risk factors for thrombosis recurrence in patients with lower-extremity deep venous thrombosis (DVT) and high-altitude polycythemia (HAPC). Furthermore, the nomogram model constructed using these factors demonstrated promising applications in predicting thrombosis recurrence after discharge, thereby providing a reference for predicting thrombosis recurrence in patients with lower-limb DVT combined with HAPC in clinical practice.
- Citation: Zhao MX, Li GJ. Establishment of a nomogram model for predicting therapy complications in patients with polycythemia and deep venous thrombosis. World J Clin Cases 2024; 12(22): 4881-4889
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/4881.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.4881
Low-pressure hypoxia and low ambient temperatures at high altitudes significantly increase the risk of thrombotic disease in the local population[1]. Patients residing at high altitudes (> 3000 m) for 11 months have a 30-fold increased risk of venous thrombosis compared to those residing at low altitudes (< 800 m)[2]. The prevalence of deep venous thrombosis (DVT) is higher in highland regions than in plains[3,4]. Numerous studies have established a correlation between high-altitude environments and the activation of the coagulation cascade, resulting in a hypercoagulable state that predisposes individuals to thrombosis. This hypercoagulable state is intricately linked to high-altitude polycythemia (HAPC), which contributes to vascular endothelial damage and platelet activation, further exacerbating the risk of thrombosis[5]. In clinical practice, it has been observed that many cases of lower-extremity DVT in highland areas miss the optimal window for surgical intervention or thrombolysis at the time of consultation, thus relying solely on anticoagulation therapy. Additionally, there is a higher incidence of lower-extremity DVT complicated with HAPC in highland areas, which complicates DVT treatment and elevates the risk of recurrent thrombosis[6-8].
An essential step in safeguarding patient well-being is the effective identification of high-risk groups for thrombosis recurrence and implementation of targeted interventions. In this study, we aimed to investigate the factors associated with thrombosis recurrence in patients with lower-extremity DVT complicated by HAPC who underwent anticoagulation therapy after hospital discharge.
A total of 123 patients with HAPC and lower-extremity DVT who were admitted to the hospital between January 2019 and January 2021 were enrolled in this study. The inclusion criteria were as follows: Residence at high altitudes (> 3000 m) for over five years, meeting the diagnostic criteria for HAPC according to the 2004 Qinghai Criteria for Chronic Plateau Disease[9], having a confirmed HAPC diagnosis, being newly diagnosed with DVT, having DVT in the lower extremity for > 15 days and being unsuitable for catheter thrombolysis or surgical retrieval, receiving anticoagulant medication, and being available for a post-discharge follow-up. The exclusion criteria included the presence of a combination of other coagulation disorders, inadequate clinical information, and the absence of a follow-up.
Treatments for HAPC included intermittent oxygenation, volume expansion, and hemodilution. Anticoagulation therapy for lower-extremity DVT comprised the subcutaneous administration of low-molecular-weight heparin sodium (5000 IU) every 12 hours for seven days. Starting on the second day after admission, patients began receiving oral warfarin sodium tablets (National Medicine Permission Number H19993692; Henan Zhongjie Pharmaceutical Co., Ltd., Henan, China) once daily at an initial dose of 5.0 mg, followed by 2.5 mg for the next two days. Subsequently, the dosage was adjusted every 72 hours based on each patient’s international normalized ratio to maintain anticoagulation within the target range. Before discharge, patients were instructed to wear elastic stockings or bandages while ambulating, elevate the affected limb, and adhere to the scheduled oral anticoagulant therapy.
The patients underwent follow-up 6-12 months after discharge from their initial treatment. Recurrence was assessed using imaging examinations upon the emergence of symptoms and signs indicative of lower-extremity DVT during the follow-up period. The patients were classified into recurrence and non-recurrence groups according to thrombosis recurrence.
To collect and compare data, we assessed age, sex, education level, occupation, presence of malignancy, breaking history, medication compliance, compliance with wearing elastic stockings, and laboratory indices between the two groups. The laboratory indices included D-dimer (D-D) levels, red blood cell (RBC) count, white blood cell count, platelet count, activated partial thromboplastin time, fibrinogen level, prothrombin time, and thrombin time.
Statistical analyses were conducted using SPSS software (version 19.0; IBM Corp., Armonk, NY). Quantitative data were expressed as means ± SD, and comparisons between the two groups were performed using the independent samples t-test. Enumeration data were expressed as frequencies or composition ratios, and comparisons between groups were conducted using the χ2 test. Logistic regression model analysis was employed for multi-factor analysis, with variables showing significant results in single-factor analysis included in the multi-factor regression analysis. Independent risk factors were screened, a risk prediction model was established, and a nomogram was created using R software (R Software for Statistical Computing, Vienna, Austria). The accuracy of the model was evaluated by comparing the predicted and actual nomogram probabilities. Bootstrap re-sampling (1000 times) was used to draw the calibration curve, and the fitting degree of the model was evaluated using the Hosmer-Lemeshow (H-L) test. Receiver operating characteristic (ROC) curve analysis was performed to assess the predictive value of the model, with the area under the ROC curve used to evaluate predictive accuracy. Internal verification was conducted using the caret package and the bootstrap self-sampling method. The consistency index (C-index) was calculated using the RMS package. The level of significance was set at P < 0.05.
After seven days of treatment, all 123 patients with HAPC and lower-extremity DVT showed a reduction in the circumference of the affected knee, with the measurements of positions 10 cm above and 10 cm below the knee showing a difference of < 1 cm compared with those on the healthy side. The degree of swelling in the affected limbs significantly decreased, pain was alleviated, and the patients experienced no significant discomfort after activity. Interestingly, no serious complications, such as pulmonary embolism or bleeding occurred in any of the patients. All patients were discharged after completing the seven-day treatment period and were followed-up by telephone, supplemented with in-hospital follow-up information. The follow-up duration was 6-12 months after discharge, with an average follow-up time of 8.46 months ± 1.69 months. Of the 123 patients, 10 were lost to follow-up because of relocation or changes in contact information. However, the complete follow-up data were available for the remaining 113 patients, 33 of whom were diagnosed with recurrent DVT in the lower extremities. These patients exhibited clinical manifestations, such as swelling and distension of the lower limbs, increased skin temperature, and increased pain after activity. The diagnosis of DVT recurrence in a lower extremity was confirmed through non-invasive vascular color ultrasound, ultrasound B-mode imaging, and other imaging examinations, indicating the reappearance of a thrombus at the original thrombus site or adjacent sites.
Univariate analysis of the factors affecting thrombotic recurrence in patients with lower-extremity DVT complicated by HAPC is shown in Table 1. The results indicated that age, breaking history, medication compliance, compliance with wearing elastic stockings, peripheral blood D-D levels, and fibrin degradation product (FDP) levels were significantly associated with thrombotic recurrence in patients with HAPC and lower-extremity DVT (P < 0.05).
| Factor | Group | Recurrence group (n = 33) | Non-recurrence group (n = 80) | χ2/t | P value |
| Age (year) | 7.646 | 0.006 | |||
| < 60 | 13 | 54 | |||
| ≥ 60 | 20 | 26 | |||
| Sex | 0.039 | 0.843 | |||
| Male | 18 | 42 | |||
| Female | 15 | 38 | |||
| Smoking history | 0.184 | 0.668 | |||
| Yes | 7 | 20 | |||
| No | 26 | 60 | |||
| Alcohol consumption history | 0.136 | 0.713 | |||
| Yes | 6 | 17 | |||
| No | 27 | 63 | |||
| Education level | 1.221 | 0.543 | |||
| Primary or below | 7 | 13 | |||
| Middle school level | 20 | 45 | |||
| Junior college or above | 6 | 22 | |||
| Residence | 2.091 | 0.148 | |||
| Urban areas | 10 | 36 | |||
| Rural areas | 23 | 44 | |||
| Complicated with malignant tumor | 0.001 | 0.970 | |||
| Yes | 2 | 5 | |||
| No | 31 | 75 | |||
| Breaking history | 14.152 | < 0.001 | |||
| Yes | 7 | 1 | |||
| No | 26 | 79 | |||
| Medication compliance | 11.502 | < 0.001 | |||
| Good | 17 | 66 | |||
| Poor | 16 | 14 | |||
| Compliance with wearing elastic stockings | 9.184 | 0.002 | |||
| Good | 20 | 69 | |||
| Poor | 13 | 11 | |||
| D-D (mg/L) | 1.26 ± 0.31 | 0.41 ± 0.11 | 21.512 | < 0.001 | |
| RBC (× 1012/L) | 4.96 ± 0.85 | 4.99 ± 1.02 | 0.146 | 0.884 | |
| WBC (× 109/L) | 6.51 ± 0.79 | 6.49 ± 0.83 | 0.118 | 0.906 | |
| PLT (× 109/L) | 187.44 ± 33.57 | 189.37 ± 31.58 | 0.290 | 0.772 | |
| PT (second) | 13.77 ± 1.81 | 13.68 ± 1.75 | 0.243 | 0.808 | |
| ATPP (second) | 30.45 ± 2.89 | 31.07 ± 3.54 | 0.890 | 0.375 | |
| TT (second) | 18.44 ± 2.03 | 18.73 ± 2.17 | 0.658 | 0.512 | |
| FIB (g/L) | 2.83 ± 0.39 | 2.86 ± 0.43 | 0.346 | 0.730 | |
| FDP (mg/L) | 4.84 ± 1.07 | 4.17 ± 1.12 | 2.925 | 0.004 |
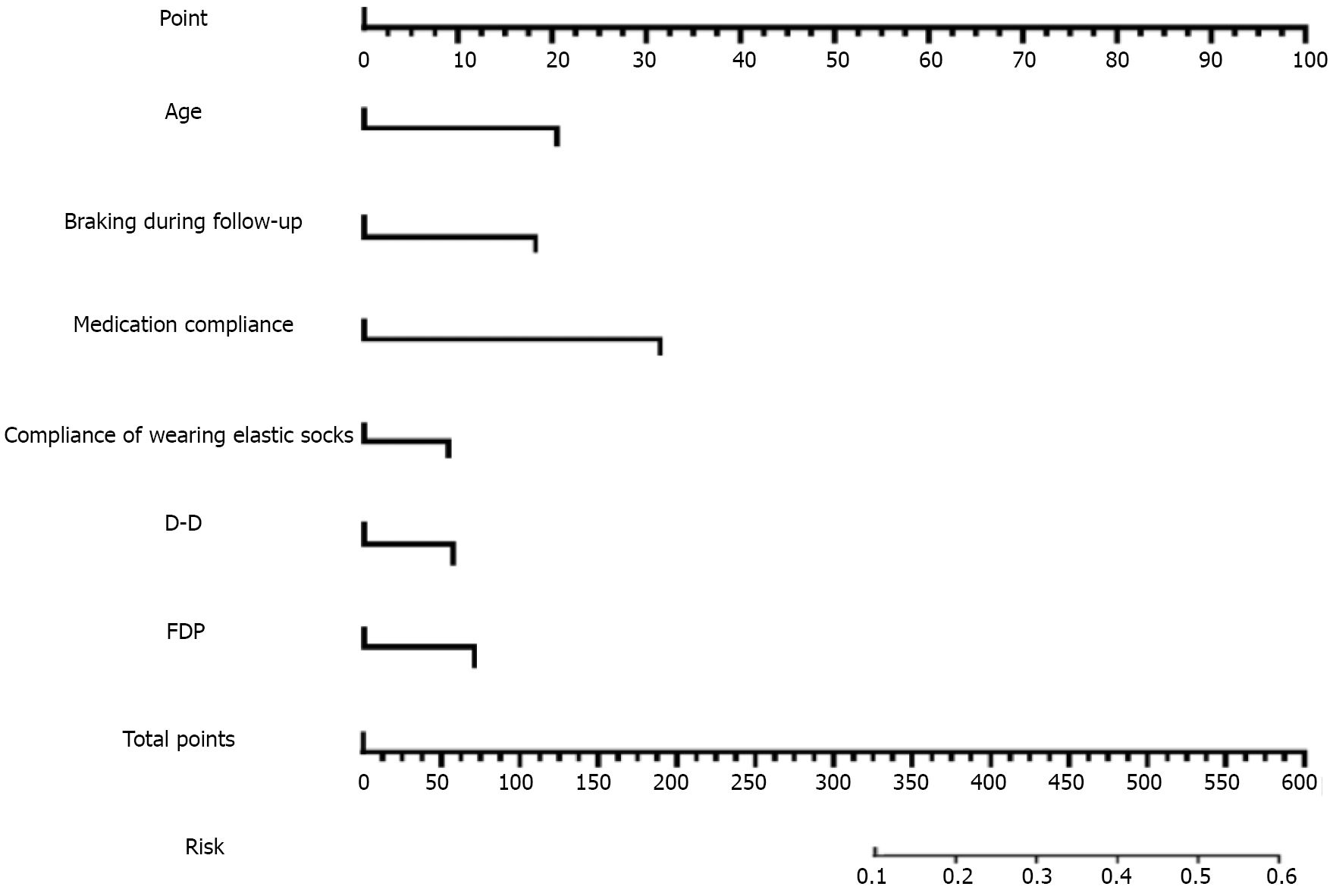
Assignment table are shown in Table 2. Multifactorial regression analysis of these factors are shown in Table 3. Meaningful indicators identified in the univariate analysis were incorporated into the multiple-factor regression analysis model. The dependent variable was thrombosis recurrence during the follow-up period, whereas factors such as age and occurrence of braking during follow-up were considered independent variables. Binary multivariate logistic regression analysis revealed that age, braking during follow-up, medication compliance, compliance with wearing elastic stockings, peripheral blood D-D levels, and FDP levels were independent risk factors for thrombosis recurrence in patients with lower-extremity DVT complicated by HAPC.
| Variable | Variable name | Assignments |
| Y | Thrombosis recurrence | No = 0, Yes = 1 |
| X1 | Age (year) | < 60 = 0, ≥ 60 = 1 |
| X2 | Braking during follow-up | No = 0, Yes = 1 |
| X3 | Medication compliance | Good = 0, Poor = 1 |
| X4 | Compliance with wearing elastic stockings | Good = 0, Poor = 1 |
| X5 | D-D (mg/L) | < 0.84 = 0, ≥ 0.84 = 1 |
| X6 | FDP (mg/L) | < 4.53 = 0, ≥ 4.53 = 1 |
| Factor | β | SE | Wald χ2 | OR | P value | 95%CI |
| Age | 0.783 | 0.245 | 10.214 | 2.188 | 0.001 | 1.354-3.537 |
| Braking during follow-up | 0.634 | 0.189 | 11.253 | 1.885 | < 0.001 | 1.302-2.730 |
| Medication compliance | 0.893 | 0.232 | 14.816 | 2.442 | < 0.001 | 1.550-3.849 |
| Compliance with wearing elastic stockings | 0.369 | 0.047 | 61.639 | 1.446 | < 0.001 | 1.319-1.586 |
| D-D | 0.455 | 0.119 | 14.619 | 1.576 | < 0.001 | 1.248-1.990 |
| FDP | 0.518 | 0.224 | 5.348 | 1.679 | 0.021 | 1.082-2.604 |
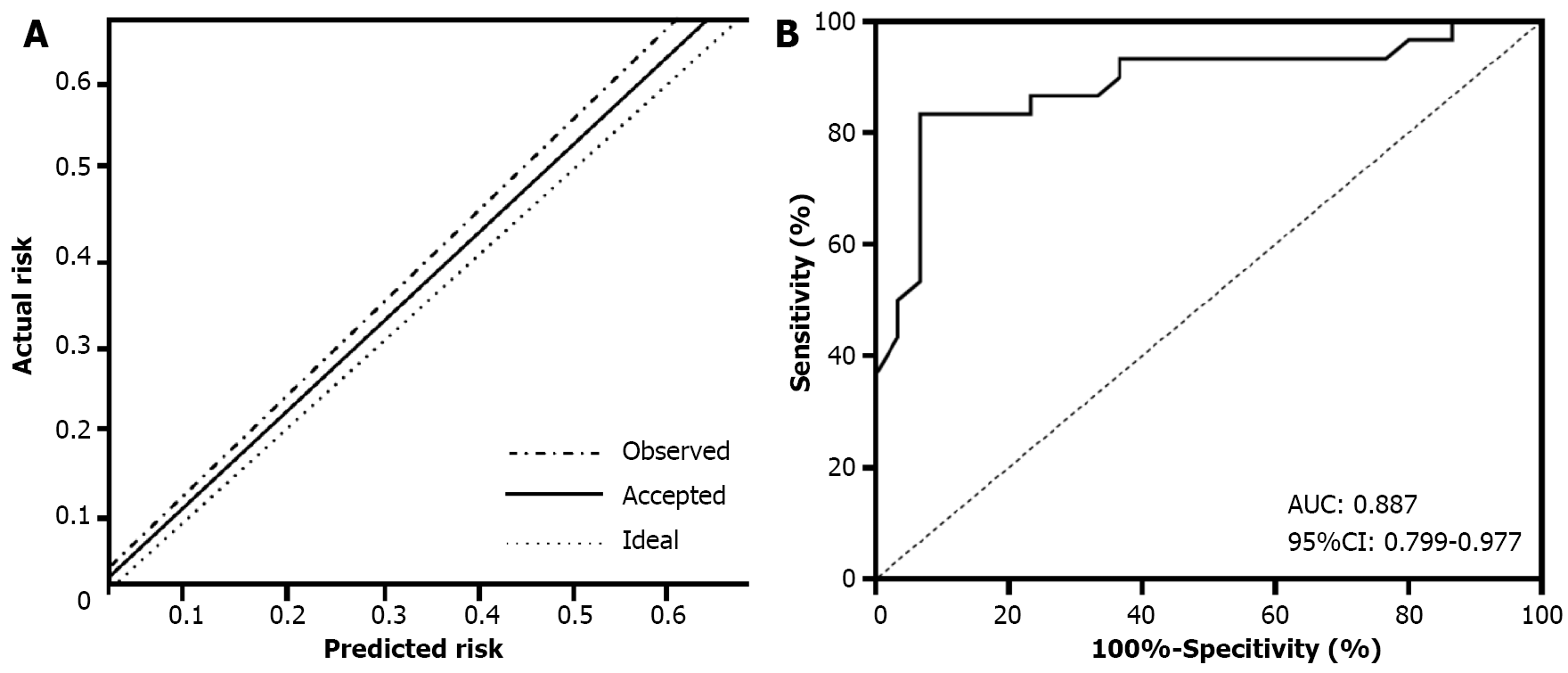
The establishment of the nomogram model is shown in Figures 1 and 2. Based on the results of the binary logistic multiple factor regression analysis, we used R software and the relevant packages to construct a prediction model for thrombosis recurrence in patients with lower-extremity DVT complicated by HAPC, which is presented in the form of a nomogram. Following 1000 iterations of re-sampling of the original data, the predictive accuracy and discriminative ability of the nomogram model was evaluated using the H-L deviation test and the area under the ROC curve, res
Various factors trigger DVT in the lower extremities, including long-term braking, lower-extremity phlebitis, and chronic pulmonary heart disease. Additionally, HAPC are common contributors to lower-extremity DVT in plateau areas[10,11]. When individuals from low-altitude areas enter high-altitude environments (3-7 days after arrival), their bodies undergo a compensatory stress response to maintain normal tissue oxygen levels, leading to an increased RBC count and hemoglobin (Hb) concentration[12].
HAPC affects 5%-18% of the population living in the Qinghai-Tibetan Plateau region[13]. It occurs as the body adapts to the low-pressure, oxygen-deprived environment at high altitudes, characterized by the excessive production of RBCs in the blood and the excessive production of Hg (Hb ≥ 190 g/L in women and Hb ≥ 210 g/L in men)[14]. When Hb levels reach 220-250 g/L, abnormal enhancement of RBC aggregation occurs, leading to plasma-RBC separation in blood vessels, intravascular blood cell stasis, and thrombus formation[15,16]. DVT is a common complication of HAPC.
In our hospital, it is common to encounter patients with lower-extremity DVT in plateau areas who also have HAPC. HAPC complicates the treatment of lower-limb DVT and may increase the risk of late thrombotic recurrence.
Clinical observations suggest that most patients with lower-extremity DVT and HAPC in plateau areas seek medical attention over 15 days after symptom onset, missing the optimal window for surgical and thrombolytic interventions. Anticoagulation therapy is the primary treatment option for patients under these conditions. Common anticoagulation regimens include low-molecular-weight heparin and sodium warfarin tablets. Following anticoagulation treatment in the hospital, the symptoms and signs associated with lower-limb DVT in the 123 included patients significantly improved without the occurrence of serious complications, such as pulmonary embolism and bleeding. All patients were safely discharged after a seven-day hospitalization period.
However, the risk of DVT recurrence peaks 6-12 months after the initial treatment. DVT recurrence increases treatment expenses and exposes patients to the risk of disability and mortality.
Predicting the risk of DVT recurrence after anticoagulant therapy in patients with lower-extremity DVT combined with HAPC in plateau areas is clinically important, as it allows for targeted interventions to improve patient outcomes. After excluding cases with missing follow-up data, we found 33 cases of DVT recurrence among 113 patients during the 6-12-month post-discharge period. The DVT recurrence rate was 29.20%. Binary multivariate logistic regression analysis identified age, braking during follow-up, medication compliance, compliance with wearing elastic stockings, and peri
Concerning age, our study revealed that patients aged ≥ 60 years had a 2.188-times higher risk of DVT recurrence than those aged < 60 years. With advancing age, blood viscosity increases and vascular elasticity declines, potentially contributing to lower-extremity DVT recurrence[17,18].
Regarding braking during follow-up visits, eight patients required bed rest for post-discharge surgical procedures, and seven experienced DVT recurrence. Meta-analysis data from 24181 patients with venous thrombosis showed that braking increased the risk of venous thrombosis by approximately two-fold[19]. A previous study found that a bed rest period of > 3 days was an important risk factor for developing DVT[20]. Prolonged bed rest has been found to elevate the intra-abdominal pressure, impede venous return, and promote blood stasis, thereby increasing DVT recurrence risk[21].
Moreover, the timely administration of anticoagulant drugs after discharge is crucial for preventing DVT recurrence. However, our study found poor medication compliance in 26.55% of patients, with 48.48% experiencing DVT recurrence. To address this, enhanced patient education regarding the importance of medication adherence after discharge is recommended.
Regarding compliance with wearing elastic stockings, medical elastic stockings are designed to reduce pressure gradients in deep veins, aiding in DVT prevention. However, evidence from large placebo-controlled trials over a two-year period has shown no significant benefit of wearing elastic stockings[22]. Consequently, current clinical practice guidelines do not recommend their routine use for the prevention of DVT.
Peripheral blood D-D levels, which indicate fibrin degradation, increase during acute thrombosis and may also increase with age, infection, or other inflammatory conditions[23]. These levels have some certain value in predicting DVT. In fact, Jiang et al[5] found that plasma D-D levels in 100 healthy participants were significantly higher in the high-altitude group than in the low-altitude group (103 participants). Patients with peripheral blood D-D levels ≥ 0.84 mg/L at discharge had an increased risk of late DVT recurrence.
Finally, abnormal FDP elevation often indicates the active degradation of fibrin in the body. Our study found that peripheral blood FDP levels ≥ 4.53 mg/L were predictive of later DVT recurrence. Therefore, clinicians should closely monitor patients with high peripheral blood D-D and FDP levels at discharge, and provide comprehensive disease education.
These results suggest that the nomogram model intuitively indicates the contribution of each variable to the final outcome, offering insights into the risk of thrombotic recurrence in patients with lower-extremity DVT complicated by HAPC in highland areas. The H-L deviation test of the model resulted in a Chi-squared value of 0.873 and P > 0.05, suggesting satisfactory goodness-of-fit. The C-index was 0.887 (95%CI: 0.799-0.997), indicating high accuracy and differentiation capabilities of the nomogram model.
In conclusion, our study identified age, braking during follow-up, medication compliance, compliance with wearing elastic stockings, and peripheral blood D-D and FDP levels as independent risk factors for thrombotic recurrence in patients with lower-extremity DVT complicated by HAPC. Moreover, the nomogram model constructed based on these findings demonstrated efficacy in predicting thrombosis recurrence in patients after discharge. However, the patients in the current study were all from the same hospital, and the number of samples was limited. In the future, larger samples and multicenter studies are expected to further improve the accuracy of the nomogram model to help predict the recurrence of thrombosis in patients with lower-extremity DVT combined with HAPC.
| 1. | Parati G, Agostoni P, Basnyat B, Bilo G, Brugger H, Coca A, Festi L, Giardini G, Lironcurti A, Luks AM, Maggiorini M, Modesti PA, Swenson ER, Williams B, Bärtsch P, Torlasco C. Clinical recommendations for high altitude exposure of individuals with pre-existing cardiovascular conditions: A joint statement by the European Society of Cardiology, the Council on Hypertension of the European Society of Cardiology, the European Society of Hypertension, the International Society of Mountain Medicine, the Italian Society of Hypertension and the Italian Society of Mountain Medicine. Eur Heart J. 2018;39:1546-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Anand AC, Jha SK, Saha A, Sharma V, Adya CM. Thrombosis as a complication of extended stay at high altitude. Natl Med J India. 2001;14:197-201. [PubMed] |
| 3. | Ma YH, Li YL. A case of misdiagnosis of thromboembolic pulmonary hypertension in the highlands. Zhonghua Quanke Yixue. 2020;18:1063-1065. [DOI] [Full Text] |
| 4. | Jiang YR, Niu LL, Feng N, Fan HL, Jin QQ, Du QX, Cao J, Wang YY, Sun JH. Correlation between the Polymorphism of Coagulation-Related Genes and Lower Extremity Deep Venous Thrombosis. Fa Yi Xue Za Zhi. 2021;37:145-150. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Jiang P, Wang Z, Yu X, Qin Y, Shen Y, Yang C, Liu F, Ye S, Du X, Ma L, Cao H, Sun P, Su N, Lin F, Zhang R, Li C. Effects of long-term high-altitude exposure on fibrinolytic system. Hematology. 2021;26:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Zhang SX, Lu XW, De Y, Yang L, DuoJi ZM, Song JY, Liang Z, Basang ZM. Molecular mechanism of high altitude polycythemia:a Meta analysis. Chongqing Yixue. 2020;49:1149-1154, 1158. [DOI] [Full Text] |
| 7. | Yuang L, Zheng X, Liu DS, Wang Y. Coagulation characteristics of patients with high altitude polycythemia. Shaanxi Yixue Zazhi. 2020;49:623-625. [DOI] [Full Text] |
| 8. | Wei S, Xiao PC. Research on the effects of complicated polycythemia on the risk of cerebral hemorrhage in patients with high altitude hypertension. Haijun Yixue Zazhi. 2020;41:681-684. [DOI] [Full Text] |
| 9. | Liu Y. Chronic plateau disease Qinghai diagnostic criteria. Qinghai Yixueyuan Xuebao. 2005;1:3-5. |
| 10. | Gao WX, Chen YW, Cai GJ, Hu LJ, Qian MP, Yang JF, Zhang J, Hou LC. Risk Management Effect of Deep Vein Thrombosis (DVT) Among High-Risk Hospitalized Patients. Jiefangjun Yiyuan Guanli Zazhi. 2021;28:1035-1037. [DOI] [Full Text] |
| 11. | Dai QL, Fu CM, Zhu MQ, Zhu DH, Fan L. Construction and validation of risk factors and risk prediction model for lower extremity deep venous thrombosis in orthopedic inpatients. Quanke Huli. 2023;21:1400-1403. [DOI] [Full Text] |
| 12. | Li C, Li X, Liu J, Fan X, You G, Zhao L, Zhou H, Li J, Lei H. Investigation of the differences between the Tibetan and Han populations in the hemoglobin-oxygen affinity of red blood cells and in the adaptation to high-altitude environments. Hematology. 2018;23:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Yi H, Yu Q, Zeng D, Shen Z, Li J, Zhu L, Zhang X, Xu Q, Song H, Kong P. Serum Inflammatory Factor Profiles in the Pathogenesis of High-Altitude Polycythemia and Mechanisms of Acclimation to High Altitudes. Mediators Inflamm. 2021;2021:8844438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Wang Z, Liu F, Ye S, Jiang P, Yu X, Xu J, Du X, Ma L, Cao H, Yuan C, Shen Y, Lin F, Zhang R, Li C. Plasma proteome profiling of high-altitude polycythemia using TMT-based quantitative proteomics approach. J Proteomics. 2019;194:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera: historical oversights, diagnostic details, and therapeutic views. Leukemia. 2021;35:3339-3351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 16. | Fox S, Griffin L, Robinson Harris D. Polycythemia Vera: Rapid Evidence Review. Am Fam Physician. 2021;103:680-687. [PubMed] |
| 17. | Han LZ, Yin QN, Bian Y, Huang XF, Lei Y, Tong RS. Management and treatment of deep vein thrombosis and pulmonaryembolism: interpretation of 2020 ASH guidelines for management of venous thromboembolism. Zhongguo Xinyaoyulinchuang Zazhi. 2021;40:784-788. [DOI] [Full Text] |
| 18. | Huang LW, Lu Z, Tang JL, Li G, A Z, Na J, Yang ZL, Huang GY. Analysis of factors associated with the occurrence of DVT in patients with fractures in highland areas. Zhongguo Guyuguanjie Sunshang Zazhi. 2018;33:890-891. [DOI] [Full Text] |
| 19. | Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 372] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 20. | Duffett L. Deep Venous Thrombosis. Ann Intern Med. 2022;175:ITC129-ITC144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 21. | Lin P, Tian LY, He AL, Gao HM, Su YN, Yang QH, Li W, Tiang HY, Li YL. Risk assessment tools for venous thromboembolism recurrence: current status and research progress. Zhongguo Putong Waike Zazhi. 2020;29:1391-1398. [DOI] [Full Text] |
| 22. | Subbiah R, Aggarwal V, Zhao H, Kolluri R, Chatterjee S, Bashir R. Effect of compression stockings on post thrombotic syndrome in patients with deep vein thrombosis: a meta-analysis of randomised controlled trials. Lancet Haematol. 2016;3:e293-e300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. 2021;398:64-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 380] [Article Influence: 95.0] [Reference Citation Analysis (0)] |