Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4813
Revised: May 22, 2024
Accepted: June 13, 2024
Published online: July 26, 2024
Processing time: 79 Days and 0.3 Hours
Hepatoid adenocarcinoma of the lung (HAL) is a rare type of non-small cell lung cancer (NSCLC), histologically similar to hepatocellular carcinoma. HAL has high malignancy and poor prognosis, and a better treatment plan needs further study.
In order to deeply understand the occurrence and development of HAL, here we report a case of HAL with extensive metastasis of alpha fetoprotein negative KRAS A146T mutation. The patient refused chemotherapy and received one course of treatment (immune checkpoint inhibitors), and died three months later due to progressive disease.
HAL is a special type of NSCLC. The surgical treatment of HAL in the limited stage can achieve long-term survival, but most of them were in the advanced stage when they were found, and the prognosis was poor, which requires multi
Core Tip: Hepatoid adenocarcinoma of the lung is a special type of non-small cell lung cancer, which is mainly seen in men who smoke heavily. It usually occurs in the upper lobes of both lungs, showing huge masses.
- Citation: Mo YJ, Lin LN, Tao JL, Zhang T, Zhang JH. Hepatoid adenocarcinoma of the lung: A case report. World J Clin Cases 2024; 12(21): 4813-4819
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4813.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4813
Hepatoid adenocarcinoma (HAC) refers to adenocarcinoma that occurs in organs or tissues outside the liver with hepatocyte like differentiation and similar morphological characteristics to hepatocellular carcinoma, most of which occur in rectum, pancreas, gallbladder, kidney, lung and other organs[1]. Hepatoid adenocarcinoma of the lung (HAL) is a HAC subtype with a short survival period, which is rare clinically, and the expression of alpha fetoprotein (AFP) is uncertain[2]. Although computed tomography (CT) images can diagnose HAL to a certain extent, morphological and immunohistochemical evidence is still needed to finally diagnose HAL[3]. As this kind of disease is rare, most patients are diagnosed in late stage, and there is no standard treatment plan at present[4]. Some scholars also believe that the treatment of HAL adopts the commonly used treatment strategies of non-small cell lung cancer (NSCLC), including chemotherapy, immunotherapy, etc., but the overall prognosis of HAL is poor[5].
He was admitted to the hospital for 5 days because of right chest pain, and the weight had dropped by 5 kg in the last month.
Right chest pain, and the weight had dropped by 5 kg in the last month.
The patient was 76 years old, male, and had been smoking heavily for 50 years (360 pack years).
No specific personal and family history.
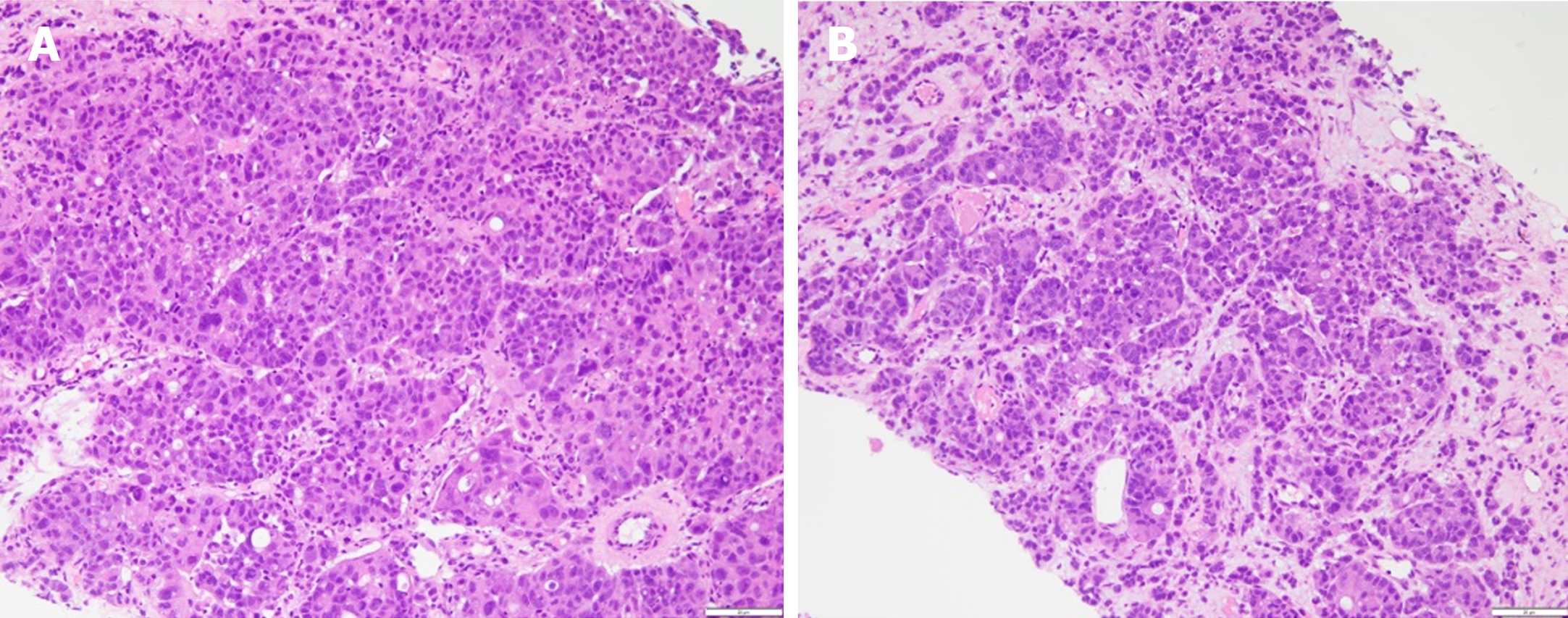
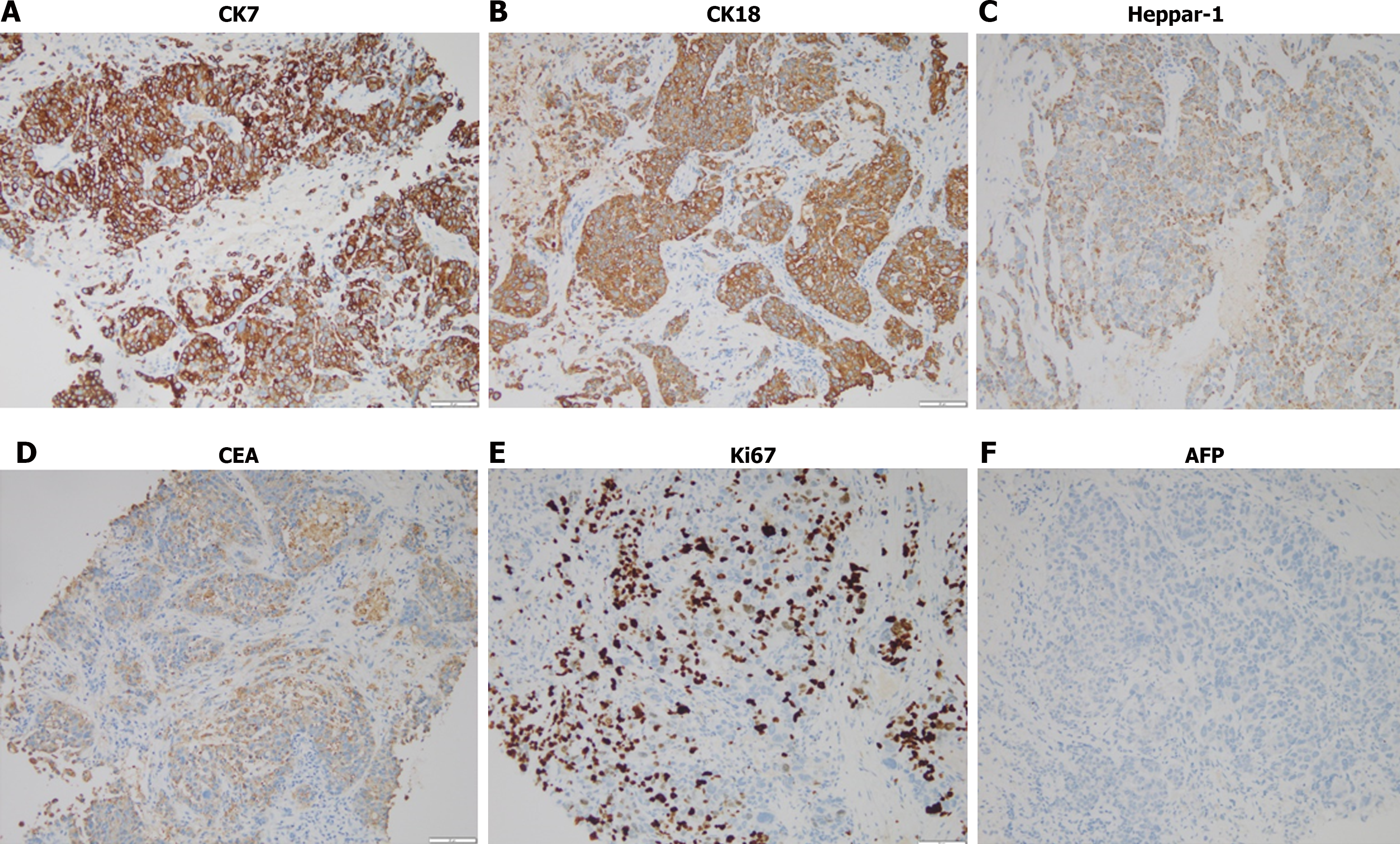
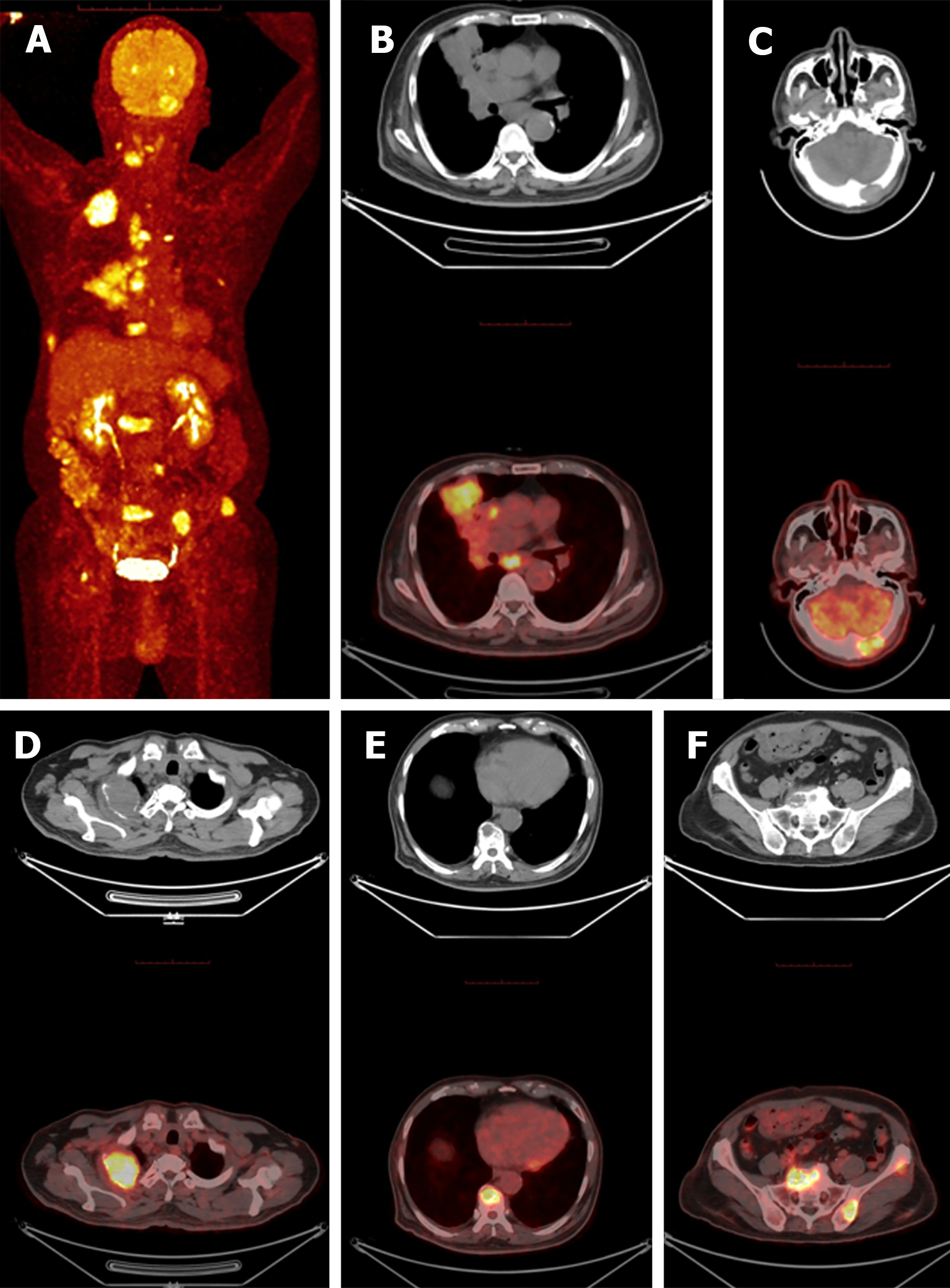
Percutaneous lung biopsy showed adenocarcinoma, the immunohistochemical results were shown in Table 1. Combined with positron emission tomography (PET)/CT imaging examination, hematoxylin-eosin staining morphology and immunohistochemical examination of tumor cells (Figures 1 and 2), the patient was diagnosed as primary HAL. According to the eighth edition of The American Joint Committee on Cancer cancer staging manual, the patient was staged as cT4N2M1c IV B.
| Immunohistochemical stain | Positive | Negative |
| CK | + | |
| CK7 | + | |
| CK18 | + | |
| CK19 | + | |
| Heppar-1 | + | |
| CDX2 | + | |
| Ki67 | 60%, + | |
| MLH1 | + | |
| MSH2 | + | |
| MSH6 | + | |
| PMS2 | + | |
| CEA | + | |
| Napsin | - | |
| P63 | - | |
| TTF1 | - | |
| P40 | - | |
| AFP | - | |
| CD34 | - | |
| Glypican-3 | - | |
| Villin | - | |
| CK20 | - | |
| Muc2 | - |
In order to formulate a standardized treatment plan, we used Next Generation Sequencing (NGS) to detect 8 genes (EGFR, KRAS, ALK, ROS1, BRAF, NTRK1/2/3, MET, RET, ERBB2) recommended by Non-Small Cell Lung Cancer, Version 4.2022, National Comprehensive Cancer Network (NCCN) guidelines in tissues, and found KRAS A146T mutation, TPS = 2%, CPS = 2, and MSI is MSS type. Because there was no effective drug for KRAS A146T mutation, we suggested that patients receive chemotherapy combined with immune checkpoint inhibitors (ICIs). The patient refused chemotherapy and received one course of ICIs treatment, and the patient died 3 months later.
PET/CT examination confirmed that: Right central lung tumor (51 mm × 36.7 mm), middle lobe tumor of right lung (71 mm × 47 mm) with mediastinal lymph node metastasis and occipital bone metastasis (31 mm × 20 mm). Osteolytic bone destruction area can be seen locally in C3 spinous process, right attachment of C4 vertebral body, thoracic 1, 3, 9 vertebral body, lumbar 2, 4 vertebral body, sacral 1 vertebral body, left iliac bone, right ribs 2, 8, left ribs 4, 6 and right femoral head, and obvious soft tissue mass formation can be seen in some lesions. The size of metastatic tumor in the right second rib was 56.0 mm × 46.0 mm. The left adrenal gland metastasized, and no malignant tumor was found in the liver (Figure 3).
The patient was diagnosed as primary HAL.
Because there was no effective drug for KRAS A146T mutation, we suggested that patients receive chemotherapy combin
The patient refused chemotherapy and received one course of ICIs treatment, and the patient died 3 months later.
Hepatoid adenocarcinoma is a rare extrahepatic tumor. Its morphological characteristics are similar to those of hepatocellular carcinoma[6]. About 5% of the primary sites are in the lungs[7]. According to the literature, 63% of hepatoid adenocarcinoma originated from stomach, and other sources include ovary (10%), lung (5%), gallbladder (4%), pancreas (4%) and uterus (4%)[8]. Lung is one of the least common sites of origin for hepatoid adenocarcinoma. Grossman's research HAL morphologically mimics Hepatocellular Carcinoma, Immunohistochemical stains-CK7, CK19, HEA 125, MOC31, EpCAM positive[9]. Zhuansun et al[10] analyzed 42 cases of HAL, the median age was 58.5 years. 85.7% patients were male 61.9%patients had a history of smoking, the median amount of smoking was 40 pack years. The most common site of the primary tumor was the right upper lobe (52.3%) and the left upper lobe (23.8%). Fifty percent patients had pre-treatment serum AFP increased, 76.2% (III, IV) in the progressive stage, medium overall survival of 14 months. Many characteristics are similar to those of Grossman[9].
Ishikura et al[2] first proposed the concept of hepatoid adenocarcinoma of lung. They studied five cases of primary purple adenocarcinoma with AFP expression. They adopted two criteria for diagnosis: Typical acinar or papillary adenocarcinoma and a component of carcinoma that resembles hepatocellular carcinoma and produces AFP. Kishimoto et al[11] reports the microscopic analysis of the surgical specimen revealed a large cell neuroendocrine carcinoma with occasional hepatoid foci. Haninger et al's research found that an immunohistochemical panel that includes a variety of cyclokeratins, monoclonal CEA and EpCAM markers (HEA125 and MOC31) facilitates distinction of hepatoid adenocarcinoma of lung from hepatocellular carcinoma metastatic to lung, especially when correlated with clinical and radiologic findings[12]. Haninger et al[12] propose modification of Ishikura’s diagnostic criteria1 for hepatoid adenocarcinoma of lung: (1) The tumor can be pure hepatoid adenocarcinoma or have components of typical acinar or papillary adenocarcinoma, signet-ring cells or neuroendocrine carcinoma; and (2) AFP expression is not mandatory for diagnosis as long as other markers of hepatic differentiation are expressed.
Radical resection is the preferred treatment for early NSCLC, as is HAL. When HAL presents as localized disease, resection for long-term cure is possible[13]. HAL, like other types of lung cancer, requires early detection in order to achieve better results through surgery. Chen et al[14] found that males might exhibit an increased risk of developing HAL and poorer prognosis than females, and surgical resection combined with chemotherapy might prolong the survival of patients with HAL. As summary of the research finding, the survival benefits for patients receiving chemoradiotherapy or chemotherapy alone appeared to be limited, while radical surgery could significantly improve patient prognosis[15]. Targeted therapy is an important method to treat advanced NSCLC, about 50% of cases of conventional adenocarcinoma of lung harbor somatic mutations in genes that encode proteins in the EGFR signaling pathway: KRAS proto-oncogene, GTPase, EGFR, erb-b2 receptor tyrosine kinase 2, erb-b2 receptor tyrosine kinase 4, BRAF and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha[16].
Of the 42 cases of HAL, only one case exhibited anaplastic lymphoma kinase (ALK) rearrangement[17]. No article has reported patients had EGFR 19 DEL and 21 L858R mutations. We speculated that the reason was that HAL mostly occurred in heavy smoking men, while EGFR mutations mostly occur in non-smoking women. In addition, the incidence rate of HAL was low, and there were fewer cases for gene testing. This problem needs more test cases to confirm. We used NGS to detect 8 genes (ALK, BRAF, EGFR, ERBB2, KRAS, MET, RET, ROS1) recommended by NCCN guidelines in tissues. The patient was a heavy smoking male, and only KRAS A146T mutation was detected. KRAS mutation is a comm
There have been a few reports on immunotherapy for HAL, and more evidence is needed to support whether there is a definite effect[20]. For NSCLC patients with EGFR/ALK/ROS1/BRAF/NTRK/ METex14/RET negative, the 2022 NCCN guidelines recommend Pembrolizumab, Nivolumab, Atezolizumab and other single drugs to be used for first-line treat
There are still shortcomings in this study. Only one HAL patient was included in this study and died after 3 months of treatment. Not enough information was collected. We will collect more patients and conduct in-depth analysis in future studies.
To sum up, HAL is a special type of NSCLC, which is mainly seen in men who smoke heavily. It usually occurs in the upper lobes of both lungs, showing huge masses. The surgical treatment of HAL in the limited period can achieve long-term survival, but most of them are in the advanced stage when they are found, and the prognosis is poor, requiring multidisciplinary comprehensive treatment.
| 1. | Chen L, Han X, Gao Y, Zhao Q, Wang Y, Jiang Y, Liu S, Wu X, Miao L. Anti-PD-1 Therapy Achieved Disease Control After Multiline Chemotherapy in Unresectable KRAS-Positive Hepatoid Lung Adenocarcinoma: A Case Report and Literature Review. Onco Targets Ther. 2020;13:4359-4364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Ishikura H, Kanda M, Ito M, Nosaka K, Mizuno K. Hepatoid adenocarcinoma: a distinctive histological subtype of alpha-fetoprotein-producing lung carcinoma. Virchows Arch A Pathol Anat Histopathol. 1990;417:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Terracciano LM, Glatz K, Mhawech P, Vasei M, Lehmann FS, Vecchione R, Tornillo L. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2003;27:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Bonis A, Dell'Amore A, Verzeletti V, Melan L, Zambello G, Nardocci C, Comacchio GM, Pezzuto F, Calabrese F, Rea F. Hepatoid Adenocarcinoma of the Lung: A Review of the Most Updated Literature and a Presentation of Three Cases. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Liu M, Luo C, Xie ZZ, Li X. Treatment of gastric hepatoid adenocarcinoma with pembrolizumab and bevacizumab combination chemotherapy: A case report. World J Clin Cases. 2022;10:5420-5427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
| 6. | Miyama Y, Fujii T, Murase K, Takaya H, Kondo F. Hepatoid adenocarcinoma of the lung mimicking metastatic hepatocellular carcinoma. Autops Case Rep. 2020;10:e2020162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie. 2010;33:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Mao JX, Liu C, Zhao YY, Ding GS, Ma JQ, Teng F, Guo WY. Merged hepatopulmonary features in hepatoid adenocarcinoma of the lung: a systematic review. Am J Transl Res. 2021;13:898-922. [PubMed] |
| 9. | Grossman K, Beasley MB, Braman SS. Hepatoid adenocarcinoma of the lung: Review of a rare form of lung cancer. Respir Med. 2016;119:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Zhuansun Y, Bian L, Zhao Z, Du Y, Chen R, Lin L, Li J. Clinical characteristics of Hepatoid adenocarcinoma of the lung: Four case reports and literature review. Cancer Treat Res Commun. 2021;29:100474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Kishimoto T, Yano T, Hiroshima K, Inayama Y, Kawachi K, Nakatani Y. A case of *-fetoprotein-producing pulmonary carcinoma with restricted expression of hepatocyte nuclear factor-4* in hepatoid foci: a case report with studies of previous cases. Hum Pathol. 2008;39:1115-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Haninger DM, Kloecker GH, Bousamra Ii M, Nowacki MR, Slone SP. Hepatoid adenocarcinoma of the lung: report of five cases and review of the literature. Mod Pathol. 2014;27:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Hayashi Y, Takanashi Y, Ohsawa H, Ishii H, Nakatani Y. Hepatoid adenocarcinoma in the lung. Lung Cancer. 2002;38:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Chen Z, Ding C, Zhang T, He Y, Jiang G. Primary Hepatoid Adenocarcinoma of the Lung: A Systematic Literature Review. Onco Targets Ther. 2022;15:609-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Hou Z, Xie J, Zhang L, Dai G, Chen Y, He L. Hepatoid Adenocarcinoma of the Lung: A Systematic Review of the Literature From 1981 to 2020. Front Oncol. 2021;11:702216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Gu K, Shah V, Ma C, Zhang L, Yang M. Cytoplasmic immunoreactivity of thyroid transcription factor-1 (clone 8G7G3/1) in hepatocytes: true positivity or cross-reaction? Am J Clin Pathol. 2007;128:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Khozin S, Roth MJ, Rajan A, Smith K, Thomas A, Berman A, Giaccone G. Hepatoid carcinoma of the lung with anaplastic lymphoma kinase gene rearrangement. J Thorac Oncol. 2012;7:e29-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Schabath MB, Welsh EA, Fulp WJ, Chen L, Teer JK, Thompson ZJ, Engel BE, Xie M, Berglund AE, Creelan BC, Antonia SJ, Gray JE, Eschrich SA, Chen DT, Cress WD, Haura EB, Beg AA. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene. 2016;35:3209-3216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 19. | Jänne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C, Franke FA, Grinsted L, Zazulina V, Smith P, Smith I, Crinò L. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 20. | Basse V, Schick U, Guéguen P, Le Maréchal C, Quintin-Roué I, Descourt R, Simon H, Uguen A, Quéré G. A Mismatch Repair-Deficient Hepatoid Adenocarcinoma of the Lung Responding to Anti-PD-L1 Durvalumab Therapy Despite no PD-L1 Expression. J Thorac Oncol. 2018;13:e120-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |