Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4783
Revised: May 15, 2024
Accepted: June 4, 2024
Published online: July 26, 2024
Processing time: 90 Days and 20.2 Hours
Large cell neuroendocrine carcinoma (LCNEC) of the bladder is a rare non-urothelial tumor of the bladder. The treatment of LCNEC of the bladder is dif
We report a 72-year-old female patient who presented with gross hematuria for 3 mo. A solitary tumor located in the anterior wall of the bladder was found by cystoscopy. Pathological examination after biopsy suggested UC of the bladder in the absence of immunohistochemical assessment. The patient underwent partial cystectomy and was finally diagnosed with LCNEC (pT2bN0M0) based on the results of postoperative immunohistochemical examination. During the 10-mo follow-up, no signs of tumor recurrence or metastasis were found.
Immunohistochemical examination is essential for diagnosing LCNEC of the bladder. Accurate diagnosis and multidisciplinary treatment in the early stage of the disease are crucial for improving the prognosis.
Core Tip: Large cell neuroendocrine carcinoma (LCNEC) of the bladder is a rare non-urothelial tumor of the bladder. As LCNEC is a rare disease and its clinical symptoms and radiographic features mimic those of urothelial tumors, it may be misdiagnosed as urothelial carcinoma. In this article, we report a patient with LCNEC of the bladder, who was not accurately diagnosed due to the absence of immunohistochemical examination before surgery. We summarize the methods for diagnosing and treating LCNEC of the bladder and highlight the importance of early diagnosis and multimodal treatment.
- Citation: Bai LL, Guo YX, Song SY, Li R, Jiang YQ. Primary large cell neuroendocrine carcinoma of the bladder: A case report. World J Clin Cases 2024; 12(21): 4783-4788
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4783.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4783
Neuroendocrine carcinoma (NEC) is commonly found in the lung and gastrointestinal tract but rarely found in the urinary system[1]. NEC of the bladder accounts for less than 1% of bladder tumors[2], and large cell NEC (LCNEC) is the rarest subtype[3,4]. Few cases of LCNEC of the bladder have been reported previously, and studies on its biological and clinicopathological features are lacking.
LCNEC of the bladder has a poor prognosis, with the majority of patients being diagnosed at an advanced stage[5,6]. However, due to the rarity of the disease, there is currently no standard treatment. The treatment for urothelial carcinoma (UC) of the bladder is not applicable to this disease. In the existing studies, the most commonly used treatment was comprehensive treatment including surgery[2,5]. The therapeutic effect of new antineoplastic drugs, such as targeted drugs and immune checkpoint inhibitors, on this disease is still uncertain[5,7,8]. More data are needed to develop guidelines for the diagnosis and treatment of this disease.
As the clinical symptoms and radiographic features of LCNEC of the bladder are similar to those of urothelial tumors, it may be misdiagnosed as UC; thus, clinicians cannot choose the appropriate treatment in a timely manner. The diagnosis of this disease is challenging for clinicians. In this article, we report a patient with LCNEC of the bladder, who was not accurately diagnosed due to the absence of immunohistochemical examination before surgery. The treatment process and clinical data can improve clinicians' awareness of LCNEC of the bladder and facilitate its accurate diagnosis and treatment. We also summarize the diagnostic and treatment approaches for LCNEC of the bladder. We suggest that attention should be paid to the identification of rare pathological types of bladder tumors before surgery to adopt timely and appropriate treatment.
A 72-year-old female patient who had experienced intermittent gross hematuria in the last 3 mo came to our hospital.
The patient experienced gross hematuria 3 mo before her visit, with obvious terminal hematuria accompanied by a burning sensation in the lower abdomen, without urinary frequency or urgency symptoms. After treatment with oral cefixime for 1 wk, there was no change in hematuria; therefore, she came to the Department of Urology of the Hebei Medical University Third Hospital for treatment. Ultrasonography revealed a hypoechoic mass in the anterior wall of the bladder.
The patient had no history of chronic diseases or other diseases.
The patient was exposed to leather tanning reagents for more than 10 years. The patient did not mention any family history.
Physical examination showed no obvious positive signs.
Urine analysis showed that red blood cell count was 418.40/μL and white blood cell count was 46.50/μL.
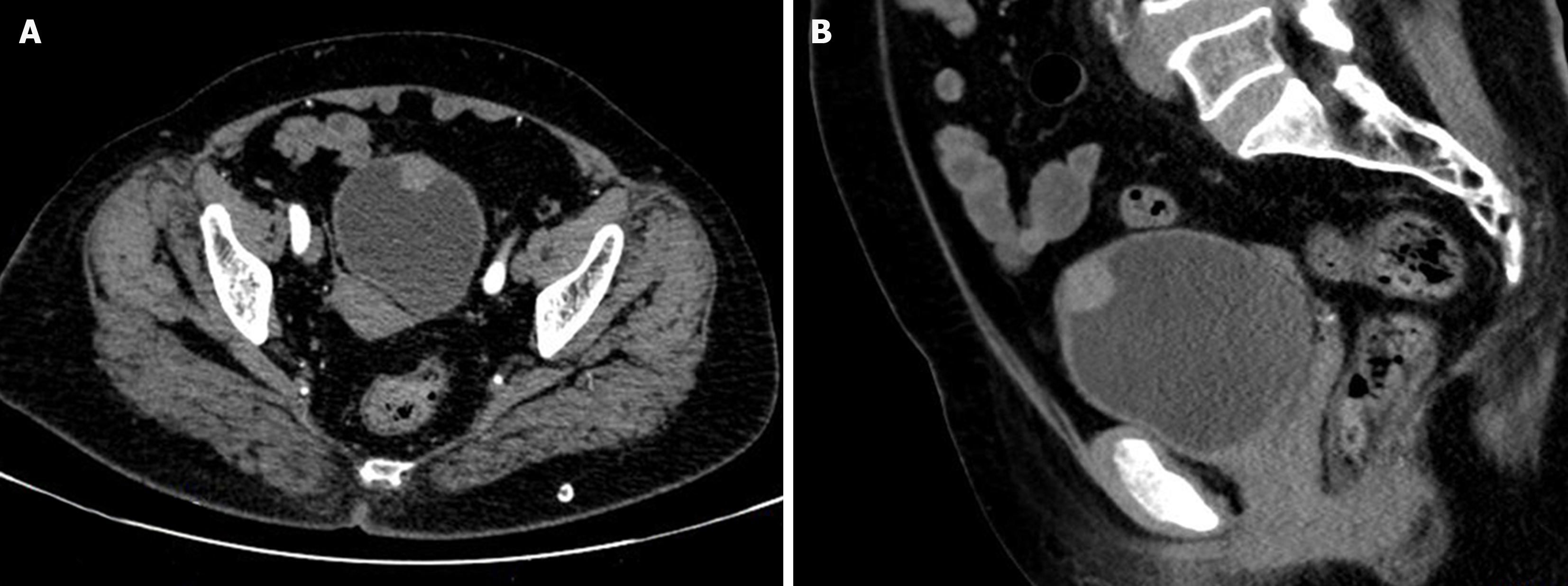
Contrast-enhanced computed tomography (CT) revealed that the tumor involved almost the whole layer of the bladder wall and exhibited mild enhancement, similar to what is observed in common infiltrative UC of the bladder (Figure 1).
LCNEC of the bladder (pT2bN0M0).
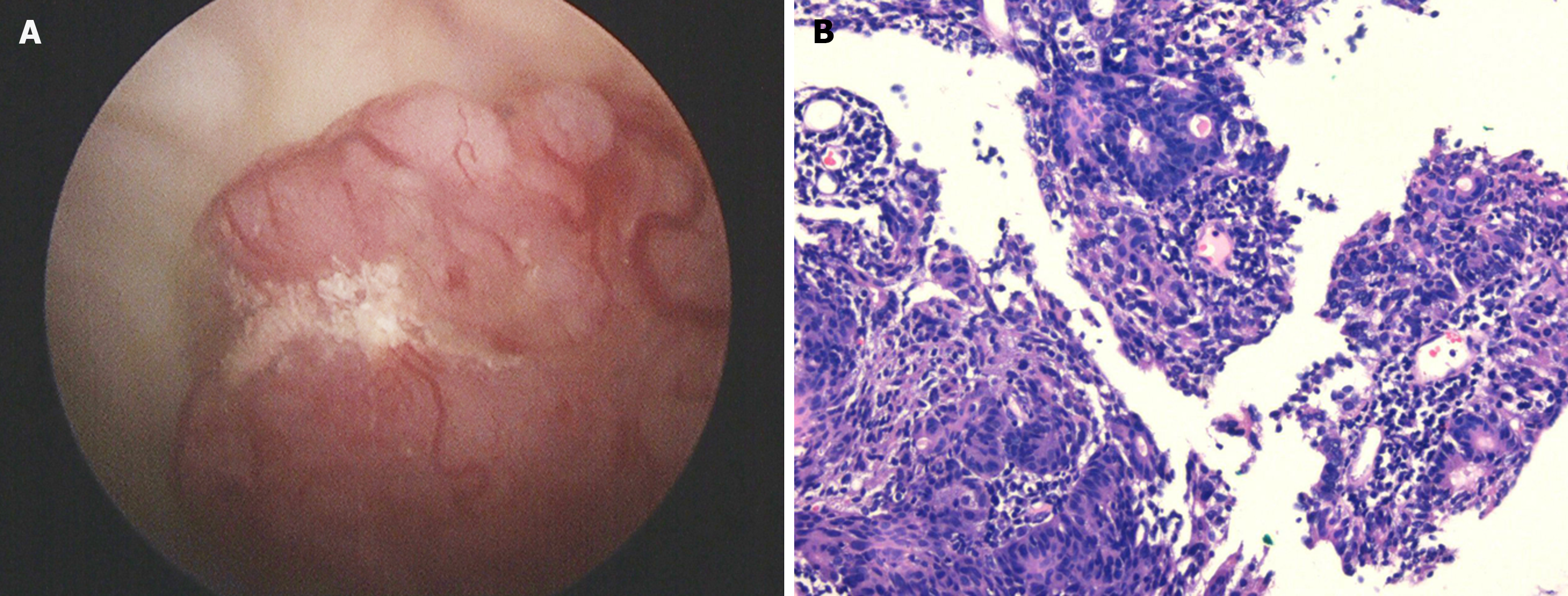
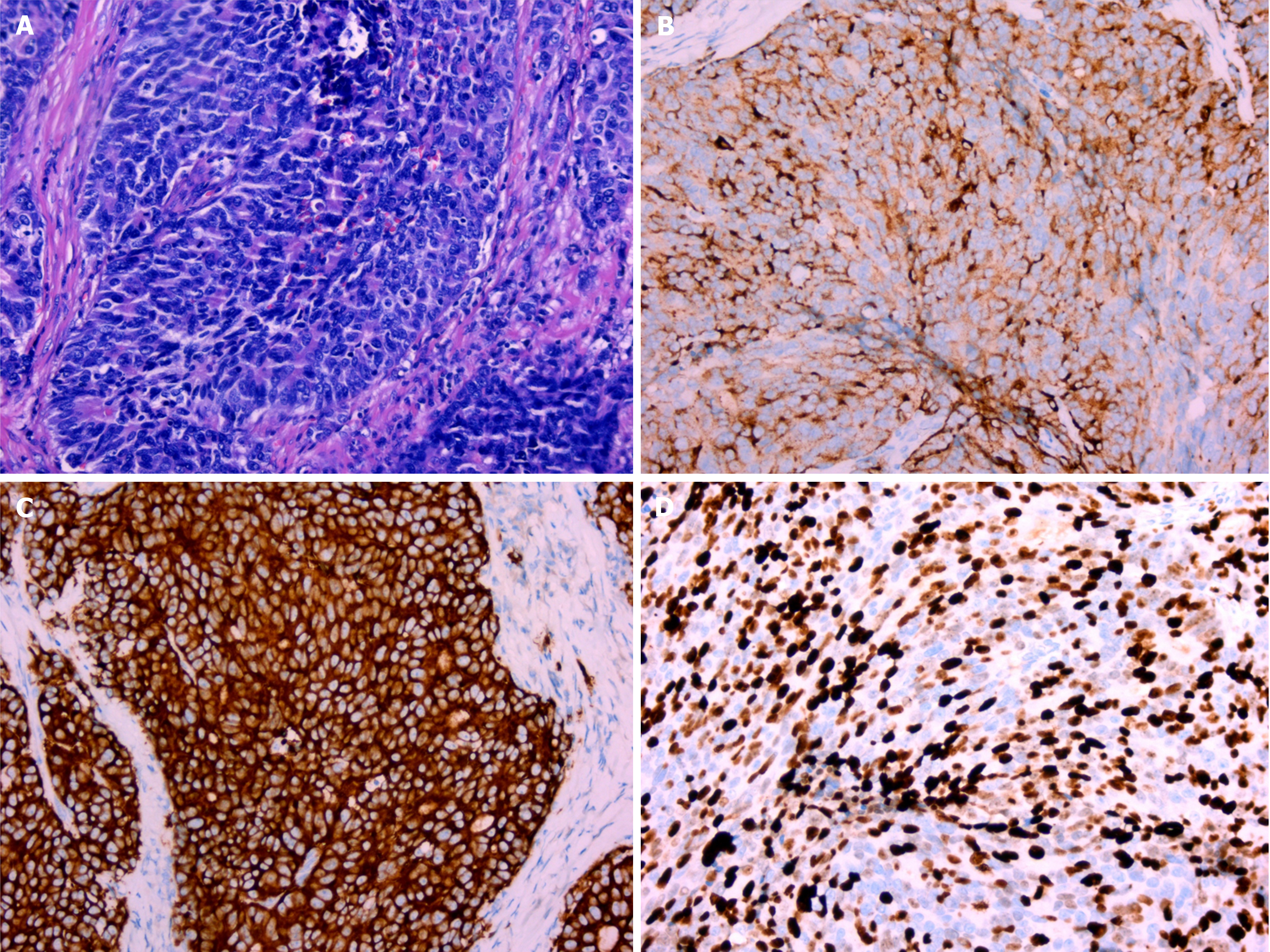
After admission, cystoscopy revealed a pink mass with a maximum diameter of approximately 2 cm on the anterior wall of the bladder (Figure 2A). A small amount of tumor tissue was obtained by cystoscopy and sent for pathological examination. The pathological section showed pronounced atypia, large and differently shaped cells and nuclei, and deeply stained nuclei (Figure 2B). Tumor cells invaded the muscularis layer. The pathologist diagnosed the patient with a malignant tumor of the bladder, suggesting an invasive UC. The patient was unwilling to pay for immunohistochemical examination; thus, the pathologist could not determine the origin of the tumor. The preliminary clinical diagnosis was bladder UC (cT2N0M0). As the patient wished to retain her bladder, we opted for laparoscopic partial cystectomy rather than radical cystectomy. During the operation, no tumor invasion or adhesions were found in the adipose tissue outside the bladder wall. Pathological examination of the complete tumor showed that the tumor invaded the deep muscular layer rather than the vessels or nerves, and no tumor tissue was found at the incisal margin or base. The morphology of the cells revealed large nuclei, coarse-grained chromatin, a nucleolus, mitosis, and trabecular growth patterns (Figure 3A). Immunohistochemical examination revealed that the cells were positive for chromogranin-A (CgA) and synaptophysin (Syn) (Figure 3B and C) and negative for CD56. The Ki-67 index reached 70% (Figure 3D). The patient was finally diagnosed with LCNEC of the bladder (pT2bN0M0) based on the results of immunohistochemical examination.
The patient refused further chemotherapy after the surgery. During the 10-mo follow-up, no indications of tumor recurrence or metastasis were found.
The tissue origin of LCNEC of the bladder is still unclear. Recent studies have suggested that metaplasic neuroendocrine cells, pluripotent stem cells, or urothelial cells beneath the bladder mucosa are possible origins[9]. Since nearly half of all LCNECs in the bladder contain tumor cells from other tissue types, such as UC, small cell carcinoma, or adenocarcinoma[2,10], pluripotent stem cells have become a popular origin because of their origin. Recent studies have identified mutations of multiple genes, such as P53, RB1, NTRK, RET, and BRAF, in LCNEC of the bladder. Inactivation of TP53 and RB1 is the most common genetic alteration in the LCNEC of the bladder, but unfortunately, neither of these genes can be a target for precision therapy[11]. Future studies on gene targets may bring new treatment options for patients with LCNEC of the bladder.
The average age at diagnosis of LCNEC of the bladder is 61.5 years, and the male-to-female ratio is approximately 4:1[2]. Smoking and personal or family history of cancer are risk factors for this cancer[4], but our patient did not mention any of these factors. However, whether her exposure to leather tanning chemicals contributed to her disease development remains unclear. Hematuria is the most common clinical symptom of LCNEC of the bladder[2,10]. At the time of diagnosis, most patients have muscle layer invasion or distant metastasis[2,10], which may be related to its high invasi
CT and magnetic resonance imaging (MRI) can aid in the diagnosis and staging of LCNEC of the bladder. Some studies have recommended 18F-FDG-PET/CT scanning to measure the systemic change in 18F-FDG uptake since tumor cells in NEC have high metabolic activity and high 18F-FDG uptake[3,5]. As a functional imaging modality, 18F-FDG-PET/CT is more reliable than CT or MRI for staging the disease[11]. This advanced method is not available in most hospitals and cannot be fully applied.
Currently, the diagnosis of LCNEC relies on histopathological examination and immunohistochemical staining. The pathomorphological characteristics of LCNEC of the bladder include large cells with pleomorphism, abundant cytoplasm, coarse granular chromatin, and prominent nucleoli. In addition, LCNEC of the bladder sometimes shows special structures, such as rosettes, palisading pattern, and trabecular architecture[8,10,11]. The most important method for diagnosing NEC is immunohistochemical examination. The expression of Syn, CgA, and CD56 and a Ki-67 index > 40% indicate high sensitivity and specificity for detecting NEC compared to UC[5,8-10]. In the present case, palisade-like structures, large nuclei, coarse granular chromatin, and nucleoli were observed in the surgically resected tumor tissue (Figure 3A), and immunohistochemical staining showed positive Syn and CgA and a Ki-67 index of 70%, all supporting the diagnosis of LCNEC (Figure 3B-D), even though CD56 was negative. For this patient, the pathological sections of the tissue obtained during cystoscopy showed the general characteristics of malignant tumor cells, including large cells and nuclei, deep staining of nuclei, and lack of cell polarity. Rosette and palisade-like structures were not observed possibly because the tissue amount was too small and could not represent most of the diseased tissue. It is difficult to make an accurate diagnosis based on limited histological and morphological information. Immunohistochemical examination serves as a reliable method to distinguish NEC from other tumors. Cystoscopy and urine cytology are common methods to obtain pathological specimens. Urine cytology has a relatively high false negative rate, even though it is a non-invasive test. In comparison, cystoscopy is a more reliable method. However, in certain scenarios, such as when imaging examination confirms the presence of bladder tumors, cystoscopy may be bypassed. Although UC is the most common type of bladder tumor, the differential diagnosis of rare tumors should not be overlooked. Patients with bladder tumors confirmed by imaging examination should undergo cystoscopic biopsy to accurately determine the origin and stage of the tumor. Accurate diagnosis is crucial, as non-urinary epithelial tumors necessitate different treatment modalities compared to urothelial cancer.
Currently, there is no standard treatment for LCNEC of the bladder. The common clinical treatments include surgery, chemotherapy, and radiotherapy. Compared to surgery alone, multimodal treatment is more conducive to prolonging survival[4,10,12]. There was no significant difference in overall survival between patients who underwent radical cystectomy and those who underwent bladder-sparing surgery[2]. The same chemotherapy regimens used for small cell NEC of the lung, such as etoposide and platinum drugs, are adopted for treating LCNEC of the bladder. Cisplatin combined with etoposide or carboplatin combined with etoposide is the treatment choice[9]. A study showed that cisplatin-based chemotherapy was superior to carboplatin in terms of prolonging the survival time[13]. For our patient, preoperative imaging revealed no extravesicular invasion or metastasis. Compared to radical surgery, bladder-sparing surgery may lead to similar survival times but has less impact on the quality of life. Our patient refused postoperative chemotherapy or radiotherapy. Single-modal treatment may be a risk factor for poor prognosis. We conducted a review of the follow-up data of patients with bladder LCNEC, comparing the prognosis of various surgical procedures. We observed that similar to those undergoing partial cystectomy, 33% of patients treated with radical cystectomy suffered from metastasis or recurrence during follow-up. Patients treated with trans-urethral resection of the bladder tumor had the worst prognosis, with 50% having metastasis or recurrence during follow-up[5]. We recommend radical surgery combined with platinum chemotherapy for patients with LCNEC of the bladder. For localized tumors that can be completely removed from the bladder, partial cystectomy may be an alternative option, particularly for patients refusing radical surgery. However, currently, few patients undergo partial cystectomy whose reliability needs more studies. Our patient had a relapse-free survival of 10 mo after partial cystectomy, without any additional treatments. She had a favorable prognosis, showing the value of partial cystectomy. We will continue to closely monitor her condition.
The overall prognosis of LCNEC of the bladder is poor, with a 1-year survival rate of less than 55% and 3-year survival rate of less than 25%. The average survival time of patients with metastasis is less than 3 mo. The postoperative survival time of patients with this disease is closely related to the T stage at the time of diagnosis. The median survival times of patients with T1-2 and T3-4 tumors are 23.9 mo and 16.0 mo, respectively[2]. Early diagnosis is therefore particularly important for survival.
Primary LCNEC of the bladder is an extremely rare malignant bladder tumor. Immunohistochemical examination is indispensable for diagnosing LCNEC of the bladder. For patients whose tissue biopsy has been obtained before surgery, we recommend immunohistochemical examination to determine the source of the tumor. Early diagnosis and multimodal treatment are highly important for prolonging patients’ survival.
| 1. | Dasari A, Mehta K, Byers LA, Sorbye H, Yao JC. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer. 2018;124:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 2. | Xia K, Zhong W, Chen J, Lai Y, Huang G, Liu H, Dong W, He W, Lin T, Huang J. Clinical Characteristics, Treatment Strategy, and Outcomes of Primary Large Cell Neuroendocrine Carcinoma of the Bladder: A Case Report and Systematic Review of the Literature. Front Oncol. 2020;10:1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Radović N, Turner R, Bacalja J. Primary "Pure" Large Cell Neuroendocrine Carcinoma of the Urinary Bladder: A Case Report and Review of the Literature. Clin Genitourin Cancer. 2015;13:e375-e377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Bhatt VR, Loberiza FR Jr, Tandra P, Krishnamurthy J, Shrestha R, Wang J. Risk factors, therapy and survival outcomes of small cell and large cell neuroendocrine carcinoma of urinary bladder. Rare Tumors. 2014;6:5043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Sun Z, Liang X, Zhang C, Song S, Gao J. Primary pure large cell neuroendocrine carcinoma of the urinary bladder: a case report and literature review. Front Oncol. 2024;14:1337997. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Halabi R, Abdessater M, Boustany J, Kanbar A, Akl H, El Khoury J, El Hachem C, El Khoury R. Large Cell Neuroendocrine Carcinoma of the Bladder with Adenocarcinomatous Component. Case Rep Urol. 2020;2020:8827646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Guleria P, Kumar S, Malik PS, Jain D. PD-L1 Expression in Small Cell and Large Cell Neuroendocrine Carcinomas of Lung: an Immunohistochemical Study with Review of Literature. Pathol Oncol Res. 2020;26:2363-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Williams JF, Vivero M. Diagnostic criteria and evolving molecular characterisation of pulmonary neuroendocrine carcinomas. Histopathology. 2022;81:556-568. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Sanguedolce F, Calò B, Chirico M, Tortorella S, Carrieri G, Cormio L. Urinary Tract Large Cell Neuroendocrine Carcinoma: Diagnostic, Prognostic and Therapeutic Issues. Anticancer Res. 2020;40:2439-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Wang G, Yuan R, Zhou C, Guo C, Villamil C, Hayes M, Eigl BJ, Black P. Urinary Large Cell Neuroendocrine Carcinoma: A Clinicopathologic Analysis of 22 Cases. Am J Surg Pathol. 2021;45:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Stumpo S, Formelli MG, Persano I, Parlagreco E, Lauricella E, Rodriquenz MG, Guerrera LP, Zurlo IV, Campana D, Brizzi MP, Cives M, La Salvia A, Lamberti G. Extrapulmonary Neuroendocrine Carcinomas: Current Management and Future Perspectives. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Mohanty P, Mohapatra AS, Sabat D, Nayak J. Unusual histomorphological spectrum of urinary bladder cancers and their treatment modalities revisited: Our experience with series of five cases. J Cancer Res Ther. 2023;19:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Sroussi M, Elaidi R, Fléchon A, Lorcet M, Borchiellini D, Tardy MP, Gravis G, Guérin M, Laguerre B, Estrade F, Delva R, Barthélémy P, Loriot Y, Lavaud P, Lebret T, Neuzillet Y, Penel N, Houede N, Pouessel D, Rousseau B, Mussat E, Gross-Goupil M, Culine S, Gauthier H, Gobert A, Roupret M, Huillard O, Tartas S, Radulescu C, Allory Y, Oudard S. Neuroendocrine Carcinoma of the Urinary Bladder: A Large, Retrospective Study From the French Genito-Urinary Tumor Group. Clin Genitourin Cancer. 2020;18:295-303.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |