Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4755
Revised: May 17, 2024
Accepted: June 3, 2024
Published online: July 26, 2024
Processing time: 99 Days and 23 Hours
Brachial artery aneurysms are defined as dilations greater than 50% of the normal diameter, which are rare among all peripheral arterial aneurysms. While they are often present as pseudoaneurysms, true brachial artery aneurysms are also detected rarely. In this case report, the surgical repair method of true brachial artery aneurysms, which is a rare condition, is explained.
Herein, we present a 61-year-old male patient with complaints of swelling and pain in the right arm antecubital region that had been progressing over 6 mo. Upon the diagnosis of a true brachial artery aneurysm associated with arteriovenous fistula, the aneurysm was surgically repaired with an autologous saphe
True brachial artery aneurysms are rare and there are not any recommendations for their management in the current literature. Even though the treatment of true aneurysms in this artery is primarily based on a surgical treatment, endovascular repair also might be an option.
Core Tip: Brachial artery aneurysm often presents as a mass in the upper extremity and is asymptomatic. Pain or neurological symptoms related to median nerve compression, as well as hand or digital ischemia due to thrombosis or distal embolization, may occur. While endovascular techniques have been described in the literature for treating pseudoaneurysms in the brachial artery, the treatment of true aneurysms in this artery is primarily based on a surgical approach involving aneurysm resection and the placement of autologous venous or prosthetic grafts. Based on our clinical experiences and as in the reported case, surgical repair is the appropriate treatment option that should be undertaken without delay to prevent upper extremity ischemic sequelae. However, due to the rarity of true brachial artery aneurysm occurrences and a lack of sufficient studies among the available treatment options, the matter remains open to discussion and maintains its relevance.
- Citation: Demirkan NA, Keskin Y, Sevinç H, Çetinkaya ÖA. Surgical treatment of a rare brachial artery aneurysm post-arteriovenous fistula closure after kidney transplant: A case report. World J Clin Cases 2024; 12(21): 4755-4761
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4755.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4755
Arterial aneurysms in the upper extremities are rare and primarily occur secondarily to recurrent blunt and penetrating trauma, infections, or iatrogenic complications, often manifesting as pseudoaneurysms.
True brachial artery aneurysms (BAAs) are even rarer, with the most common cause being recurrent blunt trauma (> 50% of cases). Additionally, they may occur congenitally due to connective tissue disorders, such as Ehlers-Danlos syndrome, Kawasaki syndrome, Buerger's disease, Kaposi's sarcoma, or cystic adventitial diseases, or may manifest idiopathically. Another factor associated with true BAAs is a history of arteriovenous fistula (AVF). The post-transplant use of steroids and immunosuppressive drugs can also cause the development of BAA.
BAAs often present as asymptomatic masses in the upper extremities, but can cause pain or neurological symptoms related to median nerve compression, as well as hand or digital ischemia due to thrombosis or distal embolization. The risk of rupture in these aneurysms is typically extremely low.
The standard treatment for BAAs remains debated owing to their rarity, and most cases have been associated with pseudoaneurysms. Although endovascular techniques for treating pseudoaneurysms in the brachial artery have been described, the treatment of true aneurysms in this artery is primarily based on a surgical approach involving aneurysm resection and placement of autologous venous or prosthetic grafts.
In this case report, we aimed to explain the surgical approach in a patient who underwent AVF closure after kidney transplantation and subsequently developed a true BAA, a rare occurrence.
A 61-year-old male patient was admitted to the Peripheral Vascular Surgery Unit with complaints of swelling and pain in the antecubital region of the right arm, which had been progressing for over 6 mo.
After experiencing chronic pyelonephritis due to recurrent kidney stones, the patient underwent hemodialysis for 11 years due to end-stage renal failure. In 2014, the patient underwent renal transplantation from a living donor. After the transplantation, early immunosuppressive treatment with methylprednisolone (4 mg), mycophenolate mofetil (250 mg), and tacrolimus (1 g) was initiated. However, following the development of avascular necrosis of the femur head, methylprednisolone treatment was discontinued, and mycophenolate mofetil was continued at a dose of 250 mg, twice daily. During early postoperative period, tacrolimus (FK 506) levels ranged from 5.5-6.8 ng/mL, while recent levels ranged from 5.9-6.5 ng/mL. Renal ultrasound (USG) performed in the early postoperative period revealed no stenosis in the renal artery or vein, with a resistive index (peak systolic velocity - end-diastolic velocity/peak systolic velocity) (RI) of 0.74. In 2023, a control renal USG did not reveal any stenosis in the renal artery or vein, and the RI was 0.67.
The patient was diagnosed with cardiac arrhythmia 4 years ago and is currently under treatment with beta-blockers and acetylsalicylic acid (ASA). A transthoracic echocardiogram obtained before aneurysm repair, revealed 1st-degree aortic and mitral insufficiency, and 3rd-degree tricuspid insufficiency. The patient's ejection fraction was calculated to be 45%- 50%, and the pulmonary artery pressure was 50 mmHg.
The patient had a past history of smoking. He had no family history of aneurysms or connective tissue diseases. Vasculitis and autoimmune diseases were not present in the family history.
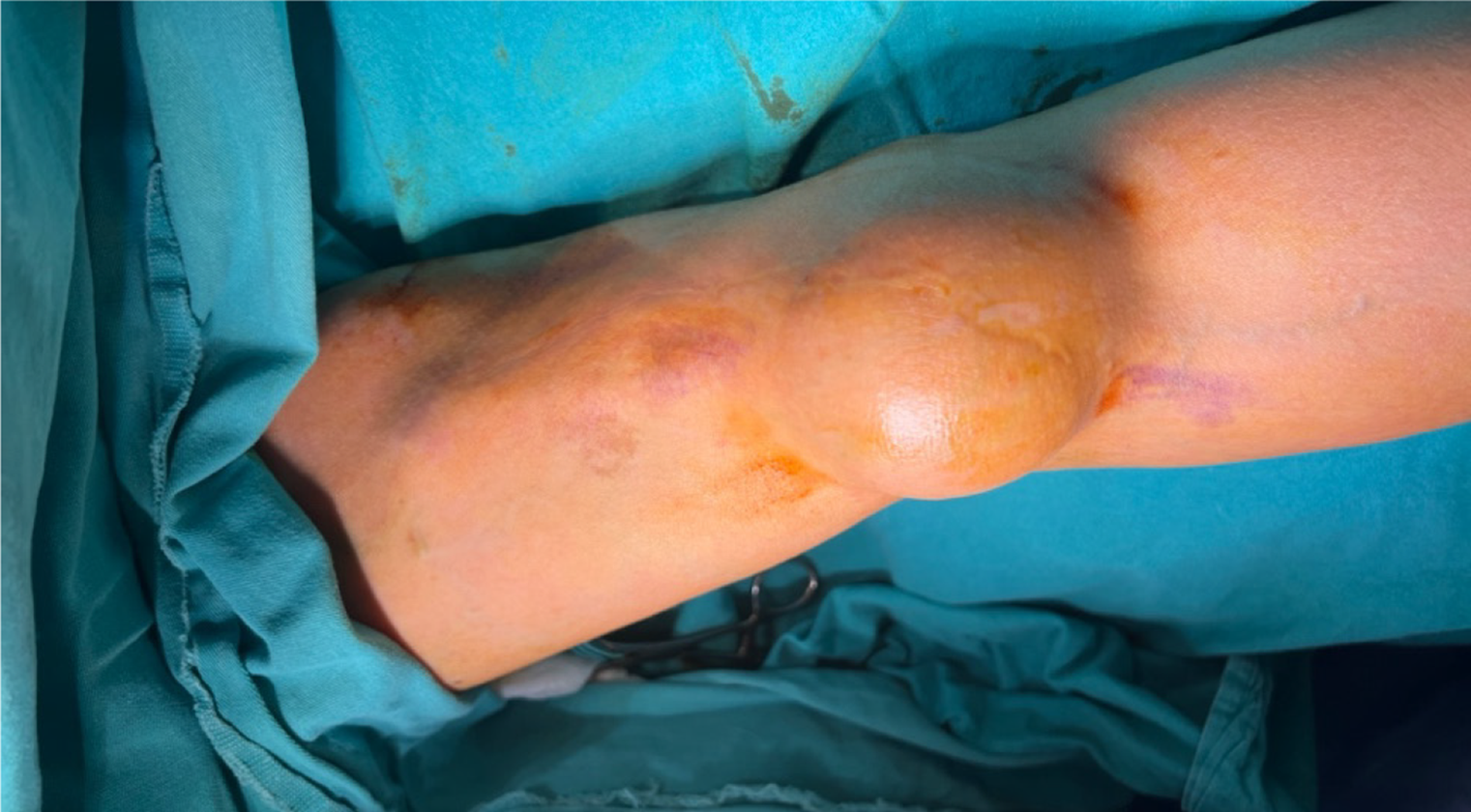
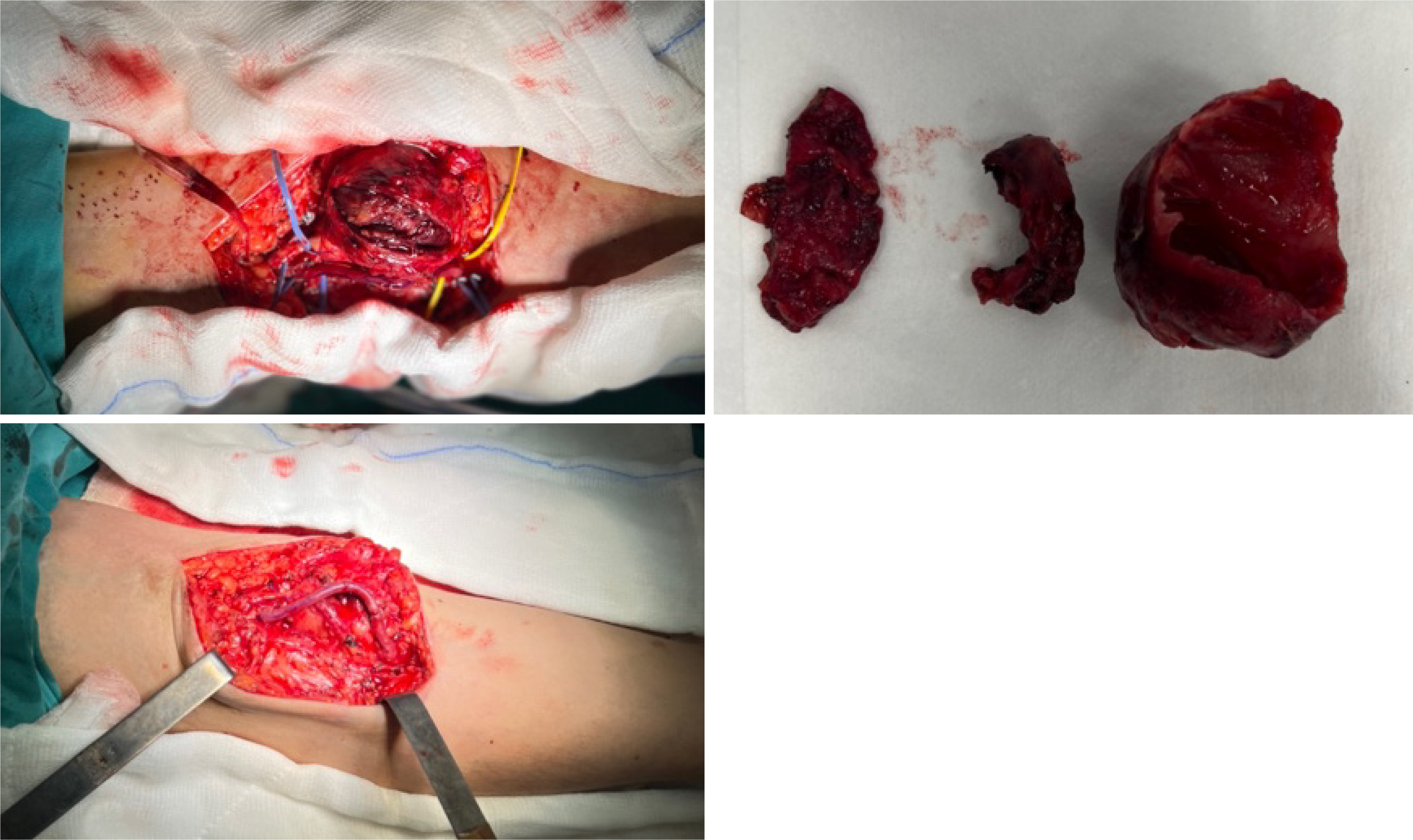
On physical examination, a circular-shaped, 5 cm × 5 cm, smooth, firm, pulsatile, and painful mass was observed in the antecubital region where the previous right brachiobasilic fistula had been closed 3 years prior (Figure 1). AVFs were created in both the right and left extremities during dialysis, and the AVF in the right arm was closed after trans
The patient's routine hemogram and biochemical tests were conducted, and no pathology was observed.
The patient's symptoms raised the suspicion of an aneurysm, and a Doppler USG was performed to confirm the diagnosis. The results were consistent with a BAA diagnosis.
The results were consistent with a BAA.
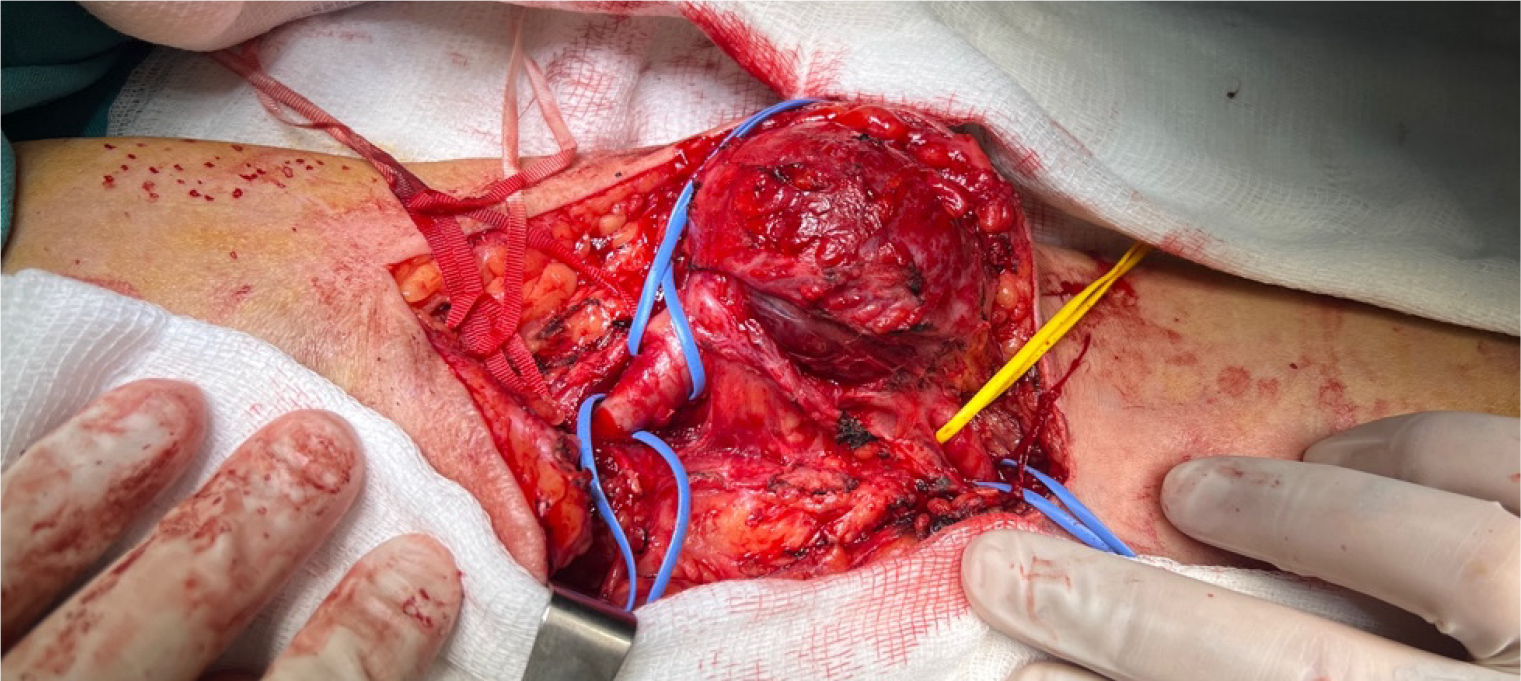
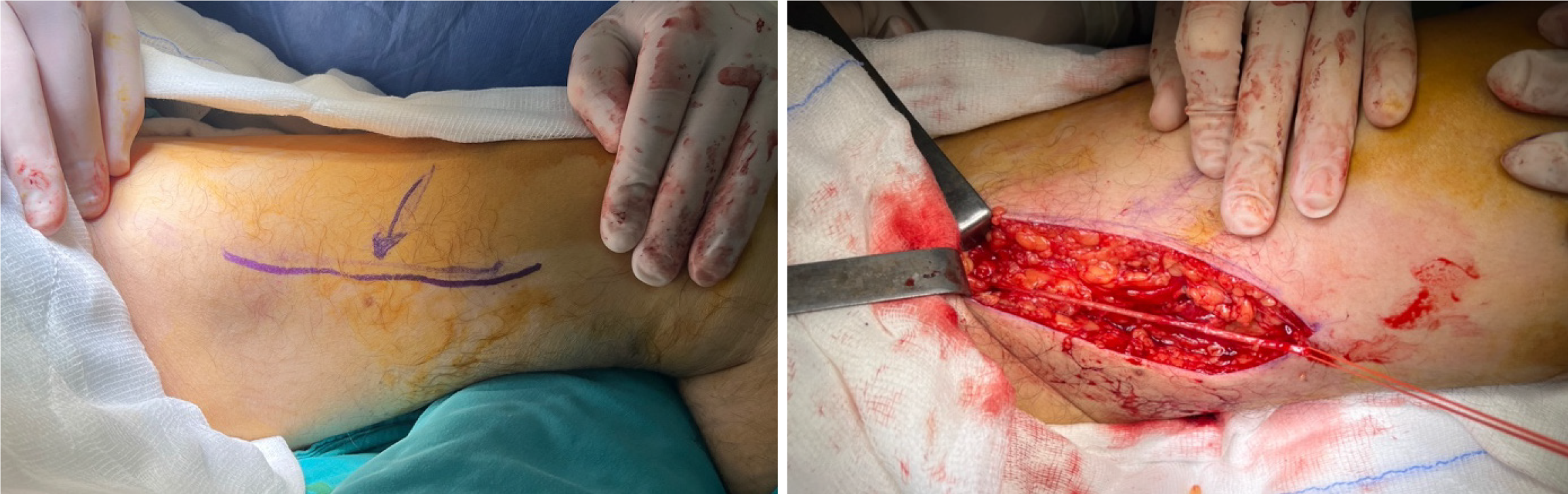
Upon diagnosing the BAA, a decision for surgical intervention was made. After administering an axillary-brachial plexus block, the surgical site was prepared, and sterile draping was performed. Access to the site of the aneurysm was achieved through an S-shaped incision in the antecubital region, traversing the skin and subcutaneous anatomical planes. Upon examination, the aneurysm and surrounding tissues, exhibited characteristics indicative of a true aneurysm.
After reaching the site of the aneurysm, the proximal and distal ends of the brachial artery were clamped using vas
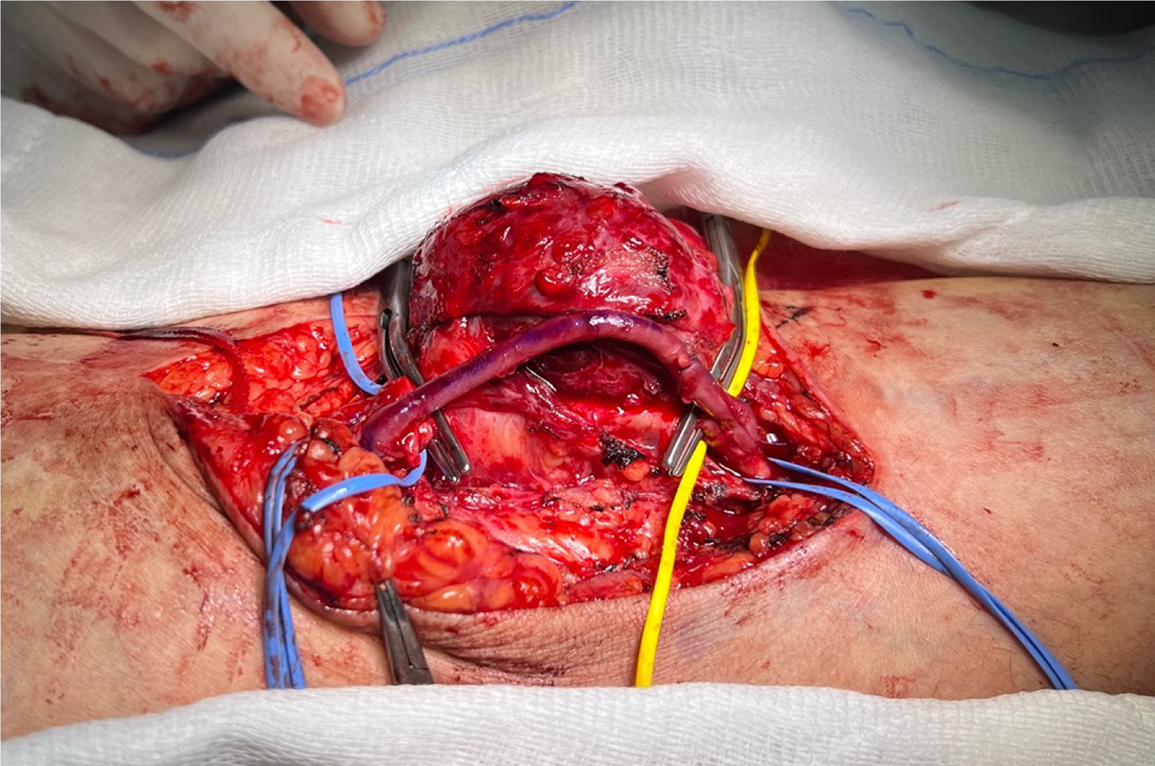
Due to challenges in clearly identifying the median nerve, excision of the aneurysm was not performed; however, the internal thrombus was evacuated (Figure 5). The operation lasted for 1 h and 30 min, with minimal intraoperative bleeding. Intraoperative ultrasonography was used to monitor venous and brachial arterial flows. Postoperatively, radial and ulnar pulses were strong and palpable. No neurological deficits were detected after the effects of regional anesthesia wore off. The patient, who was using ASA as a part of his daily regimen, was started on 4000 IU of enoxaparin after surgery.
The patient did not experience any postoperative complications and was discharged on the 7th day post-surgery. Anticoagulant and antiplatelet medications were prescribed, along with appropriate recommendations.
BAAs are defined as dilations exceeding 50% of the normal diameter. They are rare, with an incidence of 0.5% and constitute approximately 5% of all peripheral arterial aneurysms[1]. True BAAs associated with AVF account for only 0.17% of all BAAs[2].
The most common cause of true BAAs is repetitive blunt trauma (over 50%)[3,4]. Additionally, congenital connective tissue disorders, such as Ehlers-Danlos syndrome, Kawasaki syndrome, Buerger's disease, Kaposi's sarcoma, and cystic adventitial diseases, can contribute to their development[3]. Cases with no identified cause are considered idiopathic. Another factor associated with true BAAs is a history of AVF in the patient. Furthermore, post-transplant steroid and immunosuppressive drug intake can contribute to the development of BAAs[5,6].
In the present case, the patient had no history of trauma, connective tissue disorders, iatrogenic punctures, or medication use, as reported in the literature. The etiology of the BAA in this case, was associated with a history of AVF.
An association between AVFs and aneurysms has also been reported. Most cases involve the anastomotic region or are venous because of repetitive punctures for dialysis. True degenerative aneurysms with arteriomegaly, as observed in this case, are rare. The current theory suggests that AVF formation may increase blood flow in the proximal brachial artery, leading to adaptive arterial wall dilation[7]. Simultaneously, high blood flow and stress at the molecular level cause active metabolism, resulting in the production of superoxide ions and nitric oxide by endothelial cells. These substances generate peroxynitrate, which stimulates metalloproteinases to break down the extracellular matrix and damage the internal elastic lamina, leading to aneurysm formation[8,9]. Notably, closing the fistula may not completely halt the process, leading to the permanent transformation[10].
Certain collateral factors can also contribute to aneurysm formation. One such factor, as observed in the present case, is the use of steroids and immunosuppressive drugs after kidney transplantation. Steroids have been shown to induce arterial aneurysm formation and enlargement by impairing glucose tolerance and exacerbating atherosclerosis as well as by directly causing tissue fragility, primarily affecting small and medium-sized arteries. Immunosuppressants are suggested to have a synergistic effect on the damage caused by steroids[11].
Chronic mechanical stimulation is another collateral factor. Marconi et al[12] and Nishimura et al[13] reported a true BAA located near the elbow, similar to the aforementioned case, and stated that elbow joint movements may also cause brachial artery damage.
The diagnosis of BAA is often incidental, with symptoms ranging from asymptomatic pulsatile masses to compression symptoms, acute ischemia due to embolization of the forearm arteries, or rupture causing excessive bleeding[14]. Bleeding, venous edema, skin erosion, and neurological symptoms, especially compression of the median nerve or compartment syndrome, can result from aneurysm growth[3].
Diagnostic modalities included Doppler ultrasound, computed tomography angiography (CTA), and selective peripheral angiography. Doppler ultrasound is the initial choice for imaging, whereas selective arteriography is the gold standard[15]. CTA can also be used for surgical planning.
Treatment of BAAs is not well-established due to their low incidence. Surgical treatment has long been the preferred method for treating upper extremity arterial aneurysms. However, most experiences have been with pseudoaneurysms. Although endovascular techniques have been described for treating pseudoaneurysms in the brachial artery, the treatment of true aneurysms in this artery is primarily based on surgical treatment with aneurysm excision and the placement of autologous venous or prosthetic grafts[14].
In contemporary practice, surgical treatment is recommended for a 1.5-2 times increase in the normal diameter of the brachial artery[1]. The preferred surgical approach involves aneurysm resection and interposition of an autologous venous graft. This method has shown excellent results in terms of long-term vascular patency and low postoperative morbidity. In most cases, the great saphenous vein is used as the graft, demonstrating excellent outcomes in terms of graft patency and absence of complications.
The ipsilateral cephalic or basilic vein is also used to reduce surgery time and the number of incisions, as well as to preserve the veins in the lower extremities for probable future vascular reconstructions[16]. In the reported case, the great saphenous vein was preferred because of the patient's history of bilateral upper extremity AVF creation.
In some cases, an endovascular approach may be considered. Due to the serious surgical risks associated with open surgical treatment, such as major blood loss or potential damage to adjacent neurovascular structures, endovascular treatment may be preferred for patients with multiple medical comorbidities[17,18]. Similarly, endovascular therapy can be used in patients with inflammatory pathologies, such as Behçet's disease, where there is an elevated risk of aneurysm recurrence after surgical treatment[18]. Endovascular treatment can be performed if the affected vascular segment is straight and does not involve the joint[19]. However, its feasibility depends on the patient's anatomy and arterial diameter in the proximal and distal segments of the aneurysm[18].
The type of aneurysmal sac, presence of intraluminal thrombosis, and mobility of the affected arterial segments may limit endovascular treatment. Issues such as stent fracture, malpositioning, and distortion may occur in areas with continuous movement and extremity flexion. Although endovascular techniques have been explored and successfully used for treating pseudoaneurysms, their use in true BAAs has not yet been confirmed[20].
In recent years, non-invasive methods, such as ultrasound-guided thrombin injection, have also been used, but their effectiveness is debatable and may be considered for small aneurysms.
Distinguishing the natural course of untreated BAAs can be challenging due to their low incidence. In cases with small tumor size, asymptomatic presentation, and spontaneous thrombosis, a nonoperative follow-up can be considered. Initial medical treatment may be attempted in rare conditions, such as endocarditis and vasculitis.
High blood flow associated with AVF, steroid and immunosuppressive drug use after kidney transplantation, and predisposing factors such as atherosclerosis and chronic mechanical stimulation can contribute to the development of true BAA. These conditions may lead to arterial embolisms, potentially resulting in limb-threatening ischemia. Prompt diagnosis and early surgical intervention can prevent limb loss. Based on our clinical experience and as observed in the reported case, surgical repair remains the appropriate treatment option that should be undertaken without delay to prevent upper extremity ischemic sequelae. However, owing to the rarity of true BAA and the lack of sufficient studies among the available treatment options, the matter remains open to discussion and warrants further investigation.
| 1. | Schunn CD, Sullivan TM. Brachial arteriomegaly and true aneurysmal degeneration: case report and literature review. Vasc Med. 2002;7:25-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Clarke M, Waterland P, Bahia S, Asquith J, Pherwani A, Wong J. True Brachial Artery Aneurysm: A Rarity. EJVES Extra. 2012;23:e27-e28. [DOI] [Full Text] |
| 3. | Gray RJ, Stone WM, Fowl RJ, Cherry KJ, Bower TC. Management of true aneurysms distal to the axillary artery. J Vasc Surg. 1998;28:606-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Yetkin U, Gurbuz A. Post-traumatic pseudoaneurysm of the brachial artery and its surgical treatment. Tex Heart Inst J. 2003;30:293-297. [PubMed] |
| 5. | Ferrara D, Di Filippo M, Spalla F, Giribono AM, Viviani E, Santagata A, Bracale U, Santangelo M, Del Guercio L, Bracale UM. Giant true Brachial Artery Aneurysm after Hemodialysis Fistula Closure in a Renal Transplant Patient. Case Rep Nephrol Dial. 2016;6:128-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Dinoto E, Bracale UM, Vitale G, Cacciatore M, Pecoraro F, Cassaro L, Lo Monte AI, Bajardi G. Late, giant brachial artery aneurysm following hemodialysis fistula ligation in a renal transplant patient: case report and literature review. Gen Thorac Cardiovasc Surg. 2012;60:768-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Eugster T, Wigger P, Bölter S, Bock A, Hodel K, Stierli P. Brachial artery dilatation after arteriovenous fistulae in patients after renal transplantation: a 10-year follow-up with ultrasound scan. J Vasc Surg. 2003;37:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Basile C, Antonelli M, Libutti P, Teutonico A, Casucci F, Lomonte C. Is there a link between the late occurrence of a brachial artery aneurysm and the ligation of an arteriovenous fistula? Semin Dial. 2011;24:341-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Chung AW, Yang HH, Kim JM, Sigrist MK, Chum E, Gourlay WA, Levin A. Upregulation of matrix metalloproteinase-2 in the arterial vasculature contributes to stiffening and vasomotor dysfunction in patients with chronic kidney disease. Circulation. 2009;120:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Mellière D, Hassen-Khodja R, Cormier JM, Le Bas P, Mikati A, Ronsse H. Proximal arterial dilatation developing after surgical closure of long-standing posttraumatic arteriovenous fistula. Ann Vasc Surg. 1997;11:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Sato O, Takagi A, Miyata T, Takayama Y. Aortic aneurysms in patients with autoimmune disorders treated with corticosteroids. Eur J Vasc Endovasc Surg. 1995;10:366-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Marconi M, Adami D. Giant true brachial artery aneurysm after hemodialysis fistula closure. Eur J Vasc Endovasc Surg. 2015;49:531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Nishimura K, Hamasaki T, Fukino S. A Case of True Brachial Artery Aneurysm with Severe Left Upper Limb Ischemia. Int J Angiol. 2016;25:e180-e182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Shaban Y, Elkbuli A, Geraghty F, Boneva D, McKenney M, De La Portilla J. True brachial artery aneurysm: A case report and review of literature. Ann Med Surg (Lond). 2020;56:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Fernández Prendes C, Zanabili Al-Sibbai AA, González Gay M, Carreño Morrondo JA, Alonso Pérez M. True brachial artery aneurism following vascular access for haemodialysis in renal trasplant patient. Two case reports. Nefrologia. 2017;37:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Simson R, Jacobs T, Kulkarni SR. Mycotic Aneurysm of Brachial Artery Secondary to Infective Endocarditis. EJVES Short Rep. 2020;46:9-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Park SK, Hwang JK, Park SC, Kim SD. Endovascular treatment of a spontaneous aneurysm in the axillary artery. Interact Cardiovasc Thorac Surg. 2015;20:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Yiğit G, Özen S, Özen A, İşcan HZ. Isolated brachial artery aneurysm successfully treated with a covered stent in a patient with Behçet's disease. Turk Gogus Kalp Damar Cerrahisi Derg. 2019;27:565-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Maynar M, Sanchez-alvarez E, Qian Z, López-benitez R, Long D, Zerolo-saez I. Percutaneous Endovascular Treatment of a Brachial Artery Aneurysm. EJVES Extra. 2003;6:15-19. [DOI] [Full Text] |
| 20. | Vesely TM. Use of stent grafts to repair hemodialysis graft-related pseudoaneurysms. J Vasc Interv Radiol. 2005;16:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |