Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4601
Revised: May 26, 2024
Accepted: June 7, 2024
Published online: July 26, 2024
Processing time: 63 Days and 10.5 Hours
Currently, the use of dienogest in clinical practice has increased significantly, and many studies have focused on its effectiveness and safety in the treatment of endometriosis and adenomyosis; however, the effects of treatment with dienogest on uterine fibroid size in patients with endometriosis or adenomyosis have not been investigated.
To explore changes in fibroid size in patients with concomitant uterine fibroids undergoing dienogest treatment for endometriosis or adenomyosis and to evaluate the effectiveness and safety of the drug.
The clinical data of patients with uterine fibroids treated with dienogest for endometriosis or adenomyosis at Peking University First Hospital from January 2021 to January 2023 were retrospectively analyzed.
The maximum uterine fibroid diameter and volume increased after 3 months, 6 months and 1 year of dienogest treatment compared with those before treatment
The use of dienogest in patients with endometriosis or adenomyosis combined with uterine fibroids can effectively relieve the patient's pain symptoms and significantly reduce the sizes of ovarian endometriotic cysts, but it cannot inhibit uterine fibroid growth.
Core Tip: This is a retrospective single-center observational study. The clinical data of patients with endometriosis or adenomyosis with concomitant uterine fibroids during treatment with dienogest were summarizes, and the effectiveness and safety of the drug were evaluated. The use of dienogest in patients with endometriosis or adenomyosis with concomitant uterine fibroids can effectively relieve the patient's pain symptoms and significantly reduce the sizes of ovarian endometriosis cysts, but it cannot inhibit uterine fibroid growth.
- Citation: Zhang DY, Huang Y, Peng C, Zhou YF. Effect of dienogest treatment on uterine fibroid volume in patients with endometriosis or adenomyosis complicated by uterine fibroids. World J Clin Cases 2024; 12(21): 4601-4608
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4601.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4601
Endometriosis refers to the implantation and growth of active endometrial tissue outside the uterine cavity, which often occurs in the ovary and uterosacral ligament[1]. Adenomyosis occurs when endometrial tissue implants and grows in the myometrium[2]. At present, effective treatments for both conditions are lacking. Research in this field has shown that the effects of dienogest in the treatment of both endometriosis and adenomyosis are good[3,4].
Dienogest, a 19-nortestosterone derivative, is a new fourth-generation synthetic progestin and has the characteristics of 19-nortestosterone and progesterone derivatives[5,6]. Its selectivity of and affinity to its target are high, so while it has efficient progesterone activity, it can also antagonize androgen activity and inhibit gonadotropin secretion; however, it has no glucocorticoid or mineralocorticoid activity[3]. Based on its pharmacological characteristics as a progestin, dienogest has been used in the conservative treatment of endometriosis and for long-term postoperative management because it can significantly reduce pain associated with endometriosis, shrink endometriotic lesions and reduce the recurrence rate[7,8]. Dienogest was approved for the treatment of adenomyosis in Japan in 2018[9]. Currently, the use of dienogest in clinical practice has increased significantly, and many studies have focused on its effectiveness and safety in treating endometriosis and adenomyosis[4,10]; however, among patients with uterine fibroids, changes in uterine fibroids during the use of dienogest have not been investigated. Therefore, the aim of this study was to summarize the clinical data of patients with endometriosis or adenomyosis complicated by uterine fibroids who were treated with dienogest at Peking University First Hospital from January 2021 to January 2023, to explore changes in fibroid size during dienogest treatment, and to evaluate the efficacy and safety of the drug.
We included patients with endometriosis or adenomyosis complicated by uterine fibroids who were treated with dienogest at Peking University First Hospital from January 2021 to January 2023. All patients underwent gynecological ultrasound before starting medical treatment, and depending on the individual treatment plan, imaging and laboratory evaluation was performed at 3 months, 6 months, and 1 year after the start of treatment. We summarized these data and analyzed the changes in the uterine volume, maximum meridian of the uterine fibroid, volume of the uterine fibroid, maximum diameter of the uterine adenomyoma, volume of the uterine adenomyoma, maximum diameter of the ovarian endometriotic cyst, and volume of the endometriotic cyst, as well as in endometrial thickness, antigen 125 (CA125) levels, and measurements of the uterus, uterine fibroids, uterine adenomyomas and ovarian cysts on three meridians, namely, D1, D2 and D3. The volume was calculated using the ellipsoid formula D1×D2×D3×0.523). The severity of dysmenorrhea during drug treatment was assessed using a numeric rating scale, and adverse drug effects during treatment were assessed using a questionnaire.
All the data conformed to a normal distribution. Continuous variables are presented as means and standard deviations, and categorical variables are presented as frequencies and percentages. The changes in the indicators before and after drug treatment were analyzed by means of paired t tests. Pearson's correlation coefficient was used to test the correlation. All the data were processed using the Statistical Package for the Social Sciences 26. Statistical significance was set at P < 0.05.
A total of 113 patients, including 42 with pure ovarian endometriosis, 41 with pure adenomyosis, and 13 with both ovarian endometriosis and adenomyosis, were included in this study. Seventeen patients underwent surgery for endometriosis. The mean age of the patients was 40.0 ± 6.9 years, and the mean body mass index was 21.5 ± 3.9 kg/m2 (Table 1).
| Characteristics | n = 113 |
| Age (years) | 40.0 (± 6.9) |
| Body mass index (kg/m2) | 21.5 (± 3.9) |
| Condition | |
| Ovarian endometriosis | 42 (37.2) |
| Adenomyosis | 41 (36.3) |
| Both | 13 (11.5) |
| Endometriosis after surgery | 17 (15.0) |
After 3 months of dienogest treatment, 73 patients underwent gynecological ultrasound examination. We analyzed the ultrasound changes of these patients before and after treatment and found that the patients' uterine fibroids continued to grow, with the maximum diameter increasing by an average of 2.04 mm (n = 73, P < 0.05) and the volume increasing by 4.96 mm3 (n = 73, P < 0.001), and there were slow nonsignificant increases in the uterine volume, maximum diameter and volume of the uterine adenomyoma. After 3 months of treatment, the average maximum diameter of the ovarian endometriotic cysts decreased by 2.35 mm (n = 40, P = 0.134), and the volume decreased by 5.45 mm3 (n = 40, P < 0.05). Similarly, the endometrial thickness decreased from 5.93 mm to 4.48 mm after 3 months of treatment (n = 73, P < 0.001) (Table 2).
| Premedication | Post medication | Value of difference (post medication- premedication) | P value | |
| Maximum diameter of myoma (n = 73, mm) | 22.79 ± 14.52 | 24.84 ± 16.22 | 2.04 | 0.002 |
| Myoma volume (n = 73, mm) | 10.71 ± 21.15 | 15.66 ± 31.65 | 4.96 | 0.001 |
| Uterine volume (n = 73, mm3) | 139.56 ± 89.68 | 142.38 ± 90.56 | 2.82 | 0.686 |
| Maximum diameter of adenomyoma (n = 30, mm) | 44.7 ± 13.57 | 45.97 ± 16.38 | 1.27 | 0.471 |
| Adenomyoma volume (n = 30, mm3) | 42.09 ± 33.27 | 47.15 ± 46.06 | 5.19 | 0.443 |
| Maximum diameter of the cyst (n = 40, mm) | 33.48 ± 13 | 31.13 ± 11.47 | -2.35 | 0.134 |
| Volume of the cyst (n = 40, mm3) | 18.18 ± 20.33 | 12.74 ± 12.06 | -5.45 | 0.022 |
| Endometrial thickness (n = 73, mm) | 5.93 ± 3.27 | 4.48 ± 2.14 | -1.45 | < 0.001 |
After 6 months of dienogest treatment, 83 patients underwent ultrasound examinations. The changes in the average maximum diameter and volume of the uterine fibroids were consistent with those at 3 months and increased by 2.4 mm and 3.31 mm3, respectively, compared with those before treatment (n = 83, P < 0.001) (n = 83, P < 0.001). Similarly, the maximum meridian and volume of the ovarian endometriotic cysts were significantly reduced to 8.25 mm (n = 44, P < 0.001) and 9.02 mm3 (n = 44, P < 0.001), respectively. In contrast to the 3-month changes, the maximum meridian and volume of the uterine adenomyoma were both lower than those before the start of treatment; however, the differences were not significant, and the CA125 levels decreased by 17.7 U/mL on average (n = 13, P < 0.01) (Table 3).
| Premedication | Post medication | Value of difference (post medication- premedication) | P value | |
| Maximum diameter of myoma (n = 83, mm) | 23.34 ± 13.63 | 25.73 ± 15.13 | 2.40 | < 0.001 |
| Myoma volume (n = 83, mm) | 10.22 ± 16.83 | 13.53 ± 19.78 | 3.31 | < 0.001 |
| Uterine volume (n = 83, mm3) | 147.06 ± 102.17 | 144.14 ± 108.64 | -2.92 | 0.67 |
| Maximum diameter of adenomyoma (n = 34, mm) | 46.97 ± 14.99 | 44.68 ± 16.48 | -2.3 | 0.19 |
| Adenomyoma volume (n = 34, mm3) | 47.82 ± 40.38 | 44.61 ± 51.88 | -3.21 | 0.59 |
| Maximum diameter of the cyst (n = 44, mm) | 35.48 ± 11.74 | 27.23 ± 12.31 | -8.25 | < 0.001 |
| Volume of cysts (n = 44, mm3) | 19.02 ± 19.48 | 9.99 ± 12.79 | -9.02 | < 0.001 |
| Endometrial thickness (n = 83, mm) | 5.89 ± 3.2 | 4.24 ± 1.94 | -1.65 | < 0.001 |
| CA125 (n = 13, U/mL) | 45.9 ± 19.5 | 28.2 ± 16.8 | -17.7 | 0.006 |
After 12 months of dienogest treatment, 66 patients underwent ultrasound examination. The maximum diameter and volume of the uterine fibroids [3.03 mm (n = 66, P < 0.01) and 6.84 mm3 (n = 66, P < 0.01), respectively] increased compared with those before treatment and the increase was greater than that at 3 months and 6 months, indicating that most fibroids slowly increased in size during the dienogest treatment. The changes in the maximum meridian and volume of uterine adenomyomas were the same as those at 6 months but were smaller than those before treatment; however, the difference was not significant. The shrinkage ratio of the ovarian endometriotic cysts peaked after 12 months. The maximum diameter of the ovarian endometriotic cysts decreased by an average of 11.97 mm compared with that before the start of treatment (n = 38, P < 0.001), and simultaneously, the volume decreased to 8.75 mm3 (n = 38, P < 0.01). CA125 levels also decreased by 19.2 U/mL compared with those before treatment (n = 12, P < 0.01) (Table 4).
| Premedication | Post medication | Value of difference (post medication- premedication) | P value | |
| Maximum diameter of myoma (n = 66, mm) | 24.05 ± 13.55 | 27.08 ± 17.3 | 3.03 | 0.017 |
| Myoma volume (n = 66, mm) | 10.31 ± 15.01 | 17.15 ± 25.22 | 6.84 | 0.002 |
| Uterine volume (n = 66, mm3) | 141.64 ± 98.63 | 143.71 ± 99.65 | 2.07 | 0.797 |
| Maximum diameter of adenomyoma (n = 26, mm) | 42.5 ± 14.15 | 41.46 ± 19.48 | -1.04 | 0.616 |
| Adenomyoma volume (n = 26, mm3) | 39.11 ± 36.22 | 37.55 ± 37.77 | -1.57 | 0.618 |
| Maximum diameter of the cyst (n = 38, mm) | 33.11 ± 12.08 | 21.13 ± 15.5 | -11.97 | < 0.001 |
| Volume of cysts (n = 38, mm3) | 16.22 ± 17.96 | 7.47 ± 8.36 | -8.75 | 0.002 |
| Endometrial thickness (n = 66, mm) | 5.94 ± 3.59 | 4.49 ± 2.78 | -1.55 | 0.006 |
| CA125 (n = 13, U/mL) | 41.6 ± 20.0 | 22.4 ± 10.9 | -19.2 | 0.002 |
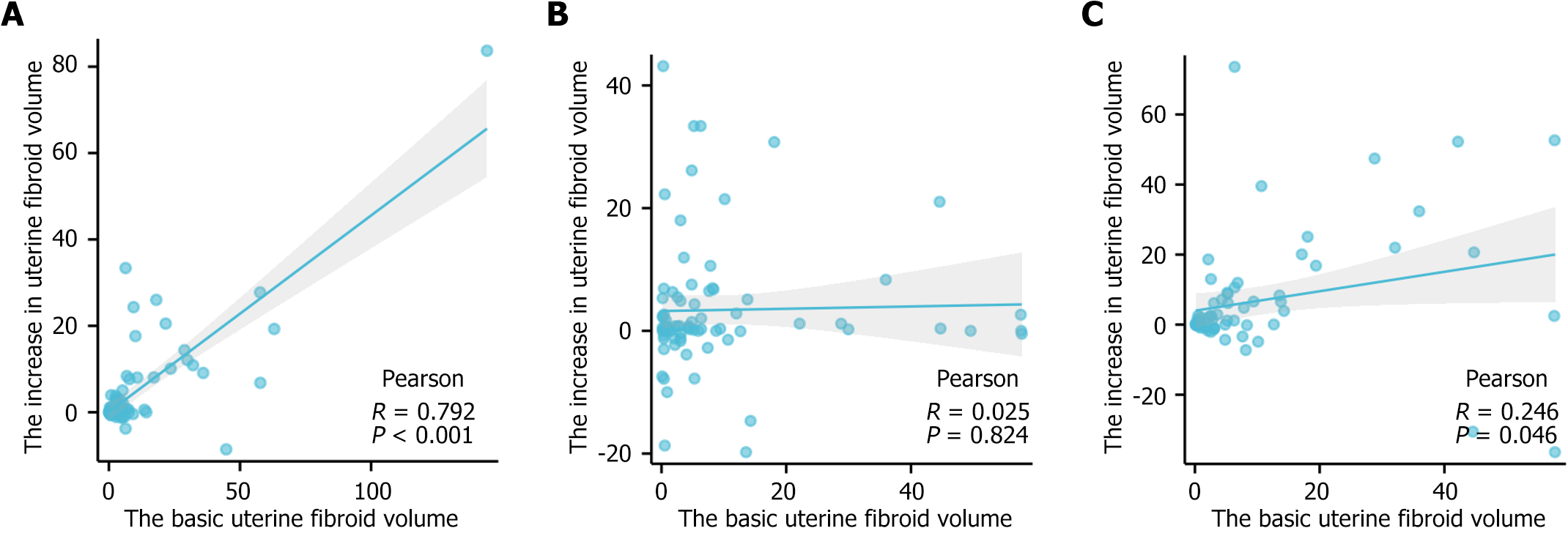
In the comparisons of the changes in uterine fibroid volume during the use of dienogest, it was found that not all patients' uterine fibroids increased in size, so we wanted to identify factors related to the changes in fibroid volume during dienogest treatment. Pearson's correlation was performed to evaluate relationships between changes in fibroid volume and uterine volume in patients without adenomyoma, uterine fibroid volume before starting treatment, the adenomyoma volume reduction value, the ovarian endometriotic cyst volume reduction value, and other variables. The results showed that the increase in uterine fibroid volume after 3 months of dienogest treatment was positively correlated with the basic uterine fibroid volume (r = 0.792, P < 0.01), but this phenomenon disappeared after 6 months and 12 months of treatment. There were no correlations with the other factors mentioned above (Figure 1).
We successfully evaluated the severity of dysmenorrhea in 81 patients using a numerical rating scale at 6 and 12 months of treatment and recorded common adverse effects using a questionnaire. The results showed that dienogest had a significant effect on dysmenorrhea caused by either endometriosis of adenomyosis. After 6 months of treatment, 63 of 64 patients with dysmenorrhea reported significant relief of dysmenorrhea symptoms, and after 12 months of treatment, all patients experienced significant relief of dysmenorrhea symptoms. The most common side effect was amenorrhea, followed by weight gain, weight loss and mood changes. A small proportion of patients had irregular vaginal bleeding and alopecia. Despite the side effects of dienogest, the drug was well tolerated by the patients as a whole, with a mean tolerance score of 9.3 ± 2.4 among 81 patients after 6 months of dienogest treatment and a score of 8.73 ± 2.51 after 12 months (Table 5).
| Medication for 6 months | Medication for 12 months | |
| Number of weight gain (n = 81) | 33/81 (40.7) | 28/81 (34.6) |
| Number of patients with remission of dysmenorrhea (n = 64) | 63/64 (98.4) | 64/64 (100) |
| Number of amenorrhea patients (n = 81) | 49/81 (56.3) | 56/81 (69.1) |
| Number of irregular vaginal bleeding (n = 81) | 10/81 (12.3) | 7/81 (8.6) |
| Number of patients with acne (n = 81) | 3/81 (3.7) | 2/81 (2.5) |
| Number of patients with breast tenderness (n = 81) | 8/81 (9.9) | 11/81 (13.6) |
| Number of patients with headache (n = 81) | 4/81 (4.9) | 5/81 (6.2) |
| Number of patients suffering from depression (n = 81) | 14/81 (17.3) | 16/81 (19.8) |
| Number of patients with decreased sexual desire (n = 81) | 1/81 (1.2) | 1/81 (1.2) |
| Number of patients with hair loss (n = 81) | 9/81 (11.1) | 9/81 (11.1) |
| Average drug tolerance score (n = 81) | 9.30 ± 2.14 | 8.73 ± 2.51 |
Endometriosis refers to the implantation and growth of active endometrial tissue outside the uterine cavity, which often occurs in the ovary and uterosacral ligament. The main clinical manifestations are dysmenorrhea and infertility, and endometriosis affects approximately 5%-10% of women of reproductive age worldwide[1]. At present, the main treatment options for endometriosis are surgery and drug treatment, but the recurrence rate is as high as 50% within 5 years after surgery[11]; therefore, surgery is often combined with hormone drugs, and some patients directly request these drugs for conservative treatment. The most commonly used drugs are recurrent oral contraceptives, dienogest and gonadotrophin-releasing hormone agonist (GnRH-a)[12]. Among them, dienogest, a new and promising drug, has been widely re-cognized for its role in endometriosis in recent years. Studies have shown that dienogest can effectively reduce the sizes of endometriotic lesions and relieve endometriosis-associated pain. Its efficacy is equivalent to that of GnRH-a, but it has fewer side effects than GnRH-a[10]. Moreover, dienogest can effectively reduce the recurrence rate when used after surgery[13].
This study revealed that the maximum diameter and volume of ovarian endometriotic cysts began to decrease after 3 months of dienogest treatment, and as the duration of drug use increased, the diameter and volume of the cysts gradually decreased. When the drug was used for 12 months, the average diameter of the cysts in 38 patients decreased from 33.11 ± 12.08 mm to 21.13 ± 15.5 mm (P < 0.001), and the cyst volume decreased from 16.22 ± 17.96 mm3 to 7.47 ± 8.36 mm3 (P < 0.01). In their study, Angioni et al[14] included 81 patients with ovarian endometriotic cysts who were given dienogest 2 mg/d for six months, and the average ovarian cyst volume decreased from 52 ± 22 mm3 to 32 ± 12 mm3, consistent with the results of our study. Therefore, more patients with ovarian endometriosis can choose conservative treatment with dienogest, as this can not only reduce the sizes of cysts and relieve pain but also avoid the negative impact of surgery on ovarian function[10,15,16].
Although dienogest was officially approved for the treatment of adenomyosis only within the past five years, there are many studies on its use for treating adenomyosis. There is no doubt that dienogest can relieve pain in patients with adenomyosis. In a previous study, 130 patients with adenomyosis were treated with dienogest for more than 52 weeks, and the visual analog scale (VAS) score for pain decreased from 65.9 ± 20.3 mm at baseline to 52.7 ± 25.7 mm at 24 weeks[17]. In another randomized controlled trial, 67 patients with adenomyosis were randomly assigned to receive dienogest or placebo. The results showed that the VAS score in the dienogest group decreased significantly from week 4 and remained stable until week 16, with a reduction of 58.4 mm by week 16 compared with the score in the placebo group. However, the VAS score decreased by only 20.6 mm in the placebo group[9]. Our results further confirmed that dienogest could alleviate the pain symptoms of patients with adenomyosis.
In addition, there is controversy about the changes in the adenomyotic lesion size during treatment with dienogest. In the above study involving 130 patients, the uterine volume decreased by 20.6 cm3 at 24 weeks and 26.0cm3 at 52 weeks, suggesting that dienogest can significantly reduce adenomyotic lesion volume[17]. However, another study revealed that uterine volume and adenomyopathy lesion size decreased after 6 months of dienogest treatment; however, the difference was not significant[18], and another study revealed that the uterine volume did not decrease significantly after 4 months of dienogest treatment[19]. Similarly, our study revealed that the volume and diameter of the adenomyoma decreased by 1.57 mm3 and 1.04 mm, respectively, after 12 months of treatment with dienogest; however, the difference was not significant, and the uterine volume did not change significantly, which further supported the latter view that dienogest could not significantly reduce adenomyotic lesion volume in the treatment of adenomyopathy.
The effect of dienogest on uterine fibroid size is the highlight of this study. There is only one study on the effect of dienogest on uterine fibroids. In this study, 55 patients with endometriosis treated with dienogest were retrospectively analyzed, and 6 of them had fibroids. Therefore, 12 patients with uterine fibroids treated with leuprolide acetate were selected as the control group, and the study revealed that the reduction in uterine volume in patients treated with dienogest was 59.7% after 6 months of treatment, which was similar to the reduction of 51.9% observed in the GnRH-a group[20]. In contrast, our study of 113 patients with endometriosis or adenomyosis treated with dienogest showed a slow increase in the size and diameter of the uterine fibroids, although decreases in these parameters were evident in a few patients at 3, 6, and 12 months, which is inconsistent with the above results. However, in this larger study, it is reasonable to assume that the vast majority of fibroids will not be controllable with dienogest.
The use of dienogest in patients with endometriosis or adenomyosis combined with uterine fibroids can effectively relieve the patient's pain symptoms and significantly reduce the sizes of ovarian endometriotic cysts, but it cannot inhibit uterine fibroid growth.
The authors wish to acknowledge the patients and their families who agreed to participate in the study.
| 1. | Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 1173] [Article Influence: 234.6] [Reference Citation Analysis (0)] |
| 2. | Bulun SE, Yildiz S, Adli M, Chakravarti D, Parker JB, Milad M, Yang L, Chaudhari A, Tsai S, Wei JJ, Yin P. Endometriosis and adenomyosis: shared pathophysiology. Fertil Steril. 2023;119:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 79] [Reference Citation Analysis (0)] |
| 3. | Muzii L, Di Tucci C, Galati G, Carbone F, Palaia I, Bogani G, Perniola G, Tomao F, Kontopantelis E, Di Donato V. The Efficacy of Dienogest in Reducing Disease and Pain Recurrence After Endometriosis Surgery: a Systematic Review and Meta-Analysis. Reprod Sci. 2023;30:3135-3143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Kobayashi H. Efficacy, Adverse Events, and Challenges of Dienogest in the Management of Symptomatic Adenomyosis: A Comparison with Different Hormonal Treatments. Gynecol Obstet Invest. 2023;88:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Foster RH, Wilde MI. Dienogest. Drugs. 1998;56:825-33; discussion 834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Barra F, Scala C, Ferrero S. Current understanding on pharmacokinetics, clinical efficacy and safety of progestins for treating pain associated to endometriosis. Expert Opin Drug Metab Toxicol. 2018;14:399-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Caruso S, Iraci M, Cianci S, Vitale SG, Fava V, Cianci A. Effects of long-term treatment with Dienogest on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain. J Pain Res. 2019;12:2371-2378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Lang J, Yu Q, Zhang S, Li H, Gude K, von Ludwig C, Ren X, Dong L. Dienogest for Treatment of Endometriosis in Chinese Women: A Placebo-Controlled, Randomized, Double-Blind Phase 3 Study. J Womens Health (Larchmt). 2018;27:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Osuga Y, Fujimoto-Okabe H, Hagino A. Evaluation of the efficacy and safety of dienogest in the treatment of painful symptoms in patients with adenomyosis: a randomized, double-blind, multicenter, placebo-controlled study. Fertil Steril. 2017;108:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Cho B, Roh JW, Park J, Jeong K, Kim TH, Kim YS, Kwon YS, Cho CH, Park SH, Kim SH. Safety and Effectiveness of Dienogest (Visanne®) for Treatment of Endometriosis: A Large Prospective Cohort Study. Reprod Sci. 2020;27:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Zakhari A, Delpero E, McKeown S, Tomlinson G, Bougie O, Murji A. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 12. | Chinese Obstetricians and Gynecologists Association; Cooperative Group of Endometriosis, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. [Guideline for the diagnosis and treatment of endometriosis (Third edition)]. Zhonghua Fu Chan Ke Za Zhi. 2021;56:812-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Lee SR, Yi KW, Song JY, Seo SK, Lee DY, Cho S, Kim SH. Efficacy and Safety of Long-Term Use of Dienogest in Women With Ovarian Endometrioma. Reprod Sci. 2018;25:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Angioni S, Pontis A, Malune ME, Cela V, Luisi S, Litta P, Vignali M, Nappi L. Is dienogest the best medical treatment for ovarian endometriomas? Results of a multicentric case control study. Gynecol Endocrinol. 2020;36:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | El Taha L, Abu Musa A, Khalifeh D, Khalil A, Abbasi S, Nassif J. Efficacy of dienogest vs combined oral contraceptive on pain associated with endometriosis: Randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2021;267:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Uludag SZ, Demirtas E, Sahin Y, Aygen EM. Dienogest reduces endometrioma volume and endometriosis-related pain symptoms. J Obstet Gynaecol. 2021;41:1246-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Osuga Y, Watanabe M, Hagino A. Long-term use of dienogest in the treatment of painful symptoms in adenomyosis. J Obstet Gynaecol Res. 2017;43:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Hirata T, Izumi G, Takamura M, Saito A, Nakazawa A, Harada M, Hirota Y, Koga K, Fujii T, Osuga Y. Efficacy of dienogest in the treatment of symptomatic adenomyosis: a pilot study. Gynecol Endocrinol. 2014;30:726-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Fawzy M, Mesbah Y. Comparison of dienogest vs triptorelin acetate in premenopausal women with adenomyosis: a prospective clinical trial. Arch Gynecol Obstet. 2015;292:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Ichigo S, Takagi H, Matsunami K, Suzuki N, Imai A. Beneficial effects of dienogest on uterine myoma volume: a retrospective controlled study comparing with gonadotropin-releasing hormone agonist. Arch Gynecol Obstet. 2011;284:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |