Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4491
Revised: April 29, 2024
Accepted: May 15, 2024
Published online: July 26, 2024
Processing time: 122 Days and 6.3 Hours
Diabetic macular edema (DME), a chronic microvascular complication of diabetes, is a leading cause of visual impairment and blindness. Pars plana vitrectomy (PPV) can restore the normal macular structure and reduce macular edema, whereas internal limiting membrane (ILM) peeling is used to treat tractional macular diseases. Despite the advantages, there is limited research on the com
To observe the effects of PPV combined with ILM peeling on postoperative central macular thickness (CMT), best-corrected visual acuity (BCVA), cystoid macular edema (CME) volume, and complications in patients with DME.
Eighty-one patients (92 eyes) diagnosed with DME at the Beijing Shanqu Liangxiang Hospital between January and December 2022 were randomly divided to undergo PPV alone (control group: 41 patients, 47 eyes) or PPV + ILM peeling (stripping group: 40 patients, 45 eyes); a single surgeon performed all surgeries. The two groups were compared preoperatively and 1 and 3 months postoperatively.
Preoperatively, both groups had comparable values of CMT, BCVA, and CME volume (P > 0.05). After surgery (both 1 and 3 months), both groups showed significant reductions in CMT, BCVA, and CME volume compared to preope
PPV with ILM peeling can significantly improve the visual acuity of patients with DME, reduce CMT, and improve CME with fewer complications.
Core Tip: This study investigates the effectiveness of combining vitrectomy with internal limiting membrane peeling in treating patients with diabetic macular edema (DME). We found that this combined approach aids in the early reduction of DME, decreases central macular thickness, and positively affects visual recovery. Further long-term observations are required to assess the impact on complications.
- Citation: Wang L, Chen CJ, Wang ML, Huang Y, Fang LJ. Effects of vitrectomy combined with internal limiting membrane peeling in patients with diabetic macular edema. World J Clin Cases 2024; 12(21): 4491-4498
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4491.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4491
Diabetic macular edema (DME) is the leading cause of vision loss in diabetics. As a complication of diabetic retinopathy (DR), it can occur at any stage of DR. In 2015, the International Diabetes Federation reported that approximately 4.15 million people worldwide were diagnosed with diabetes, with a prevalence rate of 9.7%[1]. An epidemiological survey reported that a considerable 5.2% of people with diabetes have DME[2]. Therefore, diabetic individuals must receive timely and efficient DME therapy to retain and improve their eyesight.
Lately, a combination of pars plana vitrectomy (PPV) and internal limiting membrane (ILM) peeling has become a viable treatment option for DME as a result of extensive research into the anatomy, physiology, and pathology of the vitreoretinal interface. Additionally, laser therapy, anti-vascular endothelial growth factor (VEGF) drugs, and hormone therapy have received widespread attention[3,4]. Rajkhowa et al[5] first described PPV as an effective treatment for traction DME. Owing to the rapid development of surgical devices and techniques, the combination therapy of PPV and ILM peeling has immensely reduced the need for anti-VEGF treatment in patients with stubborn DME after vitrectomy and improved their visual acuity[6-8]. Nevertheless, the efficacy of PPV combined with ILM peeling to prevent additional deterioration of macular edema and enhance central macular thickness (CMT) remains debatable. This study discusses the impact of PPV combined with ILM peeling on CMT, visual acuity, and DME complications in patients with DR.
We retrospectively reviewed the case data of 81 patients (92 eyes) diagnosed with DME at the Beijing Shanqu Liangxiang Hospital between January and December 2022. The study was approved by the local ethics committee and conducted with the consent of patients and their families.
Inclusion criteria: (1) Diagnosed with DME as per the criteria described in the Diagnosis and Control of Diabetic Ophthalmopathy[9]; (2) DME identified using ophthalmoscopy, B-ultrasound, slit-lamp, optical coherence tomography (OCT), and fundus fluorescein angiography; (3) Preoperative fasting blood glucose levels of < 7 mmoL, 2-h postprandial blood glucose < 9 mmoL, and blood pressure < 150/90 mmHg; (4) Follow-up period of ≥ 6 months; and (5) Availability of complete clinical data.
Exclusion criteria: (1) Previous history of other ophthalmopathies; (2) Systemic malignancy; (3) Unclear refractive media hindering the fundus examination; (4) Previous history of laser, anti-VEGF drug injection, vitrectomy, cataract surgery, or other intraocular surgery; (5) History of ocular trauma; (6) Presence of vitreous hemorrhage or cataract; (7) Patients with macular retinal detachment, optic neuropathy, and retinal vein occlusion; and (8) Patients with liver and kidney dys
A computerized random number generator was used to create two sequences of random numbers from 1-81; patients numbered using the first sequence were grouped as the control group while those numbered using the second sequence were labeled as the stripping group. The baseline data of the 2 groups were balanced (P > 0.05; Table 1).
| Group (number of cases/eyes affected) | Gender (n) | Age, mean ± SD (yr) | Diabetes classification (n) | Intraocular pressure, mean ± SD (mmHg) | Duration of diabetes, mean ± SD (yr) | ||
| Male | Female | Type I | Type II | ||||
| Control group (41/47) | 21 | 20 | 51.46 ± 8.41 | 8 | 33 | 15.16 ± 2.77 | 10.06 ± 2.91 |
| Stripping group (40/45) | 17 | 23 | 53.73 ± 9.56 | 11 | 29 | 15.23 ± 3.83 | 10.03 ± 3.22 |
| t/χ2 | 0.618 | 1.135 | 0.720 | 0.094 | 0.044 | ||
| P value | 0.432 | 0.260 | 0.396 | 0.925 | 0.965 | ||
Before the surgery, both groups underwent routine investigations, including liver and kidney function tests, blood and urine tests, electrocardiograms, and other assessments, with strict control over blood glucose levels. The aforementioned values for fasting and post-prandial blood glucose and blood pressure were maintained for all patients. Each patient received levofloxacin (0.5%) eye drops (Zhongshan Wanhan Pharmaceutical Co., Ltd., CMPN: H20203122; specification: 5 mL, 24.4 mg/piece) 4 times a day for 3 d before the surgery. Three hours before the surgery, 0.5% compound picamide eye drops (Tianjin Kingyork Group Hebei Univision Pharmaceutical Co., Ltd.; CMPN: H20066782; 5 mL: 25 mg; 25 mg/bottle) were administered for full mydriasis.
Following normal disinfection, sterile towels were spread; in the control group, the conjunctival sac was cleansed after applying surface anesthesia using 5 mL of 2% lidocaine (Anhui Huayuan Pharmaceutical Group Co., Ltd.; CMPN: H31021072; 5 mL: 0.1 g × 5 pieces) and 0.75% bupivacaine (Anhui Huayuan Pharmaceutical Group Co., Ltd.; CMPN: H31022839; 5 mL: 37.5 mg) and 1:1 mixed liquid eye anesthesia behind the ball, peribulbar and subconjunctival. A 23 g puncture knife with a fixed cannula was used to puncture the three-channel site at a distance of 35-4 mm from the posterior limbus during the standard vitreous surgery. The temporomandibular and upper nose housed the incision and light guide opening, while the subtemporal region housed the perfusion opening. After removing the front and central vitreous sections, 0.1 mL TA staining was injected into the vitreous cavity, the sclera was squeezed, and the peripheral basal vitreous body was entirely cut off. The loose membrane tissue was dragged to approach the retina after being separated from the edge by cutting head separation or flute needle negative pressure.
Patients experiencing recurrent bleeding or active neovascularization throughout the surgical procedure underwent treatment via enhanced perfusion or electrocoagulation to control bleeding; if required, condensation or the introduction of heavy water into the vitreous cavity effectively sealed the retinal aperture. Following the surgery, silicone oil was administered into the vitreous cavity; the cannula was extracted and the scleral incision and bulbar conjunctiva were stitched. The incision was thoroughly inspected for any signs of leakage and subconjunctival injection of dexamethasone (Anhui Huayuan Pharmaceutical Group Co., Ltd.; CMPN: H37021969; 1 mL: 5 mg) + 0.6 mL gentamicin (Fujian Gutian Pharmaceutical Co., Ltd.; CMPN: H35020138; 1 mL: 40000 units) was given. Additionally, 0.5% levofloxacin eye drops (Hubei Haobang Pharmaceutical Co., Ltd.; CMPN: H20183382; 5 mL: 24.4 mg) + tobramycin dexamethasone eye ointment (Shenyang Huayi Medicine Co., Ltd.; CMPN: H20067635; 10 mL) was injected beneath the conjunctival sac, enveloping the eye with four heads.
Peeling of the ILM was performed in the stripping group: After vitrectomy, the posterior vitreous cortex was cleaned using triamcinolone acetonide injection, and the epiretinal membrane was peeled off. The retinal ILM was stained by injecting a 0.1% indocyanine green solution into the vitreous cavity for 30-60 s; the membrane was equally stained green after the perfusate in the vitreous cavity was replaced. The ILM flap was held using ILM forceps after the valve was lifted in the temporal retinal vascular arch, away from the macular fovea. The ILM of the retina was stripped in the fovea in a concave ring shape, with a stripping range of 2DD. The stripping effect was judged by punctate bleeding and reflection spots in the stripping area.
CMT: Sitting OCT (Heidelberg, Germany) examination was performed before surgery, and 1 and 3 months after surgery using relatively transparent refractive media. The patient manually measured CMT (fovea, retinal thickness from inner limiting membrane layer to RPE layer) on OCT 3 times and the average value was used[10].
Best-corrected visual acuity (BCVA): The standard logarithmic visual acuity chart was used for examining visual acuity on postoperative days 1, 3, and 7. These values were then converted into the log-minimum angle of resolution (logMAR) for visual acuity records.
Volume of cystoid macular edema (CME): The volume of the edematous cyst in the macular region was measured using B-mode ultrasonography at 1 week, 1 month, and 3 months postoperatively.
Complications: The incidence of retinal detachment, endophthalmitis, secondary glaucoma, and macular holes in both groups was recorded by the nursing staff. The recording time was limited to 6 months after surgery.
Enrollment management: The patient’s eyes were assessed by two senior ophthalmologists who determined whether the patient fulfilled the enrollment criteria. In case of any incongruity between the assessor, the senior-most doctor’s decision was accepted. A single surgeon from the permanent team carried out the surgery.
Data acquisition: An ophthalmologist conducted the eye examination in this study, and the training was carried out prior to the examination.
The study data was analyzed using SPSS (version 26.0). Continuous data were described as mean ± SD. A two-sample t-test was used to compare continuous data between groups, while repeated-measures ANOVA was used to compare data at different time points within the groups. Categorical data were expressed using frequency or percentage, and compared using the χ2 test. Statistical significance was indicated by a P value of < 0.05.
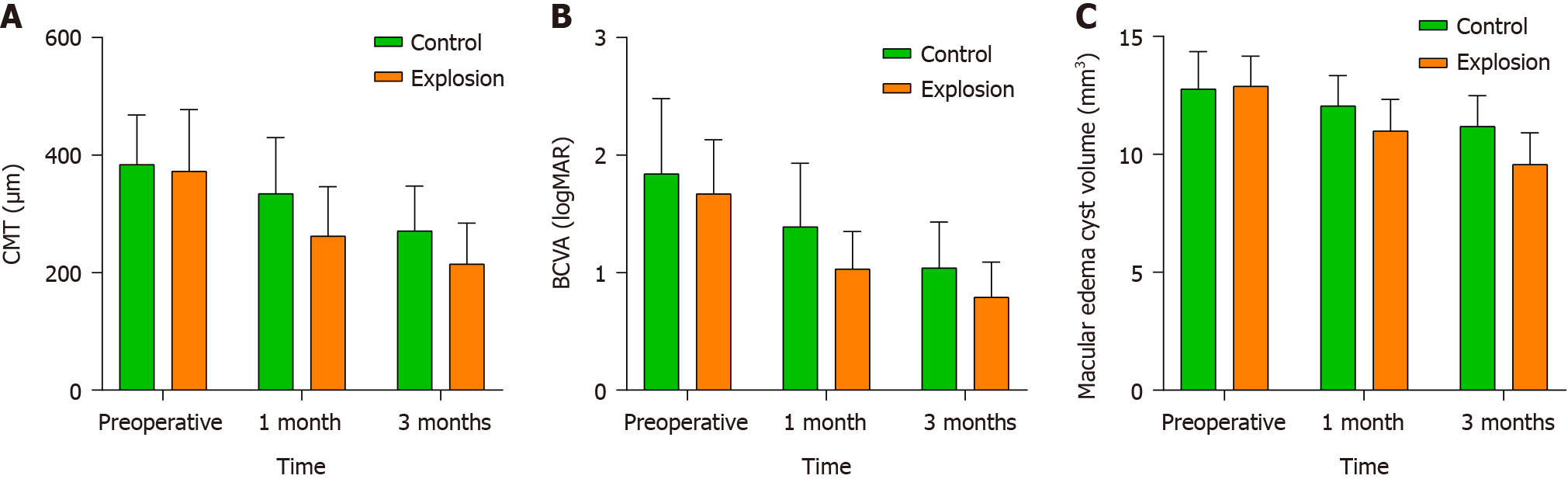
There were no statistically significant differences in the preoperative CMT levels in both groups (P > 0.05); however, both groups showed decreased CMT levels both 1 and 3 months after the surgery. Overall, both groups exhibited a decreasing trend in CMT levels (Figure 1A).
Notably, the stripping group had relatively lower CMT levels than the control group during the same period (P < 0.05). Furthermore, repeated-measures ANOVA analysis revealed significant interaction effects of CMT levels in the two groups (P < 0.05; Table 2).
| Group (number of affected eyes) | CMT (μm) (mean ± SD) | F | P value | ||
| Preoperative | 1 month after surgery | 3 months after surgery | |||
| Control group (n = 47) | 383.83 ± 84.31 | 334.37 ± 95.46 | 270.95 ± 76.24 | Fgroup = 8.854 | Pintergroup = 0.004 |
| Stripping group (n = 45) | 372.75 ± 104.76 | 262.35 ± 83.85 | 214.48 ± 69.69 | Ftime = 500.634 | Ptime < 0.001 |
| t | 0.560 | 3.838 | 3.703 | Finteraction = 7.403 | Pinteraction = 0.001 |
| P value | 0.577 | < 0.001 | < 0.001 | ||
Like CMT, both groups were statistically comparable in terms of preoperative BCVA levels (P > 0.05). Likewise, BCVA levels decreased in both groups at 1 and 3 months after the surgery compared to preoperative levels (Figure 1B), with the stripping group having considerably lower levels than the control group (P < 0.05). Furthermore, there were significant interaction effects between the group and time on the BCVA levels in both groups (P < 0.05; Table 3).
| Group (number of affected eyes) | BCVA (logMAR) (mean ± SD) | F | P value | ||
| Before surgery | 1 month after surgery | 3 months after surgery | |||
| Control group (n = 47) | 1.84 ± 0.64 | 1.39 ± 0.54 | 1.04 ± 0.39 | Fgroup = 7.978 | Pintergroup = 0.006 |
| Stripping group (n = 45) | 1.67 ± 0.46 | 1.03 ± 0.32 | 0.79 ± 0.30 | Ftime = 441.237 | Ptime < 0.001 |
| t | 1.457 | 3.868 | 0.436 | Finteraction = 17.105 | Pinteraction < 0.001 |
| P value | 0.149 | < 0.001 | < 0.001 | ||
The preoperative CME volume was comparable in both groups (P > 0.05). Postoperatively, the volume of CME in both groups steadily decreased (Figure 1C). However, at 1 month and 3 months after the surgery, the observation group had a lower volume of CME compared to the control group. The repeated ANOVA analysis showed that there were group and time interaction effects between the two groups (P < 0.05; Table 4).
| Group (number of affected eyes) | Macular edema cyst volume (mm3) (mean ± SD) | F | P value | ||
| 1 week after surgery | 1 month after surgery | 3 months after surgery | |||
| Control group (n = 47) | 12.77 ± 1.58 | 12.06 ± 1.28 | 11.19 ± 1.30 | Fgroup = 9.374 | Pintergroup = 0.003 |
| Stripping group (n = 45) | 12.89 ± 1.27 | 10.99 ± 1.34 | 9.57 ± 1.34 | Ftime = 1838.956 | Ptime < 0.001 |
| t | 0.400 | 3.917 | 5.886 | Finteraction = 210.654 | Pinteraction < 0.001 |
| P value | 0.690 | < 0.001 | < 0.001 | ||
In the control group, two eyes had retinal detachment and four eyes had endophthalmitis. In contrast, the stripping group had one eye with retinal detachment and two eyes with endophthalmitis. There were no cases of subsequent glaucoma or macular holes in any group. In terms of the incidence of complication, there were no statistically significant differences between the two groups (χ2 = 0.296, P = 0.587).
As a complication, DME can occur at any stage of DR. More than 70% of people with diabetes develop DME, making it the leading cause of visual impairment or loss of visual function in this population. Chronic hyperglycemia activates four classical oxidative stress pathways – the polyol pathway, increased advanced glycation end products, activation of protein kinase, and the hexosamine pathway. All these pathways result in retinal hypoxia, an increase in the concentration of reactive oxygen species, aggravated inflammatory response, and the up-regulation of various inflammatory and oxidative factors within the pathway; this ultimately destroys the blood-retinal barrier, resulting in augmented vascular osmolality[11]. Hyperglycemia also leads to a decrease in retinal blood flow, reduced oxygen levels, and increased autoregulatory response of retinal arterioles, resulting in dilation of the retinal capillaries and venules. These changes can amplify the hydrostatic pressure, which is detrimental to the capillaries. Simultaneously, a drop in retinal oxygen tension increases the production of VEGF and other osmotic factors, exaggerating microvascular leakage. Therefore, increased pressure within blood vessels and the augmented permeability of blood vessels result in an overall movement of water, ions, and large molecules from the inside of blood vessels to the surrounding tissues. The RPE’s pumping action can either reabsorb the extracellular fluid through retinal arteries downstream or allow it to reenter the retinal vasculature via the choroid[12].
Furthermore, in type 2 diabetes with DR, decreased subfoveal choroidal blood flow may contribute to the pathogenesis of DME. Studies have revealed that eyes with DME experience a greater decrease in choroidal blood flow compared to eyes without DME, suggesting that the retinal pigment epithelium and outer retina are relatively oxygen-deprived, leading to heightened permeability of the outer blood-retinal barrier[13]. Therefore, DME influences retinal and choroidal blood flow, which has a direct impact on the anatomy and function of the macula.
DME treatment has long been a topic of great interest in ophthalmology. Many countries and regions have produced guidelines for DME in recent years; of these, the guidelines issued by EURETINAA in 2017 are regarded as the most reliable and representative. According to the guidelines, anti-VEGF medication therapy offers promising outcomes as a first-line treatment for de novo DME[14]. However, a previous study noted that anti-VEGF medication did not work for 23% of DME patients (66/288)[15]. Additionally, anti-VEGF drug therapy requires repeated intravitreal injections, which becomes cumbersome for the patient[16]. As a result, other methods of treatment should take precedence.
Vitrectomy In contrast, PPV is regarded as an important cost-effective treatment for proliferative DR. This technique offers excellent postoperative success in DME patients with vitreous retraction, but the results are still unclear in those without vitreous retraction, hence PPV is still considered an early option for DME[17]. With advances in surgical devices and techniques, recent evidence supports the use of a combination of PPV and ILM peeling, demonstrating favorable outcomes in managing refractory DME without posterior vitreous detachment to help improve the visual acuity of patients. Nevertheless, in the absence of substantial traction following vitreous detachment, the impact of surgical intervention becomes inconsequential[18]. Since 2020, our department has performed almost 200 PPV procedures. In our clinical experience, PPV with ILM peeling effectively reduced the need for anti-VEGF treatment in patients who have undergone PPV, and improved patient outcomes. Therefore, we deemed it necessary to investigate the efficacy of this technique in patients with DME. Therefore, we evaluated postoperative changes in retinal and choroidal blood flow and macular morphology and function (in terms of postoperative BCVA, anti-VEGF drug use, alterations in retinal thickness, capillary density, choroidal thickness, choroidal vascular density, choroidal vascular index, and CME volume using OCT and OCTA) resulting from PPV in conjunction with ILM peeling in patients with DME.
Our findings also offer a new avenue for the prognostic value of assessing the ILM in the management of DME. We found that the CMT levels in both groups were similar before surgery, and these levels were significantly reduced in both groups at 1-month and 3-month postoperatively intervals. However, the stripping group exhibited significantly lower CMT levels compared to the control group during this period (P < 0.05). Likewise, the CME volume was significantly reduced postoperatively (at the 1- and 3-month follow-up), in both groups compared to preoperative levels, with the stripping group showing greater reduction in CME volumes compared to the control group. For both CMT and CME volume, the repeated ANOVA analysis revealed significant interaction effects between the two groups in terms of both group and (P < 0.05). By peeling the ILM, the residual fibroblasts on the surface of the posterior vitreous cortex and retina, as well as the scaffold of proliferative cells can be completely removed; this helps reduce the occurrence of a macular epiretinal membrane. Matsunaga et al[19] discovered that diabetic patients exhibited a thicker retinal ILM compared to individuals without diabetes, resulting in decreased oxygen diffusion from the vitreous body to the retina. Conversely, the removal of the retinal ILM may lead to a decrease in the thickness of the central macula. Our results also showed that the BCVA levels of the two groups were comparable before surgery, and both groups showed a decrease in BCVA compared to preoperative levels, with the stripping group demonstrating significantly lower BCVA than the control group. These findings concur with Ranno et al[20], who found that at the end of a 2-year follow-up of DME patients treated with PPV with or without ILM peeling, the average central macular thickness decreased significantly from 413.1 ± 84.4 µm to 291.3 ± 57.6 µm (P < 0.000). Also, the logMAR for BCVA improved over two years, from 0.6 ± 0.2 LogMAR to 0.2 ± 0.1 LogMAR (P < 0.000).
In our control group, retinal detachment occurred in two eyes and endophthalmitis occurred in four eyes, compared to only one instance of retinal detachment and two of endophthalmitis in the stripping group. None of the patients developed subsequent glaucoma or macular holes. Spinetta et al[21] discovered that DME patients had elevated levels of basic fibroblast growth factor and methylglyoxal in their retinal ILM, which enhanced the expression of VEGF. Therefore, removing the ILM can potentially decrease the concentration of VEGF within the ocular environment, thus relieving edema. Our research corroborates these findings as PPV in conjunction with ILM peeling reduced the intensity of macular edema in patients with diabetes.
PPV combined with ILM peeling aids in improving visual acuity, reducing CMT, and ameliorating macular edema in DME patients, with fewer complications.
Nevertheless, the study has certain limitations. Firstly, the strong adhesion between the macular retina and ILM makes peeling prone to cause mechanical injuries to the retina, such as retinal hemorrhage and membrane tears. Moreover, the direct toxic effect of the dye on the retina and its impact on postoperative BCVA recovery remains controversial. The small sample size and short follow-up period used in this study limit the applicability of our results and necessitate further research with extended follow-up to reliably evaluate the treatment’s efficacy and complications in DME.
| 1. | Munk MR, Somfai GM, de Smet MD, Donati G, Menke MN, Garweg JG, Ceklic L. The Role of Intravitreal Corticosteroids in the Treatment of DME: Predictive OCT Biomarkers. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 2. | Zhang J, Zhang J, Zhang C, Zhang J, Gu L, Luo D, Qiu Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 114] [Reference Citation Analysis (0)] |
| 3. | Guo J, Bi X, Chen SN, Chen S, He GH, Wu B, Zhang W, Wang J. Efficacy of internal limiting membrane peeling for diabetic macular edema after preoperative anti-vascular endothelial growth factor injection. Int J Ophthalmol. 2020;13:1758-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Liberski S, Wichrowska M, Kocięcki J. Aflibercept versus Faricimab in the Treatment of Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: A Review. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Rajkhowa S, Choudhury M, Pegu SR, Sarma DK, Hussain I. Development of a rapid loop-mediated isothermal amplification (LAMP) assay for visual detection of porcine parvovirus (PPV) and its application. Braz J Microbiol. 2021;52:1725-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Ferro Desideri L, Traverso CE, Nicolò M, Munk MR. Faricimab for the Treatment of Diabetic Macular Edema and Neovascular Age-Related Macular Degeneration. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 7. | Kim S, Park JS, Lee J, Lee KK, Park OS, Choi HS, Seo PJ, Cho HT, Frost JM, Fischer RL, Choi Y. The DME demethylase regulates sporophyte gene expression, cell proliferation, differentiation, and meristem resurrection. Proc Natl Acad Sci USA. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Barth T, Helbig H. Diabetic retinopathy. Klin Monbl Augenheilkd. 2021;238:1143-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Liu J. Diagnosis, prevention and treatment of diabetic eye disease. Jindun Chubanshe. 2013;. |
| 10. | Smiddy WE. Economic considerations of macular edema therapies. Ophthalmology. 2011;118:1827-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Hatamnejad A, Orr S, Dadak R, Khanani A, Singh R, Choudhry N. Anti-VEGF and steroid combination therapy relative to anti-VEGF mono therapy for the treatment of refractory DME: A systematic review of efficacy and meta-analysis of safety. Acta Ophthalmol. 2024;102:e204-e214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Guo H, Li W, Nie Z, Zhang X, Jiao M, Bai S, Duan N, Li X, Hu B. Microinvasive pars plana vitrectomy combined with internal limiting membrane peeling vs anti-VEGF intravitreal injection for treatment-naïve diabetic macular edema (VVV-DME study): study protocol for a randomized controlled trial. Trials. 2023;24:685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Barth T, Helbig H. Diabetic Macular Edema. Klin Monbl Augenheilkd. 2021;238:1029-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Ixcamey M, Palma C. Diabetic macular edema. Dis Mon. 2021;67:101138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Chan LKY, Lin SS, Chan F, Ng DS. Optimizing treatment for diabetic macular edema during cataract surgery. Front Endocrinol (Lausanne). 2023;14:1106706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 16. | Mansour SE, Browning DJ, Wong K, Flynn HW Jr, Bhavsar AR. The Evolving Treatment of Diabetic Retinopathy. Clin Ophthalmol. 2020;14:653-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 17. | Hunt M, Teper S, Wylęgała A, Wylęgała E. Response to 1-Year Fixed-Regimen Bevacizumab Therapy in Treatment-Naïve DME Patients: Assessment by OCT Angiography. J Diabetes Res. 2022;2022:3547461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Chi SC, Kang YN, Huang YM. Efficacy and safety profile of intravitreal dexamethasone implant vs antivascular endothelial growth factor treatment in diabetic macular edema: a systematic review and meta-analysis. Sci Rep. 2023;13:7428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 19. | Matsunaga N, Ozeki H, Hirabayashi Y, Shimada S, Ogura Y. Histopathologic evaluation of the internal limiting membrane surgically excised from eyes with diabetic maculopathy. Retina. 2005;25:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Ranno S, Vujosevic S, Mambretti M, Metrangolo C, Alkabes M, Rabbiolo G, Govetto A, Carini E, Nucci P, Radice P. Role of Vitrectomy in Nontractional Refractory Diabetic Macular Edema. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Spinetta R, Petrillo F, Reibaldi M, Tortori A, Mazzoni M, Metrangolo C, Gelormini F, Ricardi F, Giordano A. Intravitreal DEX Implant for the Treatment of Diabetic Macular Edema: A Review of National Consensus. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |