Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4419
Revised: May 8, 2024
Accepted: May 20, 2024
Published online: July 16, 2024
Processing time: 87 Days and 2.9 Hours
On June 30, 2021, China received certification from the World Health Organi
Herein, we present a case report of a 53-year-old Chinese man who worked in Africa for nearly two years. He was diagnosed with malaria in the Democratic Republic of the Congo between November 19 and November 23, 2022. After receiving effective treatment with oral antimalarial drugs, his condition impro
The successful treatment of this imported case of severe cerebral malaria provides a valuable reference for managing patients with similar malaria infections and has significant clinical implications.
Core Tip: China has achieved significant milestones in malaria prevention and control, earning recognition as a malaria-free nation by the World Health Organization. However, to safeguard against malaria resurgence and uphold this malaria-free status, China must prioritize the following malaria prevention and control efforts: (1) Surveillance and monitoring: Enhance the surveillance system for malaria cases, particularly in border regions and areas with high international mobility; (2) border area prevention and control: Strengthen collaboration and information exchange with neighboring countries, to prevent cross-border malaria transmission; (3) public education and awareness; (4) supporting malaria research; (5) international collaboration; and (6) climate change response. By implementing these measures, China can effectively prevent malaria resurgence and sustain its malaria elimination status.
- Citation: Zhu YF, Xia WJ, Liu W, Xie JM. Treatment of a patient with severe cerebral malaria during the COVID-19 pandemic in China: A case report. World J Clin Cases 2024; 12(20): 4419-4426
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4419.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4419
The world has made significant strides in combating malaria, with a 37% reduction in malaria incidence and a 60% reduction in malaria mortality between 2000 and 2015[1]. However, malaria remains a pressing public health issue, particularly in tropical regions, where malaria continues to claim millions of lives annually[2]. In China, between 2005 and 2014, a total of 228 malaria-related deaths were reported, with 203 (89.0%) attributed to plasmodium falciparum malaria[3]. Furthermore, from 2013 to 2018, a total of 763 imported cases of malaria were reported in Hubei Province, of which 69.2% (528/763) were caused by plasmodium falciparum malaria. Among these patients, three died from imported plasmodium falciparum malaria[4].
Cerebral malaria (CM) is a severe neurological syndrome and a grave complication of plasmodium falciparum infection as it has the potential to be fatal[5,6]. Globally, CM predominantly affects African children and Asian adults, with over 90% of cases occurring in children aged five years and under in sub-Saharan Africa. This demographic has the highest risk and has a mortality rate ranging from 10% to 20%[7,8]. The world has invested substantial public health resources in the pursuit of eliminating malaria altogether[1]. Currently, the pathogenic mechanism of severe CM remains unclear[6,9,10].
During the Coronavirus disease 2019 (COVID-19) pandemic in January 2023, in Huangshi, China, the Department of Intensive Care Medicine at our hospital achieved a successful treatment outcome in a critical case of CM. The patient's comprehensive data, diagnosis, and treatment were carefully analyzed to address the lack of relevant treatment data pertaining to this severe condition. The objective of this study was to collaboratively enhance the success rate of treating patients with critical CM in China.
A 53-year-old male, was admitted to the hospital on January 12, 2023, presenting with a 3-d history of fever and one day of jaundice accompanied by dark, "soy sauce" colored (deep brown) urine.
Three days prior to admission, the patient experienced intermittent fever without any identifiable triggers. The specific BT and heat potential during this time were not recorded. The patient also reported experiencing chills, a small amount of sweating, altered consciousness, and convulsions. However, symptoms such as cough, sputum production, chest tightness, or shortness of breath were not apparent.
One day prior to admission, the patient's skin and sclera demonstrated yellow discoloration, and his urine output decreased while becoming dark, similar to soy sauce. The patient had previously received treatment for malaria at the "Huangshi Infectious Disease Hospital" on January 11 and was subsequently transferred to our hospital's emergency department on January 12 upon testing positive for Plasmodium. Finally, the patient was admitted to the infectious disease department with a diagnosis of "malaria".
Following illness onset, the patient has exhibited clear consciousness but has reported low spirits, fatigue, poor appetite, and difficulty sleeping. The patient’s urine continued to be dark with a soy sauce-like appearance, while the patient’s bowel movements remained normal.
The patient's medical history included a history of tuberculosis in 2002, for which the details of the treatment were unknown. Moreover, there was a history of urinary calculi in 2010, which improved with medical intervention. The patient had been working in Africa for nearly two years until November 23, 2022, when he was diagnosed with malaria in the Democratic Republic of the Congo. After receiving antimalaria treatment with oral quinine sulfate tablets and showing improvement, the patient returned to China. The patient did not have a medical history of hypertension, diabetes, coronary heart disease, or hepatitis B.
The patient's wife is employed locally in Huangshi, while their child is attending university in China. Neither family member had a history of malaria infection in China.
On January 12, the patient was admitted to the infection department for a comprehensive physical examination. The recorded vital signs were as follows: BT: 37.5 °C; pulse rate (PR): 114 beats/min; respiratory rate (RR): 18 breaths/min: Noninvasive blood pressure (NIBP): 120/60 mmHg; and oxygen saturation (SpO2): 98% without supplemental oxygen. The patient exhibited clear consciousness but reported mental fatigue.
Upon examination, the patient presented with a soft neck without resistance. The skin and sclera of the entire body displayed moderate yellow discoloration, while the patient’s lips appeared red. Their breathing was stable, and both lungs had clear breath sounds with dry and wet rales. The patient’s heart rate (HR) was measured at 114 beats per min, and no murmurs associated with heart valve abnormalities were detected. The abdomen was flat and soft, with no tenderness or rebound pain. Furthermore, palpation of the liver, spleen, and subcostal areas did not reveal any abnor
However, at approximately 5:00 am on January 13 (the night after admission), the patient experienced a decline in consciousness, respiratory distress, occasional hiccups and tics, and bilateral dilated pupils with a dull light reflex. The neck remained soft without resistance, and bilateral pathological signs were absent. In response to this deterioration of the patient's condition, the infection department promptly transferred the patient to the intensive care unit (ICU).
The patient’s physical examination in the ICU was as follows: BT: 37.4 °C; PR: 100 beats/min and regular; RR: 40 breaths/min and characterized by shallow and rapid breathing; NIBP: 95/50 mmHg; and SpO2: 95% using an oropharyngeal ventilation tube with mask oxygen delivery at a rate of 5 L/min. Coma severity was assessed using the Glasgow Coma Scale (GCS) and the patient had a score of 3 (E1V1M1). Binocular fixation was present and the bilateral pupils were large and round in size, measuring at 2 mm and exhibiting a dull light reflex. The patient had a soft neck without resistance. Moderate yellow staining of the skin and sclera was observed, with the absence of petechiae. Tachypnea was observed without obvious signs of three concave appearances. Thick breathing sounds were noted in both lungs and minimal moist rales were detected during the inspiratory phase in both lower lungs. The patient’s HR was recorded at 100 beats/min, with no audible heart valve murmurs or abnormal sounds detected upon auscultation of all heart valves. Slight abdominal distension was observed, without tenderness or rebound pain. No palpable abnormalities of the liver, spleen, or ribs were detected. Murphy's sign was absent. Percussion pain in the kidney area was also absent. No lower limb edema was observed, low muscle tension was observed in the limbs, and the patient was negative for Bru's sign and the Babinski reflex.
On January 13, routine blood analysis revealed a white blood cell count of 8.88 × 109/L, a neutrophil percentage of 79.5%, a hemoglobin concentration of 77 g/L, a platelet count of 26 × 109/L, a hypersensitive C-reactive protein level of 266.79 mg/L, and a procalcitonin level exceeding 50 ng/mL.
Liver function assessment revealed a total bilirubin concentration of 92.1 μmol/L, a direct bilirubin concentration of 48.4 μmol/L, an indirect bilirubin concentration of 43.7 μmol/L, and an alanine aminotransferase concentration of 29.8 μmol/L.
Renal function evaluation revealed a urea nitrogen concentration of 21.56 µmol/L, a serum creatinine concentration of 222.69 μmol/L, and a serum uric acid concentration of 458 μmol/L. The myocardial enzyme profile indicated a lactate dehydrogenase level of 811 U/L and a hydroxybutyrate dehydrogenase level of 759 U/L.
Coagulation function analysis revealed a D-dimer level of 3.82 mg/L and a Neuron-Specific Enolase level of 38.84 ng/mL. The first blood gas analysis demonstrated the following results: pH: 7.51; PaCO2: 22 mmHg; PaO2: 132 mmHg; lactic acid concentration: 3.8 mmol/L; and hemoglobin level: 63 g/L.
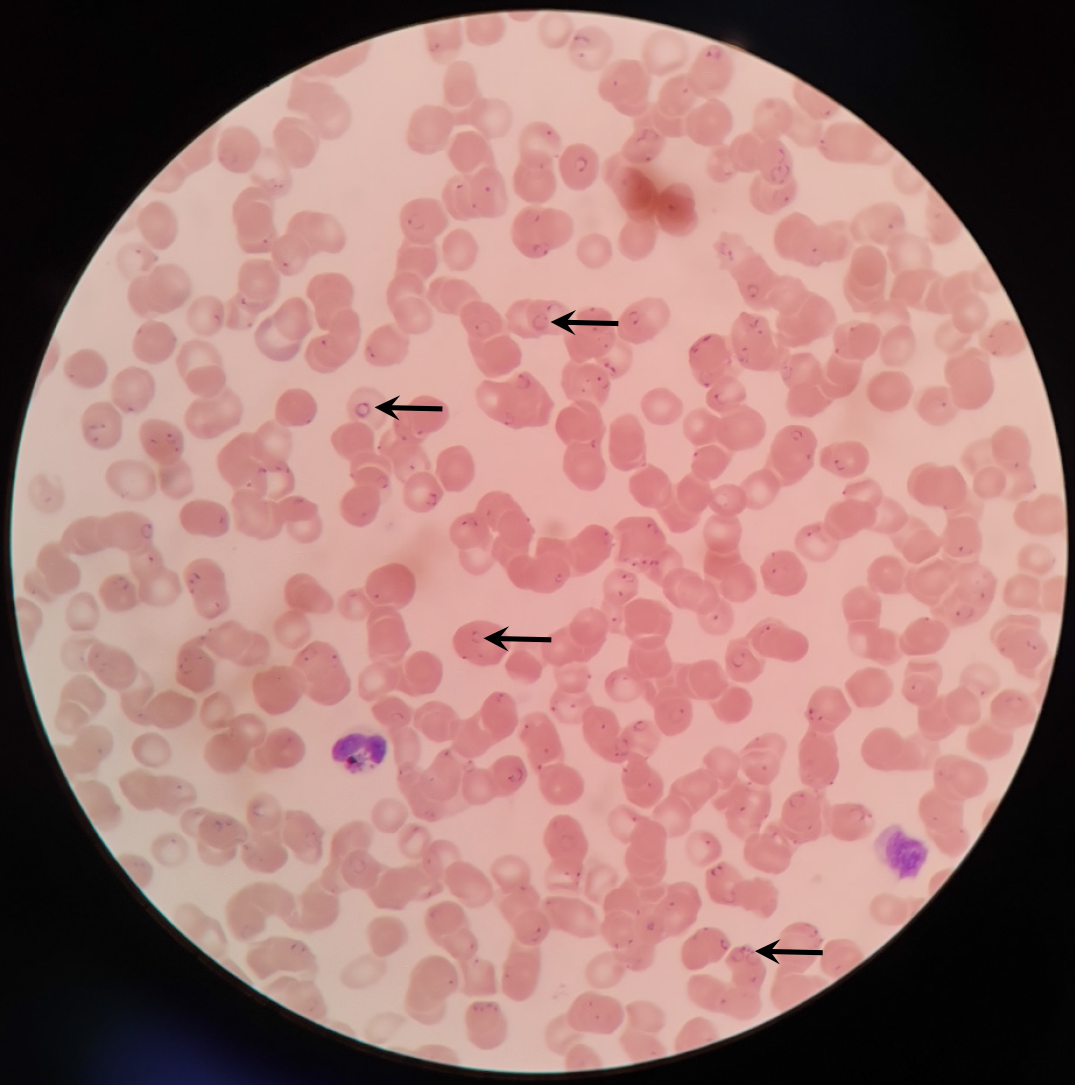
A blood smear showed a positive result for plasmodium falciparum infection (Figure 1). A brain computed tomography scan showed no abnormalities, but re-examination was recommended.
The patient was initially diagnosed with plasmodium falciparum malaria. The specific complications identified were CM, septic shock, level 3 acute kidney injury (AKI), secondary thrombocytopenia (a severe decrease in platelet count), acute liver insufficiency, and moderate anemia (hemolytic anemia).
The prescribed treatment included anti-malaria medication, specifically intravenous injections of artesunate at a dose of 120 mg per administration (given at 0, 12, and 24 h), liver protection treatment using magnesium isoglycyrrhizinate, clindamycin for infection management, and nutritional support.
At approximately 8:00 am on January 13, 2023, the patient was transferred to the ICU. Rapid tracheal intubation was performed, along with ventilator-assisted breathing to ensure airway protection and respiratory support.
Throughout the treatment period, the patient underwent tracheoscopic sputum aspiration and irrigation once to assess and address any inhalation or airway issues. The patient's lung imaging revealed that the lung infection was not severe, and bronchoscopy indicated no apparent abnormalities in the airway at any level. As a result, the oxygenation index, measured after mechanical ventilation, exceeded 300 mmHg. Despite this improvement, the patient remained in a deep coma.
To safeguard the patient's airway, percutaneous tracheotomy was performed on the 6th day following endotracheal intubation. Subsequently, the patient was successfully weaned off the invasive ventilator and transitioned to nasal high-flow tracheotomy catheter sequential oxygen therapy. This therapy involved maintaining a temperature of 37 °C, delivering oxygen at a fraction of inspired oxygen (FiO2) of 30% and a flow rate of 35 L/min. Blood gas analysis consistently revealed an oxygenation index above 300 mmHg. Over time, the FiO2 was gradually reduced to 25%, and the flow rate was reduced to 30 L/min. The patient's RR and blood SpO2 normalized, allowing gradual improvement in coughing and sputum clearance.
Following these interventions, the patient's blood gas levels were as follows: pH: 7.37; PaCO2: 35 mmHg; PaO2: 99 mmHg; HCO3-: 25.3 mmol/L; and base excess: 0.3 mmol/L. On January 21, 2023, the patient regained consciousness, demonstrating the ability to communicate fully and cooperate with movements. Consequently, on January 23, 2023, the patient was successfully discontinued from high-flow oxygen therapy and transitioned to low-flow oxygen inhalation through a nasal catheter.
Within the central nervous system, Xingnaojing was administered to promote arousal, while citicoline was administered to nourish the patient’s brain cells. Bedside ultrasound was utilized to dynamically observe and measure the width of the optic nerve sheath, as well as the middle cerebral artery pulse index and resistance index. These measurements assisted in assessing intracranial pressure. Early mannitol dehydration was implemented to reduce intracranial pressure, and dexamethasone was administered to protect the patient’s brain cells. Midazolam was given for sedation and to control hiccups and twitching. Temperature control blankets were used to regulate the patient’s BT in the event of fever. Nonsteroidal drugs were also administered as antipyretic and analgesic agents when the patient’s BT exceeded 38.5 °C. Additionally, traditional Chinese medicine treatments, such as Angong Niuhuang Pill and rhinoceros Dihuang Decoction, were used for nasal feeding and brain rejuvenation.
During episodes of shock, the patient’s cardiopulmonary function and volume status were continuously evaluated using bedside ultrasound. Guided by ultrasound, a right internal jugular vein catheter was successfully inserted despite severe thrombocytopenia. Neutral venous pressure was monitored, and rapid fluid resuscitation was performed. Norepinephrine was administered to maintain the patient’s blood pressure, while Sufentanil provided sufficient analgesia. Dexmedetomidine and midazolam were administered appropriately for sedation. Blood pressure was maintained within the range of 110-120/60-75 mmHg. The Chinese patent medicine Angong Niuhuang Pill, was used to alleviate fever and facilitate coma arousal. Immunoglobulin (20 g) was administered for three consecutive days as an impact immunotherapy. Enteral nutrition powder and enteral nutrition emulsion were administered alternately to enhance intestinal motility through enemas and moxapide citrate tablets.
Active communication and coordination took place with the clinical laboratory and blood transfusion department. Due to the shortage of blood resources during the COVID-19 pandemic, platelets were transferred from Wuhan for transfusion. The platelets were treated with recombinant human thrombopoietin at various concentrations under the supervision of competent authorities. Additionally, plasma transfusion was performed to address hypoproteinemia, and white suspended red blood cells were transfused to treat for anemia.
The antiplasmodium artesunate was intravenously injected at a dosage of 120 mg per administration (given at 0, 12, and 24 h), followed by daily administration at 120 mg for 7 d after the initial loading dose by the infection department. Subsequently, artesunate amodiaquine tablets were administered, with 1 tablet taken twice daily for 7 d, until Plasmodium microscopy showed negative results.
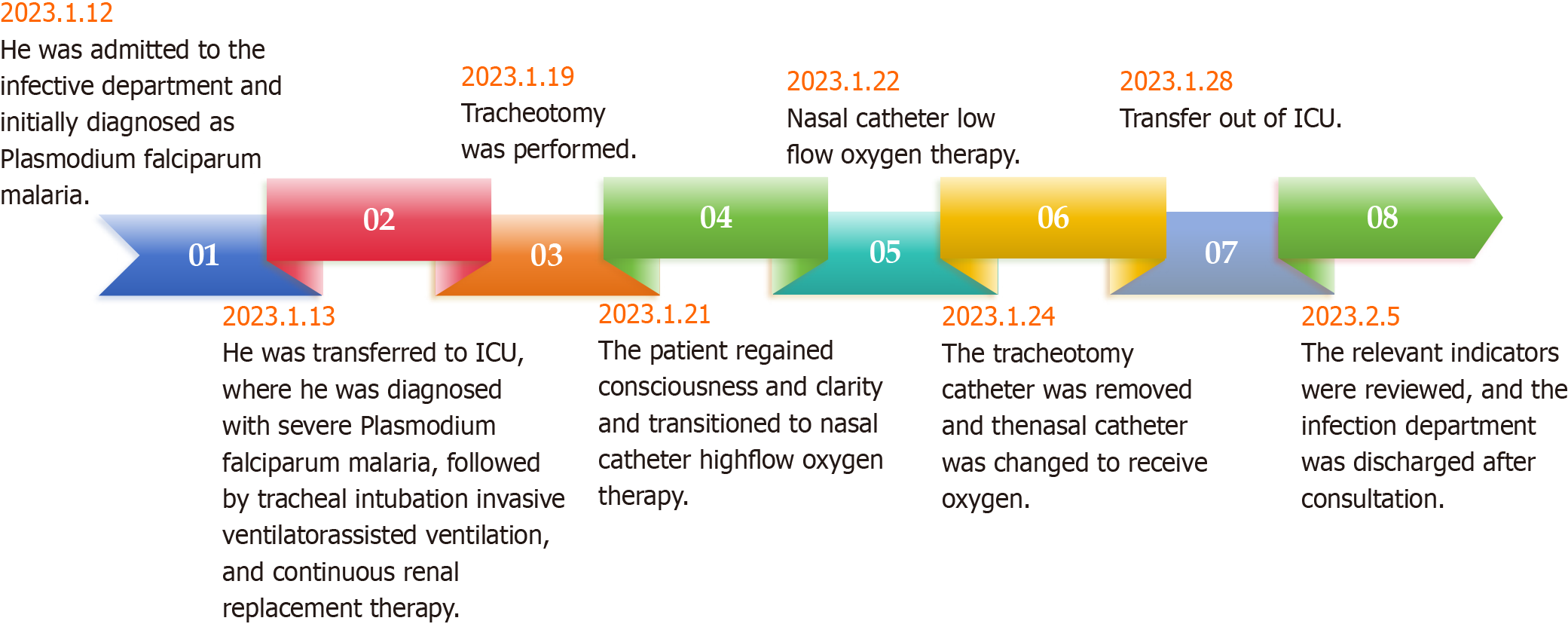
Following the comprehensive treatment and medical interventions mentioned above, the patient regained normal consciousness on January 21, as indicated by a GCS score of 10T (E4VTM6). On January 22, the patient was successfully weaned off the invasive ventilator and transitioned to nasal high-frequency oscillatory oxygen therapy. On January 24, the pneumatectomy catheter was removed, and the nasal catheter was switched to deliver oxygen inhalation. On January 28, the patient was transferred from the ICU to a common ward. On February 5, a comprehensive evaluation of relevant indicators was conducted. After consultation with the infection department, the patient was deemed eligible for discharge (Figure 2). After follow-up, it was determined that the patient had fully recovered.
CM is considered the most severe complication associated with plasmodium falciparum malaria; it presents as a comprehensive nervous system disorder, leading to unconsciousness and even life-threatening conditions, and has recently become a significant public health burden[11,12]. Despite these advances, the exact underlying mechanisms of CM remain unclear. Although antimalarial drugs can eliminate the parasite, there is currently no effective treatment available to prevent or ameliorate severe malaria or its long-term consequences[13]. Many survivors experience severe neurological issues that can persist for a lifetime, such as epilepsy and neurocognitive deficits[14,15]. For nervous system injuries, we administer a specific Chinese patent medicine called Angong Niuhuang Pill as adjunctive therapy for brain disorders. Angong Niuhuang Pill, a traditional Chinese remedy comprising ingredients such as bezoars, concentrated buffalo horn powder, and Scutellaria, is traditionally used as an emergency medicine for acute and severe conditions. It can mitigate cerebral nerve damage and is utilized as an auxiliary treatment for conditions such as cerebral infarction, cerebral hemorrhage, and other brain disorders[16,17].
Successful management of severe malaria entails maintaining a high index of suspicion, ensuring prompt diagnosis, and administering appropriate antimalarial therapy at the earliest stage[18]. The onset of CM is highly perilous, with a considerable mortality rate. Swift diagnosis and timely intervention can significantly alleviate patient suffering, enhance the likelihood of recovery, and reduce the likelihood of long-term complications. Simple and convenient diagnostic tests, such as thin blood smears, can markedly enhance the accuracy of diagnosis while also offering prognostic insights for severe malaria patients[19]. Severe malaria can give rise to a broad spectrum of syndromic conditions encompassing impaired consciousness, acidosis, hypoglycemia, severe anemia, AKI, jaundice, pulmonary edema, massive bleeding, or shock[20]. In this particular case, each symptom was managed individually, accompanied by a comprehensive evaluation of the patient's physical indicators.
The patient received anti-malaria treatment primarily through the administration of artesunate injections and oral artesunate amodiaquine tablets, which yielded positive treatment outcomes without any adverse reactions. Artesunate is effective in eradicating malaria parasites. In patients with severe malaria, intravenous artesunate is recommended as a first-line therapy. Early initiation of artesunate, along with broad-spectrum antibiotics for suspected severe malaria, can significantly reduce malaria-related mortality[21,22].
To treat accompanying bacterial infections (anti-infection treatment) in severe malaria patients, it is important to note that C-reactive protein and procalcitonin levels may increase. In such cases, the routine use of antibacterial drugs should be avoided. Instead, close clinical monitoring is required to promptly identify secondary bacterial infections. Empirical administration of antibacterial drugs may be necessary when indicated[23]. After admission, the patient presented with symptoms such as soy-sauce-colored urine, anuria, and kidney injury, which are indicative of hemolytic uremic syndrome. Consequently, hemofiltration/dialysis was employed to remove excess fluid and toxins, alleviate acidosis, lower the patient’s BT, and reduce oxygen consumption with the goal of improving AKI. Severe thrombocytopenia poses an early treatment challenge and is a commonly encountered hematological abnormality in patients in ICU[24].
Using bedside ultrasound guidance, successful placement of a central venous catheter in the right internal jugular vein was achieved for the purpose of monitoring central venous pressure, administering fluid rehydration, and delivering vasoactive drugs. Moreover, employing bedside ultrasound guidance facilitated the successful insertion of a dialysis catheter into the right femoral vein while concurrently administering platelet supplementation. This approach effectively addressed the patient’s severe thrombocytopenia, providing valuable time and opportunities for continuous renal replacement therapy (CRRT) treatment. This therapy involves the use of hemofiltration/dialysis to eliminate excess fluid and toxins, correct acidosis, and improve hemodynamics by reducing the patient’s BT and oxygen consumption. The successful execution of CRRT is also a crucial component of the overall treatment process.
This patient experienced septic shock induced by severe CM, accompanied by level 3 AKI, secondary thrombocytopenia (severe), acute liver insufficiency, and moderate (hemolytic) anemia. However, prompt detection of CM, targeted antimalarial therapy, and comprehensive initial medical interventions ensured successful treatment. The administration of Angong Niuhuang Pill can mitigate brain nerve injury and safeguard patients against conditions such as cerebral infarction and cerebral hemorrhage.
This case report serves as a valuable addition to the limited resources available for the treatment of critically ill malaria patients, aiming to increase the overall treatment success rate of this vulnerable patient population. Simultaneously, it is recommended that we prioritize strengthening health education and public awareness campaigns regarding malaria prevention for individuals traveling to and from Africa. Implementing effective preventive measures, enhancing prevention awareness, and ensuring prompt detection and timely treatment are essential steps to optimize medical staff's efficacy in managing patients, ultimately improving treatment outcomes.
Thanks to the collaborative efforts of all colleagues in the Department of Critical Care Medicine at Huangshi Hospital of Traditional Chinese Medicine (Infectious disease hospital), including the research team led by Associate Professor Ju-Min Xie from the Hubei Key Laboratory of Kidney Disease Occurrence and Intervention, for their invaluable guidance on the paper.
| 1. | Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, Fergus CA, Knox T, Lynch M, Patouillard E, Schwarte S, Stewart S, Williams R. Malaria: Global progress 2000 - 2015 and future challenges. Infect Dis Poverty. 2016;5:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Luzolo AL, Ngoyi DM. Cerebral malaria. Brain Res Bull. 2019;145:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Zhang Q, Geng QB, Sun JL, Zhang ZK, Lai SJ, Zhou S, Li ZJ. [Epidemiological analysis of the deaths of malaria in China, 2005-2014]. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Xia J, Wu D, Wu K, Zhu H, Sun L, Lin W, Li K, Zhang J, Wan L, Zhang H, Liu S. Epidemiology of Plasmodium falciparum Malaria and Risk Factors for Severe Disease in Hubei Province, China. Am J Trop Med Hyg. 2020;103:1534-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Li JL, Li K, Guo Y, Zhao Z, Chen LN, Jiang XH, Li YJ, Tu Y, Zheng XJ. [Current advance in cerebral malaria]. Zhongguo Zhong Yao Za Zhi. 2017;42:4548-4555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Song X, Wei W, Cheng W, Zhu H, Wang W, Dong H, Li J. Cerebral malaria induced by plasmodium falciparum: clinical features, pathogenesis, diagnosis, and treatment. Front Cell Infect Microbiol. 2022;12:939532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Postels DG, Birbeck GL. Cerebral malaria. Handb Clin Neurol. 2013;114:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Milner DA Jr. Malaria Pathogenesis. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 9. | Purohit D, Kumar S, Dutt R, Bhardwaj TR. An Update on Recent Advances for the Treatment of Cerebral Malaria. Mini Rev Med Chem. 2022;22:1607-1618. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Sahu PK, Mohanty S. Pathogenesis of Cerebral Malaria: New Trends and Insights for Developing Adjunctive Therapies. Pathogens. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Wassmer SC, Grau GE. Severe malaria: what's new on the pathogenesis front? Int J Parasitol. 2017;47:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Hadjilaou A, Brandi J, Riehn M, Friese MA, Jacobs T. Pathogenetic mechanisms and treatment targets in cerebral malaria. Nat Rev Neurol. 2023;19:688-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 13. | Brejt JA, Golightly LM. Severe malaria: update on pathophysiology and treatment. Curr Opin Infect Dis. 2019;32:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Nortey LN, Anning AS, Nakotey GK, Ussif AM, Opoku YK, Osei SA, Aboagye B, Ghartey-Kwansah G. Genetics of cerebral malaria: pathogenesis, biomarkers and emerging therapeutic interventions. Cell Biosci. 2022;12:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Schiess N, Villabona-Rueda A, Cottier KE, Huether K, Chipeta J, Stins MF. Pathophysiology and neurologic sequelae of cerebral malaria. Malar J. 2020;19:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Liu H, Yan Y, Pang P, Mao J, Hu X, Li D, Zhou B, Shan H. Angong Niuhuang Pill as adjuvant therapy for treating acute cerebral infarction and intracerebral hemorrhage: A meta-analysis of randomized controlled trials. J Ethnopharmacol. 2019;237:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Hui X, Wang Y, Lu X. Angong Niuhuang Pill ameliorates cerebral ischemia/reperfusion injury in mice partly by restoring gut microbiota dysbiosis. Front Pharmacol. 2022;13:1001422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 18. | Sarkar PK, Ahluwalia G, Vijayan VK, Talwar A. Critical care aspects of malaria. J Intensive Care Med. 2010;25:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | White NJ. Severe malaria. Malar J. 2022;21:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 20. | Varo R, Crowley VM, Sitoe A, Madrid L, Serghides L, Kain KC, Bassat Q. Adjunctive therapy for severe malaria: a review and critical appraisal. Malar J. 2018;17:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Daily JP, Minuti A, Khan N. Diagnosis, Treatment, and Prevention of Malaria in the US: A Review. JAMA. 2022;328:460-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 22. | Lalloo DG, Shingadia D, Bell DJ, Beeching NJ, Whitty CJM, Chiodini PL; PHE Advisory Committee on Malaria Prevention in UK Travellers. UK malaria treatment guidelines 2016. J Infect. 2016;72:635-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 23. | Plewes K, Turner GDH, Dondorp AM. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis. 2018;31:69-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Thachil J, Warkentin TE. How do we approach thrombocytopenia in critically ill patients? Br J Haematol. 2017;177:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |