Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4405
Revised: May 23, 2024
Accepted: June 3, 2024
Published online: July 16, 2024
Processing time: 84 Days and 16.7 Hours
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) significantly improve the survival of patients with Epidermal growth factor receptor (EGFR) sensitive mutations in non-small cell lung cancer (NSCLC).
A 67-year-old female patient in advanced lung adenocarcinoma suffered from drug resistance after EGFR-TKIs treatment. Secondary pathological tissue biopsy confirmed squamous cell carcinoma (SCC) transformation. Patients inevitably encountered drug resistance issues after receiving EGFR-TKIs treatment for a certain period of time, while EGFR-TKIs can significantly improve the survival of patients with EGFR-sensitive mutations in NSCLC. Notably, EGFR-TKIs resis
Squamous cell carcinoma transformation is one of the acquired resistance mechanisms of EGFR-TKIs in advanced lung adenocarcinoma with EGFR mutations.
Core Tip: Patients inevitably encounter drug resistance after receiving epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) treatment. EGFR-TKIs resistance included primary and acquired. Pathological transformation is one of mechanism of acquired resistance in EGFR-TKIs, with squamous cell carcinoma (SCC) transformation being relatively rare. This results provide more detailed results of the patients’ diagnosis and treatment process, alongside a review of literature on SCC transformation after EGFR-TKIs treatment for lung adenocarcinoma.
- Citation: Ji XZ, Liu ZD, Ye YP, Li Q, Liu XJ, Zhou MH, Jin Y. Advanced Lung Adenocarcinoma with EGFR 19-del Mutation Transformed into SCC after EGFR-tyrosine Kinase inhibitors Treatment: A Case report. World J Clin Cases 2024; 12(20): 4405-4411
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4405.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4405
Lung cancer (LC) is the primary malignant tumor with the highest incidence rate and mortality of cancer worldwide, which seriously endangers human health. According to global cancer data, the number of new lung cancer cases reached 2.207 million in 2022, ranking second among all cancer cases. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) significantly improve the survival of non-small cell lung cancer (NSCLC) patients with EGFR-sensitive mutations, but most patients develop tumor progression due to EGFR-TKI resistance. Pathological transformation is one of the rare mechanisms of EGFR-TKIs resistance, which including small cell carcinoma transformation, squamous cell carcinoma (SCC) transformation[1-3].
This case report presents a patient with advanced EGFR 19-del mutation lung adenocarcinoma that transformed into squamous cell lung cancer after treatment with EGFR-TKIs.
A 67-year-old woman was treated in the Pulmonary Disease Department of Lishui Traditional Chinese Medicine Hospital affiliated with Zhejiang University of Chinese Medicine (Zhejiang, China) due to cough and phlegm on September 16, 2020.
The patient presented with cough and sputum production 5 days prior to admission. After arrival, the patient expe
The patient with no smoking history, who occasionally experienced chest pain, difficulty breathing, and frequent coughing and phlegm accumulation before admission.
The patient’s personal and family history was unremarkable.
Initial vital signs were as follows: blood pressure, 112/80 mmHg; pulse rate, 125 beats per min; breathing rate, 60 breaths per min; and body temperature 38.1 °C. The female patient has flushed cheeks, turbulent nostrils, and rapid breathing. He keeps coughing and has a lot of phlegm.
Further immunohistochemical analysis was performed on patient tissues, including thyroid transcription factor-1 (TTF-1), Napsin A, cytokeratin 5/6, P40, and AE1AE3. Based on the results, it is preliminarily determined that the patient has lung adenocarcinoma.
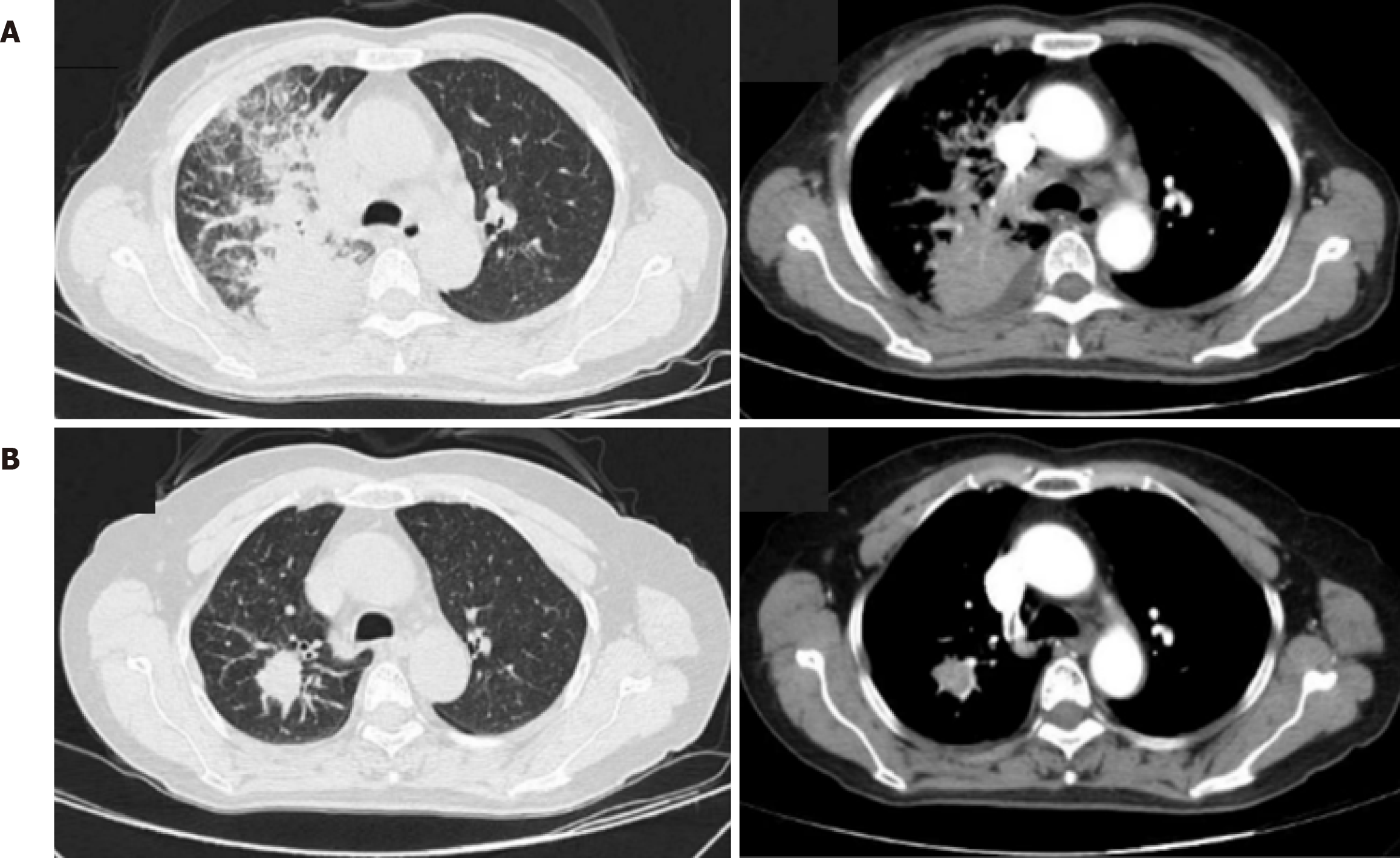
Chest computed tomography (CT) plain scan showed bronchial lesions with infectious lesions in both lungs (especially in the upper lobe of the right lung). A larger shadow in the right lung hilum, enlarged lymph nodes in the mediastinum, and pleural effusion on the right side were observed. Complete chest CT enhancement was conducted on September 17, 2020.The results showed a right lung hilum mass, right lung obstructive pneumonia, enlarged lymph nodes in the hilum and mediastinum, and right pleural effusion (Figure 1).
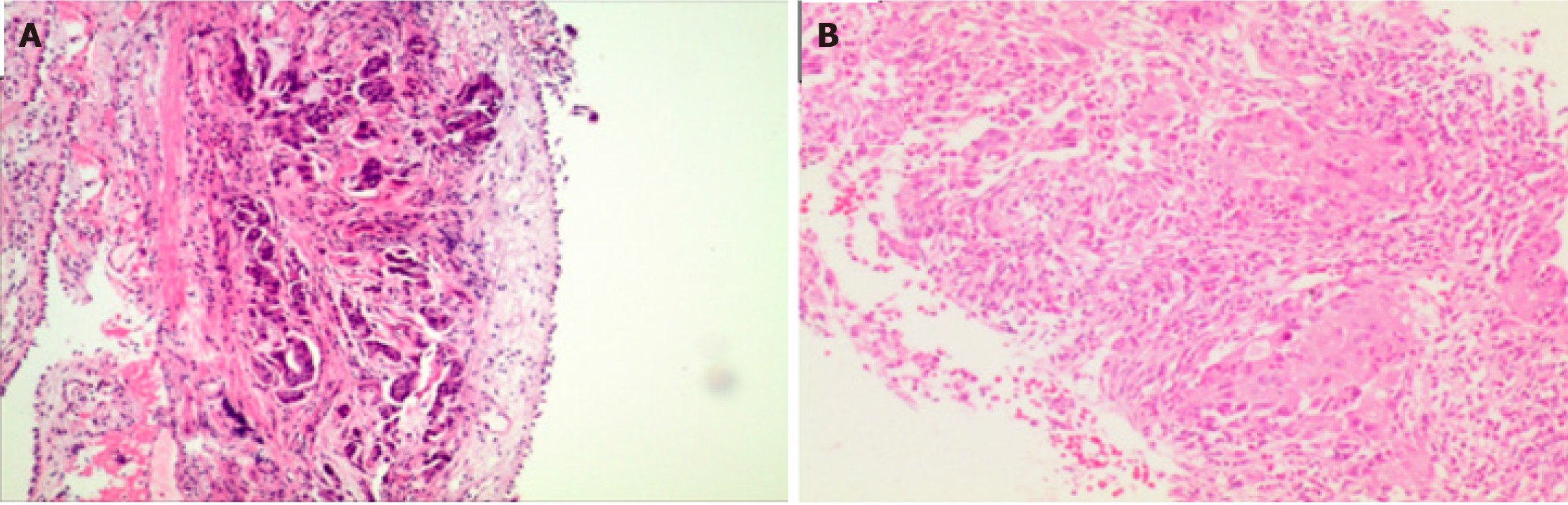
Bronchoscopy was performed on September 17, 2020, and the histopathological examination (Z202004957) (Figure 2) showed poorly differentiated carcinoma (right upper lobe bronchoscopy biopsy). On September 20, 2020, Histological genetic testing (Pathology Nos. Z202004957, FZ20200424) showed EGFR exon 19-del mutation.
Subsequently, a multidisciplinary expert group composed of MDT held a comprehensive meeting to discuss the issue. The patient was ultimately diagnosed with advanced lung adenocarcinoma with EGFR 19-del mutation.
The patient was targeted therapy with gefitinib (Yiruisha) 250 mg/d was administered starting from September 25, 2020. A follow-up CT scan of the chest and abdomen revealed enhanced pleural effusion on the right side on December 2021. Abnormally, bone signals in the right femoral head and left acetabulum suggested tumor metastasis. Peripheral blood gene testing showed no mutation in EGFR 20 exon T790M. From January 6, 2022 to April 14, 2022, a four course chemotherapy regimen consisting of pemetrexed, carboplatin, and bevacizumab was administered. The efficacy evaluation after the second course was stable. Evaluation of therapeutic effect after the fourth course showed progression (brain metastasis). Then the patient went to Lishui Central Hospital for combined cranial radiotherapy. The chemothe
The patient refused to continue intravenous chemotherapy targeted therapy with 80 mg/d fumetinib combined with 10 mg/d anlotinib was used from June 5, 2022. Whole-body bone imaging was performed at Lishui Central Hospital on December 24, 2022. T2-6 vertebral body and left acetabular bone metabolism were abnormal, and the vertebral body was a newly added lesion with possible metastasis. Chest magnetic resonance imaging plain scan showed abnormal signal in T1-6 vertebral body, with possible metastasis. Subsequently, combined with thoracic spine radiation therapy, the patient continued to receive targeted treatment with vormetinib and anlotinib. Chest enhanced CT scan was performed on October 7, 2023 (Figure 1). There were changes in the right upper lobe lung cancer with multiple thoracic vertebral metastases after treatment, compared to 14 new irregular nodules in the right upper lobe on March 14, 2023. Percu
Based on the latest lung puncture pathology and immunohistochemistry results, it was considered that EGFR-TKIs resistance may occur due to transformation to SCC. Therefore, the patient was transferred to a higher level hospital for further intervention treatment.
EGFR-TKIs are the first-line treatment for patients with advanced NSCLC with EGFR gene mutations, which can significantly improve the survival prognosis of EGFR-sensitive mutation advanced NSCLC patients. However, resistance to EGFR-TKIs is an unavoidable clinical challenge. EGFR-TKI resistance usually included primary and acquired resistance. The former refers to the tumor not responding to initial treatment, or disease progression occurring within 3 months of receiving EGFR-TKIs treatment; the latter refers to the tumor being initially sensitive to EGFR-TKIs treatment, but progressing in subsequent treatments[4]. Pathological transformation is one of the acquired resistance mechanisms of EGFR-TKIs, and about 15% of EGFR-TKIs-treated patients develop resistance due to tissue transformation, such as small cell carcinoma or SCC[5,6]. At present, research on the pathological transformation after EGFR-TKIs treatment is mostly focused on small cell carcinoma transformation, and there were few reports on the transformation of SCC.
We summarized the clinical case data, and found that the majority of patients with lung adenocarcinoma who developed SCC transformation after EGFR-TKIs treatment. Most of them were females, accounting for 82%[7]. 41% of patients with a history of smoking were non-current smokers. The time of transformation to SCC often occurs within 1 year after EGFR-TKIs treatment. The median survival of patients with SCC is only 3.5 months. The patient in this case was an elderly female with no smoking history and an EGFR 19-del mutation in advanced lung adenocarcinoma. She was given first-line targeted treatment with the first-generation EGFR-TKIs gefitinib, resulting in progression-free survival (PFS) of 15 months. After progression, peripheral blood testing showed negative T790M. She was switched to second-line treatment of pemetrexed combined with bevacizumab chemotherapy, resulting in PFS of 5 months, and the optimal therapeutic effect was stable. Third-line treatment with docetaxel combined with Sintilimab Injection stopped chemo
The mechanism of acquired drug resistance in lung cancer EGFR-TKIs included three different levels: molecular, cellular, and pathological. There are few reports on the mechanism of SCC transformation in lung adenocarcinoma treated with EGFR-TKIs[8]. The studies of genetically engineered mouse models provide some in vivo experimental evidence for the transformation of SCC[9-13]: Inactivation mutations of liver kinase B1 (LKB1) can lead to more malignant potential and distant metastasis ability of lung adenocarcinoma. At the same time, metabolic reorganization and excessive accumulation of reactive oxygen species in the body caused by LKB1 mutations promote the transformation of adenocarcinoma into SCC. When adenocarcinoma progresses due to drug resistance, the extracellular matrix is relatively in-sufficient, which prevents the activation of yes associated protein, which acts as a barrier for the transformation of adenocarcinoma to SCC, ultimately promoting the process of adenocarcinoma to SCC. At present, the transformation and drug resistance of SCC in lung adenocarcinoma have been discovered and confirmed, which is also one of the possible mechanisms of drug resistance in this patient[14]. However, reviewing the treatment process of this case, two patho
There were some shortcomings in this report. Firstly, both pathological tissue biopsies were small biopsy specimens. Due to the insufficient amount of tissue obtained and the heterogeneity of lung cancer histology and biology, small biopsy specimens have certain limitations in the pathological diagnosis of lung cancer, and there are shortcomings in the determination of the cellular composition of the entire tumor tissue[19]. Secondly, after the initial generation of EGFR-TKIs developed drug resistance, they did not undergo a timely second biopsy to obtain tissue, but instead chose circulating tumor DNA (ct-DNA) as a supplement. Compared with tissue samples, peripheral blood ct-DNA samples have slightly lower sensitivity and have certain limitations in identifying the acquired resistance types of EGFR-TKIs[20]. Once again, after pathological transformation, there was no further genetic testing to evaluate gene mutations. Given the data from SCLC transformation, approximately 95% of patients retain the initial EGFR mutation state when histopathological transformation occurs[21]. Therefore, gene mutations before and after transformation can guide treatment and help determine the presence of a secondary primary tumor.
In addition, the abnormal elevation of serum tumor markers has a clear correlation with the pathological type of lung cancer, which has reference value for the diagnosis of pathological type of lung cancer[22,23]. Serum neuron-specific enolase (NSE) and gastrin releasing peptide precursor (pro-GRP) are associated with neuroendocrine function and are specific molecular biological markers for SCLC. Serum SCC-associated antigen (SCC-Ag) is a relatively specific molecular biological marker for squamous cell lung cancer. Clinical studies have shown that most cases of lung adenocarcinoma transitioning to small cell carcinoma have elevated levels of NSE or pro-GRP, while some cases of SCC transitioning are accompanied by varying degrees of elevated levels of SCC-Ag[24-26]. This patient did not show any abnormal increase in SCC-Ag levels during the process from diagnosis of lung adenocarcinoma to transformation into SCC, indicating that there is no strong correlation between SCC-Ag levels and clinical judgment of SCC transformation. Therefore, more research samples are needed in the future to confirm .
In summary, we provide more detailed results of the patient's diagnosis and treatment process on SCC transformation after EGFR-TKIs treatment for lung adenocarcinoma. The transformation of SCC is one of the acquired resistance mechanisms of EGFR-TKIs in advanced lung adenocarcinoma with EGFR mutations. When resistance occurs in targeted therapy, secondary biopsy and genetic testing are necessary. At the same time, more tissue specimens should be obtained as much as possible to comprehensively evaluate pathological components and provide a strong basis for clinical decision-making.
| 1. | Alexander M, Kim SY, Cheng H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung. 2020;198:897-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 403] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 2. | Gao J, Li HR, Jin C, Jiang JH, Ding JY. Strategies to overcome acquired resistance to EGFR TKI in the treatment of non-small cell lung cancer. Clin Transl Oncol. 2019;21:1287-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 3115] [Article Influence: 445.0] [Reference Citation Analysis (0)] |
| 4. | He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer (Review). Int J Oncol. 2021;59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 187] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 5. | Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 895] [Cited by in RCA: 897] [Article Influence: 149.5] [Reference Citation Analysis (1)] |
| 6. | Schoenfeld AJ, Chan JM, Kubota D, Sato H, Rizvi H, Daneshbod Y, Chang JC, Paik PK, Offin M, Arcila ME, Davare MA, Shinde U, Pe'er D, Rekhtman N, Kris MG, Somwar R, Riely GJ, Ladanyi M, Yu HA. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin Cancer Res. 2020;26:2654-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 7. | Roca E, Pozzari M, Vermi W, Tovazzi V, Baggi A, Amoroso V, Nonnis D, Intagliata S, Berruti A. Outcome of EGFR-mutated adenocarcinoma NSCLC patients with changed phenotype to squamous cell carcinoma after tyrosine kinase inhibitors: A pooled analysis with an additional case. Lung Cancer. 2019;127:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Wu J, Lin Z. Non-Small Cell Lung Cancer Targeted Therapy: Drugs and Mechanisms of Drug Resistance. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 110] [Reference Citation Analysis (0)] |
| 9. | Han X, Li F, Fang Z, Gao Y, Li F, Fang R, Yao S, Sun Y, Li L, Zhang W, Ma H, Xiao Q, Ge G, Fang J, Wang H, Zhang L, Wong KK, Chen H, Hou Y, Ji H. Transdifferentiation of lung adenocarcinoma in mice with Lkb1 deficiency to squamous cell carcinoma. Nat Commun. 2014;5:3261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Zhang H, Fillmore Brainson C, Koyama S, Redig AJ, Chen T, Li S, Gupta M, Garcia-de-Alba C, Paschini M, Herter-Sprie GS, Lu G, Zhang X, Marsh BP, Tuminello SJ, Xu C, Chen Z, Wang X, Akbay EA, Zheng M, Palakurthi S, Sholl LM, Rustgi AK, Kwiatkowski DJ, Diehl JA, Bass AJ, Sharpless NE, Dranoff G, Hammerman PS, Ji H, Bardeesy N, Saur D, Watanabe H, Kim CF, Wong KK. Lkb1 inactivation drives lung cancer lineage switching governed by Polycomb Repressive Complex 2. Nat Commun. 2017;8:14922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Ivo-Dos-Santos J, Mello DL, Couto-Fernandez JC, Passos RM, Dias-Carneiro LA, Castilho EA, Galvão-Castro B. Evaluation of enzyme-linked immunosorbent and alternative assays for detection of HIV antibodies using panels of Brazilian sera. Rev Inst Med Trop Sao Paulo. 1990;32:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Li F, Han X, Li F, Wang R, Wang H, Gao Y, Wang X, Fang Z, Zhang W, Yao S, Tong X, Wang Y, Feng Y, Sun Y, Li Y, Wong KK, Zhai Q, Chen H, Ji H. LKB1 Inactivation Elicits a Redox Imbalance to Modulate Non-small Cell Lung Cancer Plasticity and Therapeutic Response. Cancer Cell. 2015;27:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 13. | Gao Y, Zhang W, Han X, Li F, Wang X, Wang R, Fang Z, Tong X, Yao S, Li F, Feng Y, Sun Y, Hou Y, Yang Z, Guan K, Chen H, Zhang L, Ji H. YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat Commun. 2014;5:4629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Hou S, Han X, Ji H. Squamous Transition of Lung Adenocarcinoma and Drug Resistance. Trends Cancer. 2016;2:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Burkart J, Shilo K, Zhao W, Ozkan E, Ajam A, Otterson GA. Metastatic Squamous Cell Carcinoma Component from an Adenosquamous Carcinoma of the Lung with Identical Epidermal Growth Factor Receptor Mutations. Case Rep Pulmonol. 2015;2015:283875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Bi XY, Li HG, Ma QY, Sun JL, Zhou L, Zhu LF, Cai PY, Tan XW. A report on pathological transformation of lung adenocarcinoma after TKI treatment. Shiyong Zhongliu Zazhi 2021; 6: 571-574. [DOI] [Full Text] |
| 17. | Zhu L, Jiang L, Yang J, Gu W, He J. Clinical characteristics and prognosis of patients with lung adenosquamous carcinoma after surgical resection: results from two institutes. J Thorac Dis. 2018;10:2397-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol. 2015;10:1240-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 1095] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 19. | Li W, Liu JB, Hou LK, Yu F, Zhang J, Wu W, Tang XM, Sun F, Lu HM, Deng J, Bai J, Li J, Wu CY, Lin QL, Lv ZW, Wang GR, Jiang GX, Ma YS, Fu D. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring. Mol Cancer. 2022;21:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 241] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 20. | Keller L, Belloum Y, Wikman H, Pantel K. Clinical relevance of blood-based ctDNA analysis: mutation detection and beyond. Br J Cancer. 2021;124:345-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 267] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 21. | Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, Mukherjee A, Paul MK. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 525] [Article Influence: 262.5] [Reference Citation Analysis (1)] |
| 22. | Yin X, Li Y, Wang H, Jia T, Wang E, Luo Y, Wei Y, Qin Z, Ma X. Small cell lung cancer transformation: From pathogenesis to treatment. Semin Cancer Biol. 2022;86:595-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Sun J, Wang Y, Yang R, Su W, Zhao Z, Liang H. Interstitial Lung Disease Combined with Lung Cancer: Current Understanding and Challenges. J Mod Med Oncol. 2024;4:1. [DOI] [Full Text] |
| 24. | Chen Y, Tang L, Huang W, Zhang Y, Abisola FH, Li L. Identification and validation of a novel cuproptosis-related signature as a prognostic model for lung adenocarcinoma. Front Endocrinol (Lausanne). 2022;13:963220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | Zhao Y, Xie Y, Jia D, Ma C, Wei D, Zhang X. Efficacy of Pemetrexed or Gemcitabine Combined with Cis-platinum on Patients with Advanced Non-small-cell Lung Carcinoma and Its Effect on Serum miR-365. J Mod Pharmacol Pathol. 2023;1:9. [DOI] [Full Text] |
| 26. | de Sousa VML, Carvalho L. Heterogeneity in Lung Cancer. Pathobiology. 2018;85:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |