Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4377
Revised: May 14, 2024
Accepted: May 27, 2024
Published online: July 16, 2024
Processing time: 98 Days and 5.8 Hours
Achromobacter xylosoxidans is a Gram-negative opportunistic aerobe, usually causing nosocomial infections in immunocompromised patients with manifestations including bacteremia, pneumonia, and catheter-related infections. However, A. xylosoxidans have not yet been reported to cause biliary system infections.
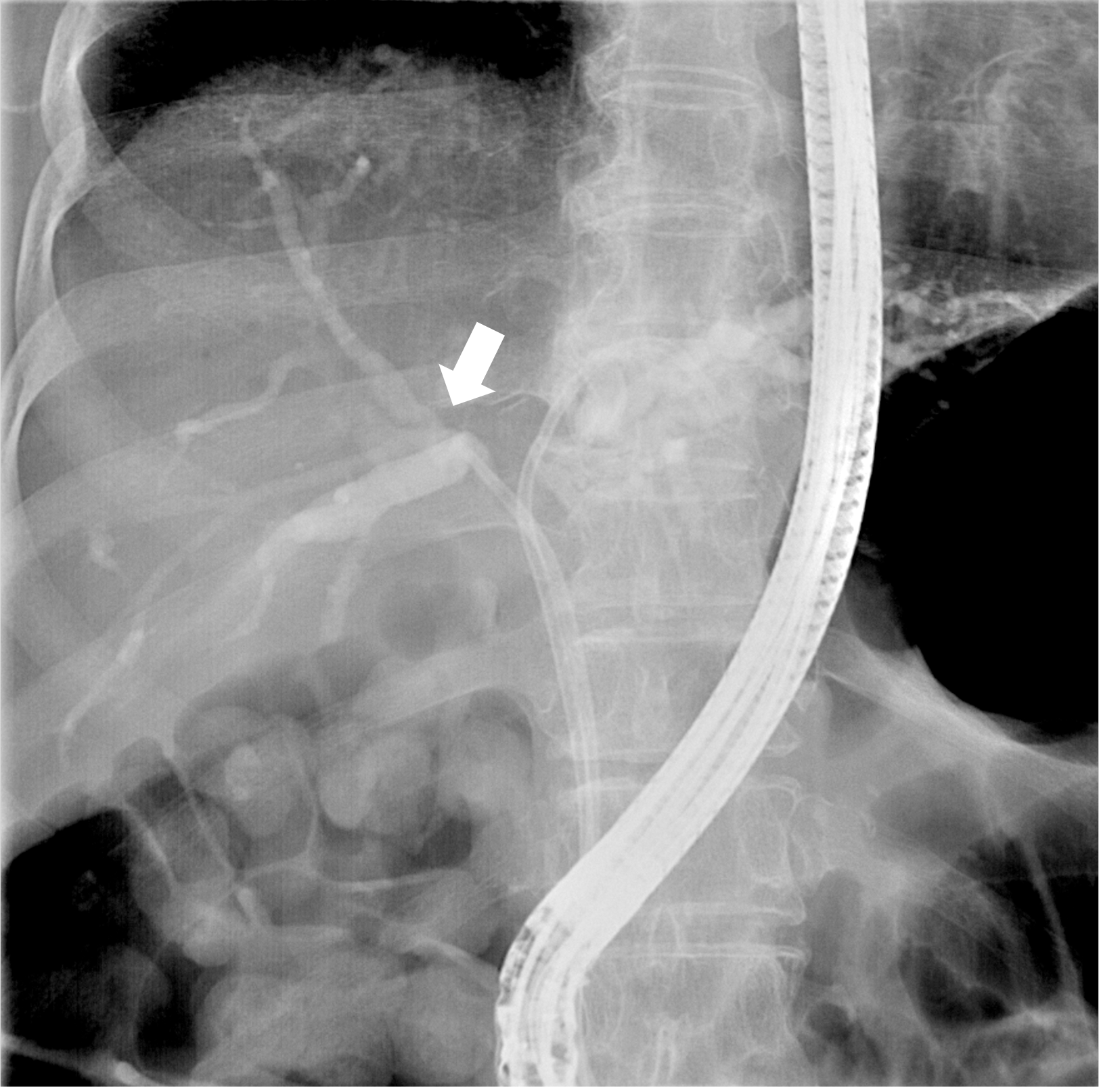
A 72-year-old woman presented to the outpatient department of our hospital with a chief complaint of jaundice. Computed tomography of her abdomen revealed the presence of a mass of approximately 2.4 cm in the hilar portion of the common hepatic duct, consistent with hilar cholangiocarcinoma. We performed endoscopic retrograde cholangiopancreatography (ERCP) to decompress the obstructed left and right intrahepatic ducts (IHDs) and placed 10 cm and 11 cm biliary stents in the left and right IHDs, respectively. However, the day after the procedure, the patient developed post-ERCP cholangitis as the length of the right IHD stent was insufficient for proper bile drainage. The blood culture of the patient tested positive for A. xylosoxidans. Management measures included the replacement of the right IHD stent (11 cm) with a longer one (12 cm) and administering culture-directed antibiotic therapy, solving the cholangitis-related complications. After the cholangitis had resolved, the patient underwent surgery for hilar cholangiocarcinoma and survived for 912 d without recurrence.
A. xylosoxidans-induced biliary system infections are extremely rare. Clinical awareness of physicians and endoscopists is required as this rare pathogen might cause infection after endoscopic procedures.
Core Tip:Achromobacter xylosoxidans-induced biliary system infections has not been previously described. We present a rare cholangitis case after endoscopic retrograde cholangiopancreatography (ERCP) caused by A. xylosoxidans. Establishing adequate drainage for obstructed bile ducts during ERCP is essential to decrease the risk of post-ERCP cholangitis. In this case, cholangitis developed due to insufficient length of the biliary stent and was resolved by susceptible antibiotic administration and adequate biliary drainage at the second ERCP. As A. xylosoxidans-induced cholangitis has not been previously reported, the A. xylosoxidans contamination source remains unclear. Further investigation is needed to identify the colonization source and prevent endoscopy-associated infections.
- Citation: Jo IH, Ko SW. Acute cholangitis with Achromobacter xylosoxidans bacteremia after endoscopic retrograde cholangiopancreatography in hilar cholangiocarcinoma: A case report. World J Clin Cases 2024; 12(20): 4377-4383
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4377.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4377
Post-endoscopic retrograde cholangiopancreatography (post-ERCP) cholangitis is among the potential adverse events after performing ERCP[1]. The most predominant bacteria identified in blood cultures of patients with post-ERCP cholangitis are the intestinal flora[2].
Achromobacter xylosoxidans, a non-fermenting, aerobic Gram-negative bacillus found primarily in contaminated water or soil[3], is a causative agent of bacteremia, pneumonia, catheter-associated infections, meningitis, cellulitis, and endocarditis[3-5] in immunocompromised patients with neoplasia, organ transplantation[6], or history of foreign device insertion (e.g., endotracheal tubes and catheters)[7]. However, A. xylosoxidans has not been reported to cause biliary system infections.
In this case report, we present a patient with cholangiocarcinoma who developed A. xylosoxidans-induced post-ERCP cholangitis and bacteremia.
A 72-year-old woman presented to the outpatient department of our hospital with a chief complaint of jaundice and recurrent epigastric pain.
The symptoms of the patient began 1 mo prior with recurrent episodes and no history of fever, chills, or vomiting.
The patient had no significant history.
The patient was a non-smoker who denied alcohol abuse and exhibited no significant family medical history.
The physical examination of the patient did not reveal tenderness or signs of peritoneal irritation. The bowel sounds of the patient were normal. At the outpatient clinic, the blood pressure, pulse rate, and temperature of the patient were 130/83 mmHg, 67 beats/min, and 36.8 °C, respectively.
Our laboratory investigations of the patient indicated increased cholestatic parameters with total bilirubin of 12.7 mg/dL (normal range: 0.3–1.2 mg/dL), aspartate aminotransferase (AST) of 227 IU/L (normal range: 0–35 IU/L), alanine aminotransferase (ALT) of 229 IU/L (normal range: 0–35 IU/L), alkaline phosphatase (ALP) of 456 IU/L (normal range: 30–120 IU/L), and gamma-glutamyl transferase of 420 IU/L (normal range: 9–64 IU/L). The white blood cell count (8600/μL) and C-reactive protein (0.5 mg/dL) values of the patient were within normal range.
Computed tomography of the abdomen revealed the presence of a mass of approximately 2.4 cm in size at the hilar portion of the common hepatic duct and a dilated intrahepatic duct. We admitted the patient and administered ceftizoxime, a third-generation prophylactic cephalosporin antibiotic, prior to performing ERCP for biliary decompres
A day after the index ERCP, the patient presented with fever, chills, severe epigastric pain, and increased body temperature (38.7 °C). Laboratory testing revealed an increased white blood cell count (15000/μL) and C-reactive protein level (9.35 mg/dL). We still recorded increased liver enzyme levels (total bilirubin 11.83 mg/dL, AST 206 IU/L, ALT 158 IU/L, and ALP 420 IU/L).
According to the guidelines of the European Society of Gastrointestinal Endoscopy, we diagnosed the patient with moderate post-ERCP cholangitis[1]. We collected two sets of blood samples in BACTECTMPlus aerobic/F culture vials (Becton Dickinson and Company, Franklin Lakes, NJ, United States) from the peripheral veins for blood culture, and administered empirical broad-spectrum antibiotics (piperacillin/tazobactam). Both blood cultures tested positive for Gram-negative bacilli (A. xylosoxidans). The pathogen exhibited susceptibility to cefepime, ceftazidime, imipenem, meropenem, piperacillin/tazobactam, ticarcillin/clavulanic acid, and trimethoprim/sulfamethoxazole while displaying resistance to amikacin, aztreonam, ciprofloxacin, gentamicin, and tobramycin (Table 1).
| Antibiotic | MIC | Interpretation CLSI 2016 |
| Amikacin | > 32 | R |
| Aztreonam | > 16 | R |
| Cefepime | 8 | S |
| Cefotaxime | 32 | I |
| Ceftazidime | 4 | S |
| Ciprofloxacin | > 2 | R |
| Gentamicin | > 8 | R |
| Imipenem | ≤ 1 | S |
| Levofloxacin | ≤ 2 | S |
| Meropenem | ≤ 1 | S |
| Piperacillin-tazobactam | ≤ 16 | S |
| Trimethoprim-sulfamethoxazole | ≤ 2/38 | S |
The final diagnosis was A. xylosoxidans-induced post-ERCP cholangitis.
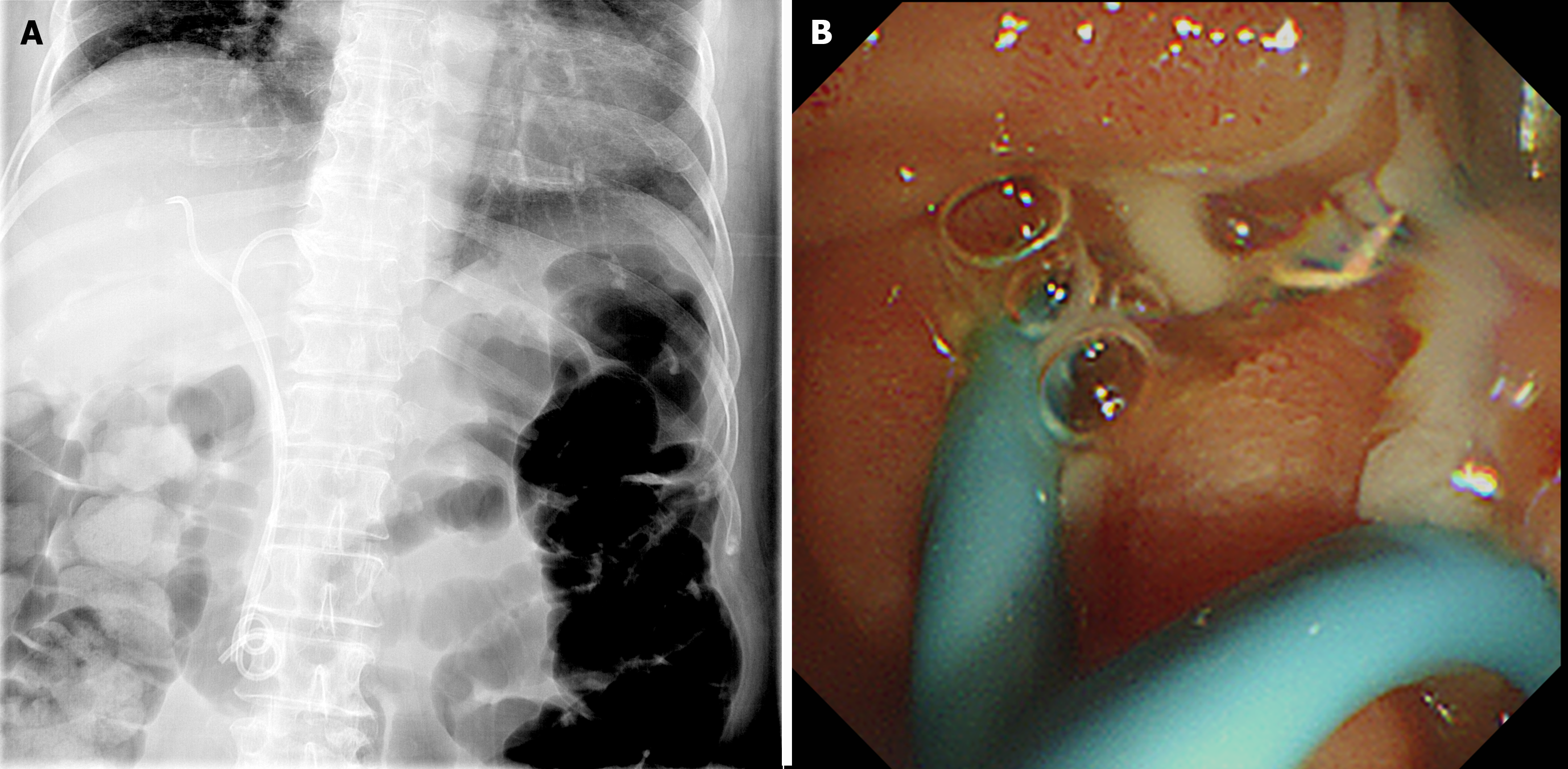
We performed a follow-up ERCP at 2 d after the index ERCP to achieve adequate biliary drainage. During the second ERCP, purulent pus, which was not visible during the index procedure, was discharged from the ampulla (Figure 2A). The 11 cm long ERBD stent placed in the right IHD was replaced with a 7 Fr, 12 cm ERBD stent (Zimmon® Biliary Stent; Cook Medical) (Figure 2B). We continued the antibiotic administration as the clinical symptoms alleviated and the laboratory results indicated improvement. We obtained subsequent blood cultures on day 5 of antibiotic treatment, testing negative for bacteremia. The patient was discharged after 14 d of antibiotic treatment.
After cholestasis had resolved, the patient was readmitted and underwent left lobectomy and bile duct resection for hilar cholangiocarcinoma. The patient was followed up for 912 d after surgery without recurrence. The disease-free survival and overall survival were 912 d.
A. xylosoxidans was first reported by Yabuuchi and Oyama[8] in 1971, isolated from the ear discharge of patients with chronic otitis media. A. xylosoxidans is an opportunistic and less virulent pathogen, with most of its caused infections being nosocomial (often associated with foreign device contaminations)[9]. The two most common clinical manifestations of A. xylosoxidans infection are catheter-associated bacteremia and pneumonia[10]. Urinary tract, soft tissue, central nervous system, and bone infections as well as endocarditis have been less commonly reported in case reports[3]. An intra-abdominal abscess caused by A. xylosoxidans after performing cholecystectomy was described in Taiwan[11]. However, to the best of our knowledge, no studies reported acute cholangitis associated with A. xylosoxidans infection, especially after ERCP.
Post-ERCP cholangitis is a rare ERCP-associated adverse event with an incidence of approximately 0.4%[12]. Inadequate biliary drainage of an obstructed bile duct filled with contrast is the major risk factor for post-ERCP cholangitis[13], considered to occur due to increased intraductal pressure causing biliary-venous reflux[14]. Therefore, ensuring intact bile and contrast flow by endoscopists during the procedure is crucial to prevent post-ERCP cholangitis, especially in cases of malignant stricture. However, various factors such as bile duct tortuosity or patient deterioration make the drainage of certain bile duct segments challenging[15-19]. In this case, we noticed that the stent in the right IHD was not long enough during the index ERCP. However, further procedures could not be performed as the patient recovered from conscious sedation and did not cooperate due to the prolonged procedure time. This incomplete procedure might have led to the development of post-ERCP cholangitis, which was resolved by replacing the previous stent with one of sufficient length.
Preoperative biliary drainage modalities in the case of hilar cholangiocarcinoma also merit discussion. The optimal biliary drainage approach, whether ERCP or percutaneous transhepatic biliary drainage (PTBD), remains unclear. The choice between these two modalities is influenced by factors such as local expertise, disease complexity, patient condition, and preference[20]. Several meta-analyses[21-23] described that ERCP is associated with higher procedure-related adverse event rates. However, the robustness of the findings might be compromised by the limited included RCT numbers[24]. Although certain studies advocate PTBD for its capacity to drain specific bile ducts[25,26], PTBD reportedly carries a higher risk of cancer seeding through the track than ERCP[27]. In contrast, compared to PTBD, ERCP is reportedly coupled with shorter hospital stays and less patient discomfort and pain[28]. Therefore, we opted for ERCP as the initial approach in the case of this patient. Although post-ERCP cholangitis developed due to the insufficient length of the biliary stent, the placement of a longer stent and appropriate antibiotic therapy solved the cholangitis-related concerns.
Enteric bacteria (e.g., Enterococcus faecium and Escherichia coli) are reportedly the most common pathogens responsible for post-ERCP cholangitis[2,14]. As A. xylosoxidans-induced cholangitis has not been reported before, the A. xylosoxidans infection source remains unclear. Foreign devices, especially intravascular catheters, have been established as major entry portals for pathogens into the bloodstream[4,7]. In this case, the ERBD stent was very unlikely the source of bacteremia as it did not break the barriers between the body and the external environment as intravascular catheters do[25].
A. xylosoxidans is a well-established nosocomial colonizer, isolated from various healthcare devices such as dialysis solutions, disinfectants, and mechanical ventilators[7,9]. Therefore, we think that the duodenoscope might be a potential infection source. In recent years, ERCP procedure-associated multidrug-resistant organisms emerged[26,27]. Numerous medical studies concluded that an elevator, the unique structure of the duodenoscope makes its cleansing difficult and inadequate[28]. Although we suspected the duodenoscope as an infection source, no massive infectious outbreak has occurred in our endoscopy suite to date, as no other patients have developed post-ERCP cholangitis associated with A. xylosoxidans, except for this one.
A. xylosoxidans is resistant to a broad spectrum of antibiotics[29]. Although wild-type strains are resistant to first- and second-generation cephalosporins, cefotaxime, ceftriaxone, and aminoglycosides, they are susceptible to piperacillin, cefepime, ceftazidime, fluoroquinolones, and colistin[3]. In particular, piperacillin-tazobactam is an agent to which A. xylosoxidans is susceptible[30]. Although strains harboring chromosomal OXA-114-like beta-lactamase, resistant to piperacillin and carbapenems have been reported[31,32], the strain in this case was susceptible to piperacillin-tazobactam. Therefore, A. xylosoxidans bacteremia was resolved without the need for combination therapy or broad-spectrum agents.
In this study, we presented the first case of A. xylosoxidans-induced post-ERCP cholangitis and bacteremia. Clinical awareness of physicians and endoscopists would be essential as this rare pathogen might cause infection after endoscopic procedures. Further investigations would be required to determine colonization sources and prevent endoscopy-associated infections.
| 1. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 491] [Article Influence: 98.2] [Reference Citation Analysis (1)] |
| 2. | Du M, Suo J, Liu B, Xing Y, Chen L, Liu Y. Post-ERCP infection and its epidemiological and clinical characteristics in a large Chinese tertiary hospital: a 4-year surveillance study. Antimicrob Resist Infect Control. 2017;6:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Isler B, Kidd TJ, Stewart AG, Harris P, Paterson DL. Achromobacter Infections and Treatment Options. Antimicrob Agents Chemother. 2020;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Pérez Barragán E, Sandino Pérez J, Corbella L, Orellana MA, Fernández-Ruiz M. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273. [PubMed] |
| 5. | Ramos JM, Domine M, Ponte MC, Soriano F. [Bacteremia caused by Alcaligenes (Achromobacter) xylosoxidans. Description of 3 cases and review of the literature]. Enferm Infecc Microbiol Clin. 1996;14:436-440. [PubMed] |
| 6. | Mandell WF, Garvey GJ, Neu HC. Achromobacter xylosoxidans bacteremia. Rev Infect Dis. 1987;9:1001-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Gómez-Cerezo J, Suárez I, Ríos JJ, Peña P, García de Miguel MJ, de José M, Monteagudo O, Linares P, Barbado-Cano A, Vázquez JJ. Achromobacter xylosoxidans bacteremia: a 10-year analysis of 54 cases. Eur J Clin Microbiol Infect Dis. 2003;22:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Marion-Sanchez K, Olive C, Platon MG, Cesarine M, Derancourt C, Pailla K. Achromobacter xylosoxidans in hospital environments: still waters run deep! Trans R Soc Trop Med Hyg. 2020;114:470-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Lee JH, Lee SY, Park IY, Park SY, Lee JS, Kang G, Kim JS, Eom JS. A Case of Septic Shock caused by Achromobacter xylosoxidans in an Immunocompetent Female Patient after Extracorporeal Shock Wave Lithotripsy for a Ureteral Stone. Infect Chemother. 2016;48:47-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Teng SO, Ou TY, Hsieh YC, Lee WC, Lin YC, Lee WS. Complicated intra-abdominal infection caused by extended drug-resistant Achromobacter xylosoxidans. J Microbiol Immunol Infect. 2009;42:176-180. [PubMed] |
| 12. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 769] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 13. | Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP Jr, Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW, Van Dam J, Hughes M, Carr-Locke DL. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 14. | Subhani JM, Kibbler C, Dooley JS. Review article: antibiotic prophylaxis for endoscopic retrograde cholangiopancreatography (ERCP). Aliment Pharmacol Ther. 1999;13:103-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Zhang R, Zhao L, Liu Z, Wang B, Hui N, Wang X, Huang R, Luo H, Fan D, Pan Y, Guo X. Effect of CO2 cholangiography on post-ERCP cholangitis in patients with unresectable malignant hilar obstruction - a prospective, randomized controlled study. Scand J Gastroenterol. 2013;48:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Dar FS, Abbas Z, Ahmed I, Atique M, Aujla UI, Azeemuddin M, Aziz Z, Bhatti ABH, Bangash TA, Butt AS, Butt OT, Dogar AW, Farooqi JI, Hanif F, Haider J, Haider S, Hassan SM, Jabbar AA, Khan AN, Khan MS, Khan MY, Latif A, Luck NH, Malik AK, Rashid K, Rashid S, Salih M, Saeed A, Salamat A, Tayyab GU, Yusuf A, Zia HH, Naveed A. National guidelines for the diagnosis and treatment of hilar cholangiocarcinoma. World J Gastroenterol. 2024;30:1018-1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Reference Citation Analysis (4)] |
| 17. | Liu JG, Wu J, Wang J, Shu GM, Wang YJ, Lou C, Zhang J, Du Z. Endoscopic Biliary Drainage Versus Percutaneous Transhepatic Biliary Drainage in Patients with Resectable Hilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2018;28:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Al Mahjoub A, Menahem B, Fohlen A, Dupont B, Alves A, Launoy G, Lubrano J. Preoperative Biliary Drainage in Patients with Resectable Perihilar Cholangiocarcinoma: Is Percutaneous Transhepatic Biliary Drainage Safer and More Effective than Endoscopic Biliary Drainage? A Meta-Analysis. J Vasc Interv Radiol. 2017;28:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Tang Z, Yang Y, Meng W, Li X. Best option for preoperative biliary drainage in Klatskin tumor: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e8372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Mocan T, Horhat A, Mois E, Graur F, Tefas C, Craciun R, Nenu I, Spârchez M, Sparchez Z. Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how? World J Gastrointest Oncol. 2021;13:2050-2063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 21. | Kloek JJ, van der Gaag NA, Aziz Y, Rauws EA, van Delden OM, Lameris JS, Busch OR, Gouma DJ, van Gulik TM. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2010;14:119-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Wiggers JK, Groot Koerkamp B, Coelen RJ, Rauws EA, Schattner MA, Nio CY, Brown KT, Gonen M, van Dieren S, van Lienden KP, Allen PJ, Besselink MG, Busch OR, D'Angelica MI, DeMatteo RP, Gouma DJ, Kingham TP, Jarnagin WR, van Gulik TM. Preoperative biliary drainage in perihilar cholangiocarcinoma: identifying patients who require percutaneous drainage after failed endoscopic drainage. Endoscopy. 2015;47:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Wang L, Lin N, Xin F, Ke Q, Zeng Y, Liu J. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J Surg Oncol. 2019;17:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Inamdar S, Slattery E, Bhalla R, Sejpal DV, Trindade AJ. Comparison of Adverse Events for Endoscopic vs Percutaneous Biliary Drainage in the Treatment of Malignant Biliary Tract Obstruction in an Inpatient National Cohort. JAMA Oncol. 2016;2:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Bouza E. Intravascular catheter-related infections: a growing problem, the search for better solutions. Clin Microbiol Infect. 2002;8:255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Epstein L, Hunter JC, Arwady MA, Tsai V, Stein L, Gribogiannis M, Frias M, Guh AY, Laufer AS, Black S, Pacilli M, Moulton-Meissner H, Rasheed JK, Avillan JJ, Kitchel B, Limbago BM, MacCannell D, Lonsway D, Noble-Wang J, Conway J, Conover C, Vernon M, Kallen AJ. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014;312:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 27. | Ross AS, Baliga C, Verma P, Duchin J, Gluck M. A quarantine process for the resolution of duodenoscope-associated transmission of multidrug-resistant Escherichia coli. Gastrointest Endosc. 2015;82:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Verfaillie CJ, Bruno MJ, Voor in 't Holt AF, Buijs JG, Poley JW, Loeve AJ, Severin JA, Abel LF, Smit BJ, de Goeij I, Vos MC. Withdrawal of a novel-design duodenoscope ends outbreak of a VIM-2-producing Pseudomonas aeruginosa. Endoscopy. 2015;47:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 29. | Hu Y, Zhu Y, Ma Y, Liu F, Lu N, Yang X, Luan C, Yi Y, Zhu B. Genomic insights into intrinsic and acquired drug resistance mechanisms in Achromobacter xylosoxidans. Antimicrob Agents Chemother. 2015;59:1152-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Wittmann J, Dreiseikelmann B, Rohde C, Rohde M, Sikorski J. Isolation and characterization of numerous novel phages targeting diverse strains of the ubiquitous and opportunistic pathogen Achromobacter xylosoxidans. PLoS One. 2014;9:e86935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Yamamoto M, Nagao M, Hotta G, Matsumura Y, Matsushima A, Ito Y, Takakura S, Ichiyama S. Molecular characterization of IMP-type metallo-β-lactamases among multidrug-resistant Achromobacter xylosoxidans. J Antimicrob Chemother. 2012;67:2110-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Sofianou D, Markogiannakis A, Metzidie E, Pournaras S, Tsakris A. VIM-2 metallo-beta-lactamase in Achromobacter xylosoxidans in Europe. Eur J Clin Microbiol Infect Dis. 2005;24:854-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |