Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4325
Revised: April 25, 2024
Accepted: May 22, 2024
Published online: July 16, 2024
Processing time: 124 Days and 15.5 Hours
Rectus sheath hematoma (RSH) is uncommon, and because people have limited knowledge about it, it is difficult to recognize the symptoms in time, often de-laying optimal treatment.
Herein, we report a case of a 77-year-old female with RSH. The patient was treated at our hospital for coronavirus disease 2019. Anticoagulant treatment was administered during this period because of thrombosis. On the 8th d of treatment, the patient complained of abdominal pain. Ultrasonography revealed a solid cystic mass in the pelvic cavity. An emergency laparotomy was performed, and a huge hematoma was found in the deep layer of the rectus abdominis muscle. We used anticoagulants with caution based on the patient’s condition.
Optimal management of patients with RSH s depends on timely diagnosis and when to reintroduce anticoagulants.
Core Tip: Rectus sheath hematoma (RSH) is an uncommon disease in clinical practice, often affecting older and postpartum women. Since the coronavirus disease 2019 (COVID-19) outbreak, the prevalence of RSH has increased due to patients with severe cough symptoms and the use of anticoagulants for thrombotic complications. Therefore, we report a case of a RSH in a patient with COVID-19. A systematic analysis of the clinical manifestations, diagnosis, and treatment of this disease can enhance clinicians’ understanding of it.
- Citation: Wang W, Duan J. Rectus sheath hematoma combined with COVID-19: A case report. World J Clin Cases 2024; 12(20): 4325-4330
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4325.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4325
Rectus sheath hematoma (RSH) is characterized by accumulation of blood in the rectus abdominis sheath. It is often due to hemorrhage from the epigastric artery or its branch arteries within the rectus sheath and accounts for 1%-2% of all causes of acute abdominal pain[1]. RSH can be classified into three types: Type I is unilateral and occurs within the rectus muscle; type II can be unilateral or bilateral, within the rectus muscle or between the muscle and the transverse fascia; and type III refers to hematomas that extend into the peritoneum and anterior interstitium of the bladder[2]. The causes of morbidity can be divided into two main categories: Traumatic and spontaneous. Trauma can be subdivided into medical and exogenous trauma, which are more common. Examples include blunt abdominal contusion and abdominal wall punctures, which can directly cause RSH. Spontaneous RSH is rarely reported[3]. Female sex, older age, histories of anticoagulant and antiplatelet therapies, pregnancy and persistent cough are risk factors for RSH[4]. Anticoagulation and antiplatelet therapies are evidently the most common risk factors for RSH[5]. In 2019, the world faced an outbreak of novel coronavirus pneumonia, with coronavirus disease 2019 (COVID-19) significantly increasing the risk of thrombosis, especially among patients requiring hospitalization, with up to 33% of patients experiencing thrombotic events[6]. When an infected person is in a hypercoagulable state, anticoagulants are typically used to prevent thrombosis. However, anticoagulant therapy tends to cause RSH. In this study, we analyzed a case of RSH combined with novel coronavirus pneumonia admitted to Hubei Provincial Maternal and Child Health Hospital. We also discussed and reviewed the literature in relation to the clinical manifestations, diagnostic tools, and treatment options for this disease.
A 77-year-old female patient presented to our institution with a 12-d history of cough and sputum.
The patient suddenly developed chills and fever 12 d ago without obvious inducement. It was accompanied by runny nose, nasal congestion, cough and sputum with yellow sputum color, as well as generalized weakness, chest tightness and shortness of breath.
previous history of hypertension, treated with oral metoprolol tartrate tablets and sacubitril valsartan medication. She was hospitalised several times for poorly controlled hypertension.
The patient denied any family history of malignant tumours.
Physical examination: Signs were stable, clear, no yellowing of the skin and sclera, superficial lymph nodes were not large, neck was soft, and the trachea was centred. The pharynx was congested, bilateral tonsils were not enlarged, the respiratory sounds of both lungs were clear, no dry or wet rhonchi were heard, the heart rate was 84 beats/min, rhythmic, and no pathological murmurs were heard in the valves’ auscultation area. Abdomen was soft, no pressure and rebound pain, liver and spleen were not touched under the ribs, Mumphy’s sign (-), percussion pain in both kidneys (-), bowel sounds were normal, and there was no oedema in both lower limbs.
Blood analysis (five categories) + ultrasensitive C-reactive protein: Ultrasensitive C-reactive protein 8.76 mg/L, red blood cell count 2.98 × 1012/L, haemoglobin 86 g/L. B-type natriuretic peptide: B-type natriuretic peptide 123.80 pg/mL. Liver function + renal function + cardiac enzyme profiles + electrolytes: Aspartate aminotransferase 43.56 U/L, albumin 31.36 g/L, white globule ratio 0.90, urea 20.10 mmol/L, creatinine 141.61 μmol/L, cystatin 3.01 mg/L, uric acid 584.48 μmol/L, lactate dehydrogenase 369.03 U/L, a-hydroxybutyrate dehydrogenase 280.37 U/L, prealbumin 165.15 mg/L, glomerular filtration rate 30.82 mL/min. On the 8th d of admission, the patient complained of abdominal pain, and urgent blood tests were performed: Ultrasensitive reactive protein 36.67 mg/L, leukocyte count 10.7 × 109/L, erythrocyte count 2.21 × 1012/L, haemoglobin 64 g/L, haematocrit 0.1941 L/L, and coagulation function: prothrombin time 14.0 s, prothrombin time 18.4 s, plasma D-dimer 0.67 μg/mL.
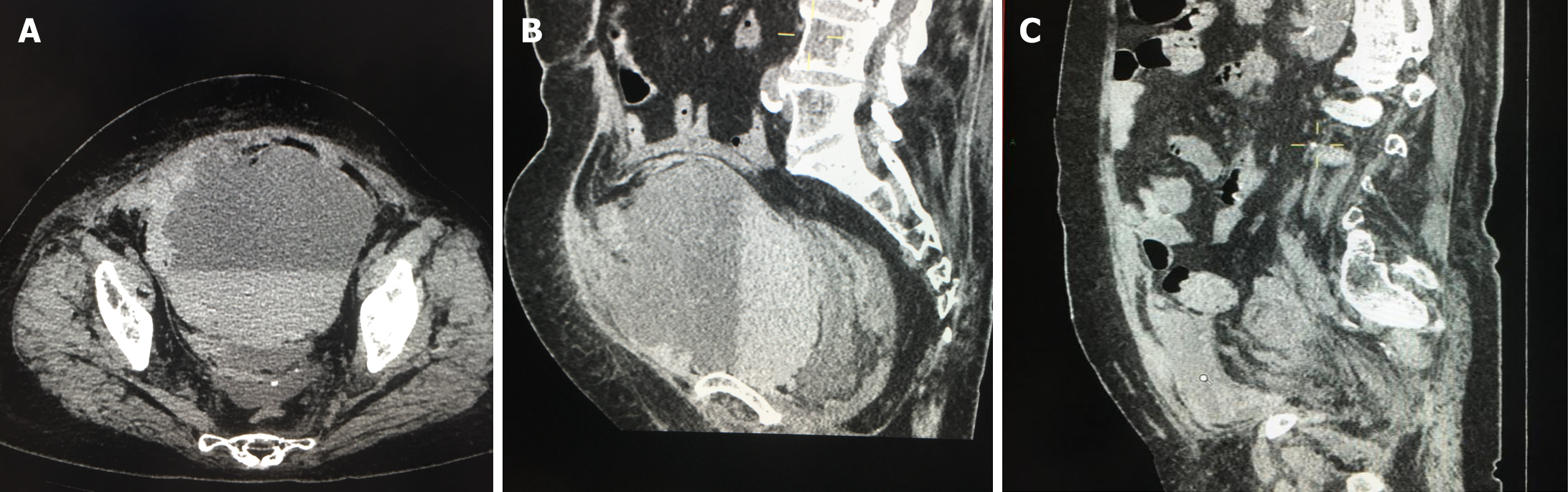
Chest computed tomography (CT) suggested lung infection, and nucleic acid test suggested positive. Bilateral lower limb deep vein ultrasound suggested: Bilateral calf intermuscular vein multiple thrombosis. On the 8th d of admission, the patient complained of abdominal pain, abdominal + pelvic CT: Masses in the pelvis (Figure 1A and B). Ultrasound: Pelvic cystic solid mass (13.6 cm × 12.1 cm × 9.1 cm echogenicity in the pelvis, and 9.3 cm × 9.5 cm × 4.3 cm slightly strong echogenicity in the pelvis).
Combined with the patient’s medical history, the final diagnosis was RSH.
He was treated with anti-infective (sulperazon 3.0 g, Q12H), anti-viral (paramivir), hormonal (dexamethasone injection 6 mg QD) anti-inflammatory, and anti-coagulant (fraxiparine 4100 UI, Q12H) therapy. On the eighth day of anticoagulation therapy, the patient had sudden onset of lower abdominal pain, and auxiliary examination suggested intra-pelvic hematoma formation. After stopping the anticoagulant drugs, emergency caesarean section was performed, during the operation, the rectus abdominis muscle was seen to have a dark red appearance, and after separation, a huge hematoma was seen in the deep layer of the rectus abdominis muscle, with a large amount of reddish haemato-hydrological exudate and 1500 mL of dark red blood in it. After the abdominal wall hematoma was removed, one intracavitary drain and one subcutaneous drain were placed, and symptomatic treatments, such as anti-infection and haemostasis, were given.
Postoperative follow-up showed improvement in various indicators. Postoperative review of the hematoma was significantly smaller than before (Figure 1C).
RSH is not a common condition and is usually caused by abdominal trauma or excessive contraction of the rectus abdominis muscle, which leads to rupture of one of the arteries in the abdominal wall or tearing of the rectus abdominis muscle[4]. Owing to the intricate distribution of the lower abdominal arteries and their stable association with the perforating branches of the muscle, they are susceptible to injury and rupture during intense muscle contractions[4]. Therefore, RSH are more likely to occur unilaterally in the lower abdomen[7]. The incidence of RSH is approximately (2-3):1 in males and females, as females have less muscle mass than males; additionally, pregnancy is a risk factor for RSH[8].
Although there is a paucity of previous cases owing to its rarity, the number of cases has begun to rise since the outbreak of COVID-19. This increase is mainly attributed to the hypercoagulable states associated with COVID-19, which elevates the risk of thromboembolism[9]. To prevent and treat the thrombotic complications due to COVID-19, physicians typically use both normal and low molecular weight heparins to prevent and treat thromboembolism. However, there are risks associated with anticoagulation, and hemorrhagic complications of anticoagulation are relatively common in patients. Although most of these are self-limiting, complications and increased mortality can still occur[10]. A retro
The clinical presentation of RSH is nonspecific; the most common clinical manifestation is abdominal pain, which may be associated with hematoma expansion between the anterior and posterior fascia of the muscle and contraction of the muscle fibers[8]. In most cases, palpable abdominal wall masses may be accompanied by nausea, vomiting, chills, and fever. Cullen and Gray-Turner signs can be detected in some cases, but they typically manifest too late in the progression of RSH to aid in the early diagnosis[12]. Depending on the size and location of the hematoma, patients may exhibit other clinical manifestations, such as hypovolemia due to rupture of a large hematoma leading to hypotension, tachycardia, or hemorrhagic shock; if the RSH originates below the arcuate line, the hematoma may bleed across the midline and move down into the anterior interstitium of the bladder, causing obstruction of the bladder outlet and difficulty in passing urine[13]. Rare cases of RSH may cause abdominal fascial compartment syndrome, which requires surgical decompre
The diagnosis of RSH is primarily based on imaging findings. Screening for RSH can be done using ultrasound, which has a sensitivity of approximately 80%-85%. However, the final result should be confirmed via abdominal and pelvic CT, which, in most cases, has a sensitivity of 100%[4]. RSH appears as a dense mass located behind the rectus muscle on CT. It should be noted that when the hematoma is chronic, it may appear isointense or even hypointense; hence, enhancement scans may help clarify the diagnosis in cases where CT scans appear atypical[17]. Magnetic resonance imaging, angiography, and radionuclide Tc-99m erythrocyte imaging are useful for diagnosing RSH[17]. Laboratory tests have low specificity and sensitivity for RSH and therefore have a low diagnostic value[4]. However, coagulation measurements are useful in patients receiving anticoagulant therapy and can be used to determine whether reversal of anticoagulant therapy should be continued[4].
The treatment options for RSH are conservative and surgical, with the current first-line treatment being conservative, including fluid resuscitation, blood supplementation, anticoagulation reversal, and bed rest[18]. Generally, the hematoma subsides completely itself within 1-2 months of conservative treatment[19]. Interventional radiological embolization, such as microcoil, n-butyl cyanoacrylate, and squid embolization, is considered the preferred therapeutic option after failure of conservative treatment. When embolization fails to control bleeding, surgical intervention is reserved as second-line treatment. However, surgery can be used as a first-line treatment for abdominal osteofascial compartment syndrome[20]. Surgery is preferred when the patient is hemodynamically unstable[21]. Interventional radiology or surgical removal of the hematoma and ligation of the blood vessels may be performed to achieve rapid hemostasis and prevent the patient from continuing to bleed and further expanding the hematoma. It should be noted that after more than two embolization procedures in the same patient, the risk of muscle ischemia is high, and surgical removal of the hematoma is the preferred option[22]. Optimal management of RSH depends on early hematoma recognition and administration of conservative treatment, and in the event that conservative treatment fails, predictors such as hematoma size, rate of hemoglobin decline, and number of red blood cell units transfused can be used to guide the next steps.
The timing of reintroducing anticoagulants in patients with RSH warrants further exploration. No guidelines or prospective studies currently exist to provide guidance on the appropriateness or timing of this question. Some studies have reported the potential for recurrent hematomas and even serious complications after reintroduction of anticoagulants[23]. Physicians need to make individualized decisions based on the needs of the patient’s condition, weighing the risk of bleeding against the risk of thromboembolic complications. RSH is generally considered a benign disease with a favorable outcome, with an estimated mortality rate of 1.6%-5%. Deaths are more prevalent among the older adults, patients on anticoagulants, and those with chronic kidney disease[20].
In this case, the patient was a female with a recent COVID-19 infection, a history of coughing, and was admitted to the hospital while on anticoagulation medication, presenting a predisposing factor for RSH. Later, the patient experienced sudden onset of abdominal pain, and ultrasound and CT findings supported the diagnosis of RSH. After discontinuing anticoagulants, emergency dissection was performed, and a large hematoma was visualized in the deep rectus abdominis muscle, confirming the diagnosis of COVID-19 infection combined with RSH.
Neocoronitis affects coagulation function, and anticoagulant therapy is often used, leading to an increased rate of bleeding. RSH is a rare condition that can be misdiagnosed or even missed and should be considered by doctors in patients with sudden onset of abdominal pain along with RSH predisposing factors. Although anticoagulation has been experimentally proven to be the most common risk factor for RSH, there was a case report of multiple abdominal wall hematomas in a patient infected with Cryptococcus neoformans who did not receive anticoagulation therapy[16]. Therefore, the use of anticoagulant therapy is not necessary for the development of RSH and should be suspected in patients who present with abdominal pain and have predisposing factors for RSH, such as pregnancy and cough, regardless of anticoagulant use. Therefore, early detection, diagnosis, and treatment are vital.
We would like to express our sincere gratitude to the patient and her parents for their support.
| 1. | Dulberger A, Streiff M, Myers SD, Sanders CS. Hernia Following Rectus Sheath Hematoma. Cureus. 2022;14:e28795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Allen M, Sevensma KE. Rectus Sheath Hematoma. 2023 Jan 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 3. | Siu WT, Tang CN, Law BK, Chau CH, Li MK. Spontaneous rectus sheath hematoma. Can J Surg. 2003;46:390. [PubMed] |
| 4. | Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg. 2015;13:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Karapolat B, Tasdelen HA, Korkmaz HAA. Conservative Treatment of Spontaneous Rectus Sheath Hematomas: Single Center Experience and Literature Review. Emerg Med Int. 2019;2019:2406873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Ottewill C, Mulpeter R, Lee J, Shrestha G, O'Sullivan D, Subramaniam A, Hogan B, Varghese C. Therapeutic anti-coagulation in COVID-19 and the potential enhanced risk of retroperitoneal hematoma. QJM. 2021;114:508-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Ben Selma A, Genese T. Spontaneous Rectus Sheath Hematoma: An Uncommon Cause of Acute Abdominal Pain. Am J Case Rep. 2019;20:163-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Buffone A, Basile G, Costanzo M, Veroux M, Terranova L, Basile A, Okatyeva V, Cannizzaro MT. Management of patients with rectus sheath hematoma: Personal experience. J Formos Med Assoc. 2015;114:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Yeoh WC, Lee KT, Zainul NH, Syed Alwi SB, Low LL. Spontaneous retroperitoneal hematoma: a rare bleeding occurrence in COVID-19. Oxf Med Case Reports. 2021;2021:omab081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Teta M, Drabkin MJ. Fatal retroperitoneal hematoma associated with Covid-19 prophylactic anticoagulation protocol. Radiol Case Rep. 2021;16:1618-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Musoke N, Lo KB, Albano J, Peterson E, Bhargav R, Gul F, DeJoy R 3rd, Salacup G, Pelayo J, Tipparaju P, Azmaiparashvili Z, Patarroyo-Aponte G, Rangaswami J. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res. 2020;196:227-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | Teske JM. Hematoma of the rectus abdominis muscle; report of a case and analysis of 100 cases from the literature. Am J Surg. 1946;71:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Gupta AK, Parker BM, Ross AS. Rectus Sheath Hematoma Causing Bladder Outlet Obstruction. Cureus. 2020;12:e7974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Drinnon K, Simpson SS, Puckett Y, Ronaghan CA, Richmond RE. Rectus Sheath Hematoma: A Rare Surgical Emergency. Cureus. 2020;12:e12156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Anwari L. Rectus sheath hematoma in pregnancy: a case report. Radiol Case Rep. 2020;15:2022-2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Hamid NS, Spadafora PF, Khalife ME, Cunha BA. Pseudosepsis: rectus sheath hematoma mimicking septic shock. Heart Lung. 2006;35:434-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Pierro A, Cilla S, Modugno P, Centritto EM, De Filippo CM, Sallustio G. Spontaneous rectus sheath hematoma: The utility of CT angiography. Radiol Case Rep. 2018;13:328-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Dağ A, Ozcan T, Türkmenoğlu O, Colak T, Karaca K, Canbaz H, Dirlik M, Sarıbay R. Spontaneous rectus sheath hematoma in patients on anticoagulation therapy. Ulus Travma Acil Cerrahi Derg. 2011;17:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Gradauskas A, Venclauskas L, Pažusis M, Karpavičius A, Maleckas A. Comparison of the Different Treatment Strategies for Patients with Rectus Sheath Haematoma. Medicina (Kaunas). 2018;54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Wong CL, So CH. Spontaneous rectus sheath hematoma associated with apixaban in an elderly gentleman with chronic obstructive airway disease - a case report. Thromb J. 2022;20:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Aktürk OM, Kayılıoğlu SI, Aydoğan İ, Dinç T, Yildiz B, Cete M, Erdoğan A, Coşkun F. Spontaneous Rectus Sheath Hematoma: an Overview of 4-Year Single Center Experience. Indian J Surg. 2015;77:1219-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Palatucci V, Lombardi G, Lombardi L, Giglio F, Giordano F, Lombardi D. Spontaneous muscle haematomas: management of 10 cases. Transl Med UniSa. 2014;10:13-17. [PubMed] |
| 23. | Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore). 2006;85:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |