Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4230
Revised: May 8, 2024
Accepted: May 27, 2024
Published online: July 16, 2024
Processing time: 97 Days and 17.3 Hours
The increasing prevalence of tuberculosis (TB) and diabetes on a global scale poses a significant health challenge, particularly due to their co-occurrence, which amplifies the severity, recurrence and mortality rates associated with both conditions. This highlights the need for further investigation into their inter-relationship.
To explore the computed tomography (CT) imaging and clinical significance of bacterium-positive pulmonary TB (PTB) combined with diabetes.
There were 50 patients with bacterium-positive PTB and diabetes, and 50 with only bacterium-positive PTB. The latter were designated as the control group. The CT imaging of the two groups of patients was compared, including lesion range, shape, density and calcification.
No significant differences were observed in age, gender, smoking and drinking history, high blood pressure, hyperlipidemia and family genetic factors between the groups. However, compared to the patients diagnosed solely with simple bacterium-positive PTB, those with concurrent diabetes showed a wider range of lesions and more complex and diverse morphology on CT images. Among them, intrapulmonary tuberculosis lesions were often accompanied by manifestations of pulmonary infection, such as cavity formation and bronchiectasis. At the same time, diabetes-related signs were often seen on CT images, such as pulmonary infection combined with diabetic pulmonary lesions. Logistic regression analysis identified age and medical history as significant factors influencing the degree of pulmonary infection and CT imaging outcomes in patients with both TB and diabetes. This suggests that older age and specific medical histories may increase the risk or severity of pulmonary damage in these patients.
CT imaging reveals more complex lesions in PTB patients with diabetes, emphasizing the need for careful evaluation and comprehensive analysis to enhance diagnostic accuracy.
Core tip: This study elucidated the distinct computed tomography imaging characteristics of pulmonary tuberculosis (PTB) in patients with diabetes, highlighting the necessity for an integrated diagnostic approach. Findings indicated that PTB-diabetes patients had more extensive, complex lesion morphologies compared to those with PTB alone. Age and medical history significantly influenced infection severity and imaging outcomes, emphasizing the importance of personalized treatment strategies. This research underscores the need for heightened clinical awareness and tailored management of this comorbidity to improve diagnostic accuracy and patient care.
- Citation: Rong XS, Yao C. Computed tomography imaging and clinical significance of bacterium-positive pulmonary tuberculosis complicated with diabetes. World J Clin Cases 2024; 12(20): 4230-4238
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4230.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4230
Tuberculosis (TB) and diabetes are two global epidemics whose prevalence in the population is on the rise and often coexist, forming a double burden. According to statistics, millions of people die from TB every year worldwide, and diabetes is also one of the leading causes of death for millions of people[1-3]. Despite progress in the research and management of both diseases in different medical fields, there are still many unanswered questions about their inter-relationship and the clinical characteristics of their combined states[4-9]. The connection between TB and diabetes has long been recognized by academics. Patients with diabetes are more susceptible to Mycobacterium tuberculosis infection due to factors such as reduced immune function and impaired glucose metabolism[10-14], and TB patients with diabetes usually have a more severe clinical course and higher recurrence rate. Among patients with diabetes, the incidence of TB is also significantly higher than that of the general population, and the mortality rate of patients with diabetes combined with TB is also relatively high. This bidirectional influence makes the joint existence of TB and diabetes a medical problem of great concern[15-17].
In the realm of clinical medicine, TB and diabetes pose significant individual challenges, with well-documented pathophysiological and clinical intricacies. However, the convergence of these diseases presents a complex clinical puzzle that remains incompletely understood. The dearth of comprehensive studies investigating the co-occurrence of TB and diabetes, particularly through the perspective of computed tomography (CT), represents a notable gap in current medical literature. This gap is particularly conspicuous given the pivotal role of CT imaging in assessing the multifaceted aspects of TB, including lesion localization, disease extent and identification of complications, which are crucial for tailoring treatment strategies[18-20]. Exploring the CT imaging traits of patients concurrently suffering from TB and diabetes not only promises to shed light on the distinct pathophysiological alterations characteristic of this comorbidity, but also aims to refine diagnostic accuracy and therapeutic interventions. Such research is poised to bridge critical knowledge gaps, facilitating a more nuanced understanding of the interplay between these conditions. The bidirectional exacerbation between TB and diabetes – where diabetes significantly heightens the risk of TB infection, while TB intensifies the metabolic dysregulation associated with diabetes – exemplifies the intricate interaction, warranting a multidisciplinary approach in clinical assessment and management[21,22].
Empirical evidence underscores the heightened susceptibility of diabetic patients to M. tuberculosis, leading to increased TB prevalence and mortality rates within this population. This alarming trend highlights the imperative for heightened clinical awareness and proactive management strategies aimed at this dual disease entity. The exploration of this synergistic relationship between TB and diabetes through advanced imaging techniques such as CT not only has the potential to enhance diagnostic precision, but also to inform more effective, individualized treatment paradigms[23]. Addressing this research gap is not merely an academic exercise but a clinical necessity that stands to affect patient care profoundly. By fostering a comprehensive understanding of the TB–diabetes nexus, healthcare professionals can anticipate and mitigate the compounded morbidity associated with these conditions, ultimately leading to improved patient outcomes and quality of life. This call to action for further research into the CT imaging of TB combined with diabetes underscores the urgent need for an integrated approach in the diagnosis, treatment, and management of patients burdened with these intersecting diseases.
This study analyzed the CT imaging of patients with bacterium-positive pulmonary TB (PTB) combined with diabetes and compared them with patients with simple bacteria-positive PTB to explore their imaging differences and clinical significance. The goal was to provide the medical community with a better understanding of the disease, offering new perspectives and references, provide clinicians with more accurate diagnosis and treatment strategies, and provide a theoretical basis for preventing and controlling the occurrence and prevalence of the disease.
There were 50 patients with bacterium-positive PTB combined with diabetes (experimental group), and 50 with bacterium-positive PTB alone (control group). All patients attended the specialist TB outpatient clinic at our hospital from 2021 to 2023, and all signed informed consent forms before enrollment. The patients' general information included age, gender, medical history, clinical symptoms, signs, and laboratory test results.
This was a retrospective cohort study that compared CT imaging differences between patients with bacterium-positive PTB combined with diabetes and patients with only bacterium-positive PTB, and explored its clinical significance.
Inclusion criteria: (1) Met clinical diagnostic criteria for TB; (2) Age > 18 years; (3) Patients had undergone CT examination with completely preserved imaging data; (4) Patients with bacterium-positive PTB and diabetes met the diagnostic criteria for both TB and diabetes; and (5) The control group had only bacterium-positive PTB.
Exclusion criteria: Other severe respiratory diseases or immune diseases; severe heart, liver, kidney and other organ dysfunction; pregnant or lactating women; mental illness or cognitive dysfunction; unable to provide complete clinical data and imaging information.
According to the research design and inclusion and exclusion criteria, eligible patients were divided into experimental and control groups, with 50 in each group. The experimental group consisted of patients with bacterium-positive PTB combined with diabetes, and the control group consisted of patients with only bacterium-positive PTB. The general information such as age, gender, medical history and clinical symptoms of the two groups of patients were compared to ensure the consistency of the baseline characteristics of the two groups of patients.
Anyone who met one of the following three criteria was diagnosed with TB: (1) The results of acid-fast bacilli test in two sputum samples were positive by microscopy; (2) A sputum smear test for acid-fast bacilli met the following criteria: positive smear microscopy; concurrently, chest radiographic manifestations were consistent with any of the following: (i) primary PTB; (ii) hematogenous disseminated PTB; (iii) secondary PTB; (iv) tracheal or bronchial TB; or (v) tuberculous pleurisy; and (3) One sputum smear for acid-fast bacilli test was positive: smear microscopy examination was positive; and one sputum specimen was positive for mycobacteria; mycobacterial culture was positive, with the species identified as part of the M. tuberculosis complex.
(1) Typical clinical symptoms of TB and chest X-ray manifestations; (2) Effective anti-TB treatment; (3) Other non-tuberculous pulmonary diseases clinically excluded; (4) Strongly positive purified protein derivative (5TU) and positive serum anti-TB antibody; (5) Positive sputum M. tuberculosis PCR+ probe test; (6) Tuberculous lesions confirmed by extrapulmonary tissue pathology; (7) Detection of acid-fast bacilli in bronchoalveolar lavage fluid; and (8) Tuberculous lesions confirmed by bronchial or pulmonary tissue pathology. Diagnosis was confirmed by having any three of criteria 1–6 or either of criteria 7 and 8.
This study was observational and did not entail specific interventions. All patients received treatment according to the standard treatment plan for TB, which included anti-TB drug treatment and supportive care. Additionally, diabetic patients underwent corresponding blood sugar control and treatment according to their diabetes type and condition.
The primary observation index of this study was CT imaging, including the scope, density, shape, cavity formation and calcification of PTB lesions. Additionally, the study records clinical symptoms, signs and pertinent laboratory test results, including blood sugar levels and inflammatory indicators.
SPSS statistical software was used for data processing and analysis. Continuous variables were compared using the t test or Wilcoxon rank sum test, and categorical variables were compared using the χ² test. In addition, logistic regression analysis and other methods were used to explore the relevant factors affecting CT imaging of patients with bacterium-positive PTB and diabetes. The significance level was set at P = 0.05.
This study comprised 100 patients who met the inclusion and exclusion criteria, including 50 patients in each of the experimental group (bacterium-positive PTB combined with diabetes) and the control group (bacterium-positive PTB only). The general information of the two groups of patients was as follows: mean age (± standard deviation) of the experimental group was 54.3 ± 8.9 years, 56% male and 44% female; the mean age of the control group was 52.8 ± 9.5 years, 60% male and 40% female. There was no significant difference in age and gender between the two groups (P > 0.05) (Table 1).
| Group | Experimental group (n = 50) | Control group (n = 50) | t/χ2 | P value |
| Mean age (yr) | 54.3 ± 8.9 | 52.8 ± 9.5 | 0.815 | 0.417 |
| Gender ratio (male/female) | 28/22 | 30/20 | 0.164 | 0.685 |
| Smoking history (yes/no) | 32/18 | 30/20 | 0.169 | 0.680 |
| Drinking history (yes/no) | 25/25 | 22/28 | 0.361 | 0.547 |
| High blood pressure (yes/no) | 42/8 | 41/9 | 0.070 | 0.790 |
| Hyperlipidemia (yes/no) | 28/22 | 26/24 | 0.161 | 0.688 |
| Family genetic factors (yes/no) | 24/26 | 26/24 | 0.160 | 0.689 |
The objective of this study was to compare the clinical symptoms and laboratory findings between patients with bacterium-positive PTB with and without concurrent diabetes. The results revealed no significant differences in clinical symptoms between the two groups. Twenty-two patients in the experimental group experienced cough and sputum, compared to 24 in the control group. The χ2 test yielded a value of 0.161, with a P value of 0.688, indicating no significant difference between the two groups for this symptom. Other common clinical symptoms included sputum production, low-grade fever, and fatigue, with similar incidences observed between the two groups, and no significant differences detected (P > 0.05) (Table 2).
| Index (%) | Experimental group (n = 50) | Control group (n = 50) | χ2 | P value |
| Cough and sputum | 22/28 | 24/26 | 0.161 | 0.688 |
| Chest distress | 12/38 | 15/35 | 0.456 | 0.499 |
| Low-grade fever | 11/39 | 10/40 | 0.060 | 0.806 |
| Hemoptysis | 10/40 | 9/41 | 0.064 | 0.798 |
| Night sweat | 12/38 | 11/39 | 0.056 | 0.812 |
| Lose weight | 11/39 | 10/40 | 0.060 | 0.806 |
| Thoracodynia | 6/44 | 5/45 | 0.102 | 0.749 |
We investigated laboratory parameters between the two groups. The average blood sugar level in the experimental group was 4.5 ± 1.3, compared to 4.4 ± 1.2 in the control group, with a t-test value of 0.400 and P value of 0.690, indicating no significant difference between the two groups. The results also revealed no significant differences in hematological parameters, including hemoglobin and white blood cell count, as well as biochemical parameters, such as blood glucose and albumin, between the two groups (P > 0.05). Erythrocyte sedimentation rate, a validation indicator, also exhibited no significant difference (Table 3).
| Index (%) | Experimental group (n = 50) | Control group (n = 50) | t/χ2 | P value |
| Blood sugar level | 4.5 ± 1.3 | 4.4± 1.2 | 0.400 | 0.690 |
| WBC | 0.578 | 0.748 | ||
| Normal | 35 | 33 | ||
| Rise | 9 | 12 | ||
| Reduce | 6 | 5 | ||
| HGB | 0.543 | 0.461 | ||
| Normal | 45 | 47 | ||
| Anemia | 5 | 3 | ||
| ESR | 0.164 | 0.685 | ||
| Normal | 20 | 22 | ||
| Accelerate | 30 | 28 | ||
| ALB | 0.360 | 0.548 | ||
| Normal | 24 | 27 | ||
| Reduce | 26 | 23 |
The range of PTB lesions in the experimental group was wide, with an average of 4.7 ± 1.2 lesions, compared with 3.2 ± 0.9 in the control group. The difference between the two groups was significant (P < 0.05).
Regarding tuberculosis lesion density, the experimental group exhibited significantly higher density compared to the control group, showing more dense imaging features, and the difference was significant (P < 0.05). The tuberculous lesions in the experimental group had various shapes, including nodular, flaky and patchy, while the lesions in the control group were single and mainly nodular. The difference between the two groups was significant (P < 0.05). The cavity formation rate in the experimental group was 68%, whereas it was 40% in the control group. The difference between the two groups was significant (P < 0.05). The detection rate of calcified lesions in the experimental group was 46%, while it was 28% in the control group. The difference between the two groups was significant (P < 0.05) (Table 4).
| Index (%) | Experimental group (n = 50) | Control group (n = 50) | t/χ2 | P value |
| Average number of lesions | 4.5 ± 1.3 | 4.4 ± 1.2 | 0.246 | 0.806 |
| Calcified lesion detection rate, % | 46 | 28 | 8.574 | 0.003 |
| Tuberculosis lesion morphology | 12.688 | 0.045 | ||
| Tubercular shadow | 6 | 5 | ||
| Patches and clumps of shadow | 32 | 12 | ||
| Striped shadow | 22 | 3 | ||
| Void shadow | 34 | 5 | ||
| Miliary shadow | 3 | 2 | ||
| Pulmonary atelectasis | 0 | 1 | ||
| Honeycomb shadow | 1 | 1 | ||
| Caseosis | 4 | 1 |
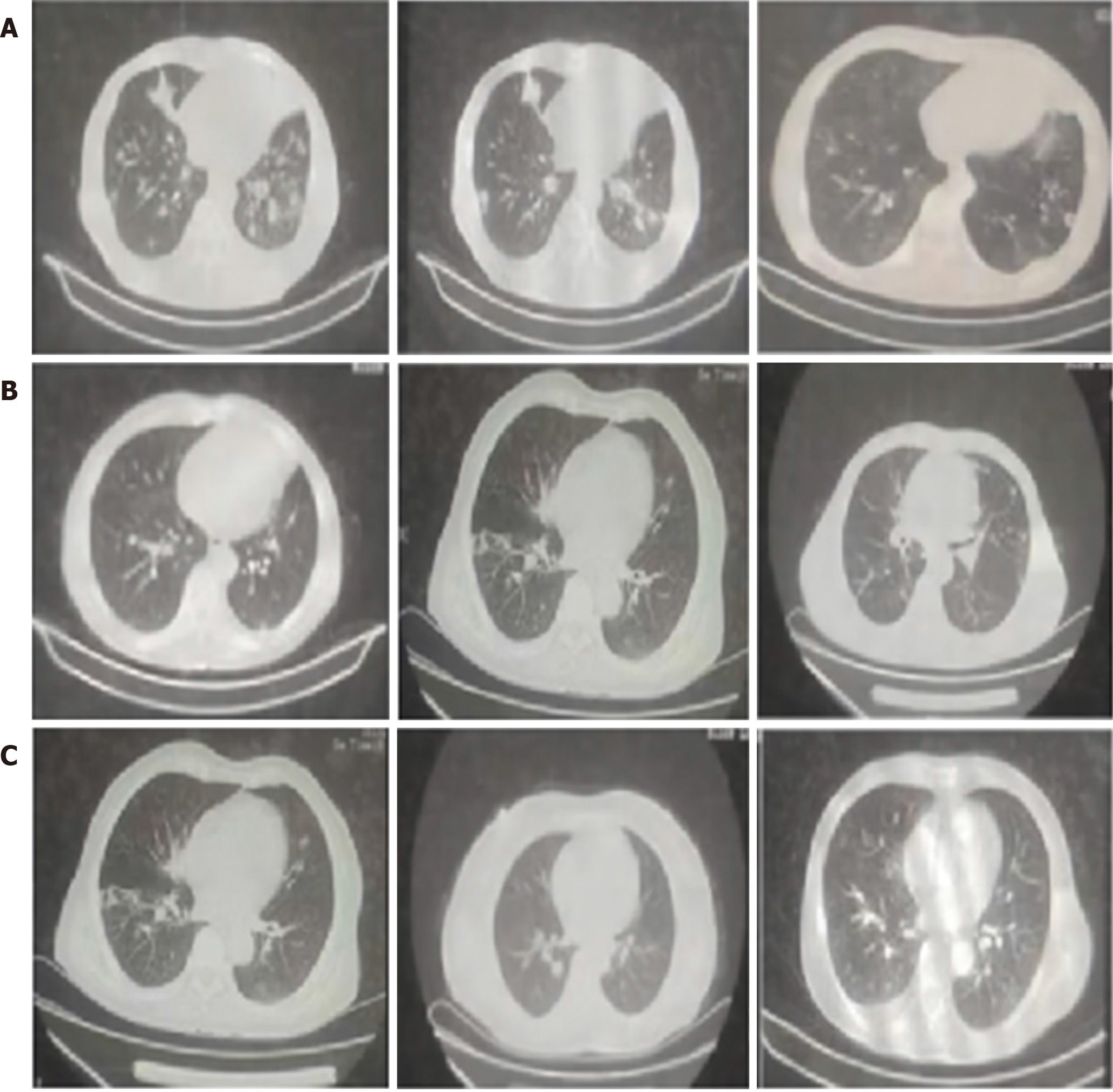
Patients with PTB and diabetes often exhibit multifocal lesions in the lungs, with certain lung segments being more prominently affected. The findings of this study suggest that the morphological features of lung lesions in PTB–diabetes patients predominantly manifest as cavities. However, the presentation of these cavities varies greatly, often occurring concurrently with other manifestations (Figure 1).
Logistic regression analysis revealed a correlation between factors such as age, gender, medical history and degree of pulmonary infection in patients with bacterium-positive PTB and diabetes and CT imaging. The effect size of age was 2.13, with a 95% confidence interval of 1.23–3.45, and P value of 0.023. This suggests that age is significantly associated with outcome, with older individuals likely having a higher risk or different outcome compared to younger individuals. Additionally, the effect size of medical history was -1.78, with a 95% confidence interval of -0.98 to -2.76, and P value of 0.032. The negative effect size indicated that a certain medical history (perhaps one without certain conditions) was associated with a lower risk or different outcome. The association was significant. Medical history and degree of pulmonary infection were factors that independently affected CT imaging (P < 0.05) (Table 5).
| Analysis factors | 95% confidence interval | P value |
| Age | 2.13 (1.23-3.45) | 0.023 |
| Gender | 1.02 (0.67-1.89) | 0.044 |
| Medical history | -1.78 (-0.98 to -2.76) | 0.032 |
| Level of lung infection | 1.13(0.92-3.36) | 0.025 |
| Tubercular shadow | 3.01 (1.55-4.32) | 0.001 |
The combination of bacterium-positive PTB with diabetes is a serious medical condition that warrants special attention. TB is caused by infection with M. tuberculosis, which usually attacks the lungs but may also attack other organs[24]. Diabetes is a chronic disease characterized by consistently elevated blood sugar levels that may lead to a variety of complications. When TB and diabetes coexist, it complicates treatment and increases associated risks.
Diabetic patients are more susceptible to M. tuberculosis infection due to compromised immune function. Conversely, the presence of TB can exacerbate the management of diabetes, potentially leading to further elevations in blood sugar levels[25]. Treatment of this dual disease requires a comprehensive approach and may require a combination of anti-TB drug therapy and diabetes management. At the same time, doctors need to closely monitor patients' conditions to ensure they receive appropriate treatment and management.
Preventive measures play a crucial role, including early screening and treatment of TB, along with regular monitoring and management of diabetes to reduce the risk of complications. Therefore, patients should actively cooperate with treatment recommendations and maintain a healthy lifestyle, including appropriate diet, exercise and medication.
This study conducted a comparative analysis of the CT imaging in patients with bacterium-positive PTB combined with diabetes and patients with only bacterium-positive PTB, and extensively discussed their clinical characteristics and diagnostic value. Lesions in patients with bacterium-positive PTB and diabetes showed wider and denser features on CT images. This phenomenon may be due in part to the compromised immune function of diabetic patients, making them more susceptible to infection and lesion expansion by M. tuberculosis. In addition, diabetes can also lead to pulmonary microcirculation disorders and immune cell dysfunction, which further aggravates the development and severity of TB. The morphology of TB lesions in patients with bacterium-positive PTB and diabetes displayed greater diversity and exhibited more varied imaging features. This may reflect that the pathophysiological changes in diabetic patients during the course of TB infection are different from those in general TB patients, and may be related to factors such as their immune status and glucose metabolism disorders. Moreover, patients with diabetes commonly present with other comorbidities such as cardiovascular disease, and abnormal liver and kidney function. This could further affect the morphological characteristics of tuberculous lesions.
We observed higher cavity formation and detection of calcified lesions in patients with bacterium-positive PTB and diabetes. This discrepancy indicates that development and evolution of lesions in diabetic patients during the course of TB infection are different from those in general TB patients. Cavity formation is often an indicator of TB severity, while calcified lesions may be related to chronic inflammatory responses and the healing process of tuberculous lesions, etc. Logistic regression analysis indicated that patient age, medical history and degree of pulmonary infection were independent factors that affected CT imaging. This finding underscores the importance of considering individual differences and clinical conditions when evaluating CT imaging in patients with bacterium-positive PTB and diabetes, necessitating a comprehensive analysis.
However, this study had several limitations. First, the small sample size may compromise the stability and reliability of the results. Second, it is important to acknowledge that retrospective studies are inherently susceptible to recall bias, as they rely on participants accurately recalling past events, which can sometimes be inaccurate or incomplete. Therefore, further prospective studies with larger samples and multiple centers need to be carried out in the future to verify the results of this study and further explore the clinical characteristics and influencing factors of bacterium-positive PTB combined with diabetes. Additionally, it is necessary to further study the pathophysiological mechanisms of patients with bacterium-positive PTB and diabetes to provide a more reliable basis for their clinical diagnosis and treatment.
Compared to patients with bacterium-positive PTB alone, the CT imaging of patients with bacterium-positive PTB complicated with diabetes showed that the lesions were wider, more dense, diversified in shape, with higher cavity formation rate and higher lesion calcification detection. Age, medical history and degree of pulmonary infection may significantly affect CT imaging in patients with bacterium-positive PTB and diabetes. Therefore, in clinical practice, when evaluating the imaging findings of such patients, it is necessary to pay special attention to factors such as the patient's age, medical history, and degree of lung infection, and formulate personalized treatment plans to improve the treatment effect and lifespan of patients.
| 1. | Eifrig DE, Hermsen V, McManus P, Cunningham R. Rubeosis capsulare. J Cataract Refract Surg. 1990;16:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Klemm M, Gesser C. [The relevance of diabetes for patients with glaucoma]. Klin Monbl Augenheilkd. 2014;231:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Chambers R. On Incontinence of Urine in Children: Fistula in Ano In Phthisis: and the Treatment of Diabetes. Prov Med Surg J. 1846;10:617-618. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Jan RL, Wang JJ, Tseng SH, Chang YS. Sociodemographic Factors and Comorbidities Including Hyperparathyroidism Are Associated With an Increased Risk of Band Keratopathy: A Population-Based Study in Taiwan. Front Endocrinol (Lausanne). 2022;13:927513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Vijitha VS, Dave TV, Murthy SI, Ali MJ, Dave VP, Pappuru RR, Narayanan R. Severe ocular and adnexal complications in dengue hemorrhagic fever: A report of 29 eyes. Indian J Ophthalmol. 2021;69:617-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Niederer RL, Sharief L, Bar A, Lightman SL, Tomkins-Netzer O. Predictors of Long-Term Visual Outcome in Intermediate Uveitis. Ophthalmology. 2017;124:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Sunil C, Irudayaraj SS, Duraipandiyan V, Alrashood ST, Alharbi SA, Ignacimuthu S. Friedelin exhibits antidiabetic effect in diabetic rats via modulation of glucose metabolism in liver and muscle. J Ethnopharmacol. 2021;268:113659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Sengupta J, Khetan A, Saha S, Banerjee D, Gangopadhyay N, Pal D. Candida keratitis: emerging problem in India. Cornea. 2012;31:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | de Gottrau P, Holbach LM, Naumann GO. Clinicopathological review of 1146 enucleations (1980-90). Br J Ophthalmol. 1994;78:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Chen YJ, Kuo HK, Huang HW. Retinal outcomes in proliferative diabetic retinopathy presenting during and after pregnancy. Chang Gung Med J. 2004;27:678-684. [PubMed] |
| 11. | Tsai JC, Feuer WJ, Parrish RK 2nd, Grajewski AL. 5-Fluorouracil filtering surgery and neovascular glaucoma. Long-term follow-up of the original pilot study. Ophthalmology. 1995;102:887-92; discussion 892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Chung PC, Lin HC, Hwang YS, Tsai YJ, Ngan KW, Huang SC, Hsiao CH. Paecilomyces lilacinus scleritis with secondary keratitis. Cornea. 2007;26:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Naumann GD, Portwich E. [Etiology and final clinical cause for 1000 enucleations. (A clinico-pathologic study) (author's transl)]. Klin Monbl Augenheilkd. 1976;168:622-630. [PubMed] |
| 14. | Fernandez ZP. Insulin treatment of diabetes in phthisis. Br Med J. 1925;1:1125. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Mastropasqua L, Carpineto P, Ciancaglini M, Zuppardi E. Long-term results of Krupin-Denver valve implants in filtering surgery for neovascular glaucoma. Ophthalmologica. 1996;210:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Kreiger AE, Sraatsma BR, Foos RY. Incisional complications in pars plana vitrectomy. Mod Probl Ophthalmol. 1977;18:210-223. [PubMed] |
| 17. | Jayne DG, Scholefield J, Tolan D, Gray R, Edlin R, Hulme CT, Sutton AJ, Handley K, Hewitt CA, Kaur M, Magill L. Anal fistula plug versus surgeon's preference for surgery for trans-sphincteric anal fistula: the FIAT RCT. Health Technol Assess. 2019;23:1-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Jayarajah U, Wickramasinghe DP, Samarasekera DN. Anal incontinence and quality of life following operative treatment of simple cryptoglandular fistula-in-ano: a prospective study. BMC Res Notes. 2017;10:572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Arroyo A, Pérez-Legaz J, Moya P, Armañanzas L, Lacueva J, Pérez-Vicente F, Candela F, Calpena R. Fistulotomy and sphincter reconstruction in the treatment of complex fistula-in-ano: long-term clinical and manometric results. Ann Surg. 2012;255:935-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 20. | Khadia M, Muduli IC, Das SK, Mallick SN, Bag L, Pati MR. Management of Fistula-In-Ano with Special Reference to Ligation of Intersphincteric Fistula Tract. Niger J Surg. 2016;22:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | García-Aguilar J, Davey CS, Le CT, Lowry AC, Rothenberger DA. Patient satisfaction after surgical treatment for fistula-in-ano. Dis Colon Rectum. 2000;43:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Ommer A. [Management of complications of fissure and fistula surgery]. Chirurg. 2015;86:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Bałaż K, Trypens A, Polnik D, Pankowska-Woźniak K, Kaliciński P. Perianal abscess and fistula-in-ano in children - evaluation of treatment efficacy. Is it possible to avoid recurrence? Pol Przegl Chir. 2020;92:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Garg P. Comparing existing classifications of fistula-in-ano in 440 operated patients: Is it time for a new classification? A Retrospective Cohort Study. Int J Surg. 2017;42:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Sahebally SM, O'Byrne L, Troy A, Byrnes KG, Burke J, McNamara D. A meta-analysis of marsupialisation versus none in the treatment of simple fistula-in-ano. Int J Colorectal Dis. 2021;36:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |