Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4154
Revised: May 17, 2024
Accepted: June 3, 2024
Published online: July 16, 2024
Processing time: 81 Days and 18.3 Hours
Accurate condition assessment is critical for improving the prognosis of neonatal respiratory distress syndrome (RDS), but current assessment methods for RDS pose a cumulative risk of harm to neonates. Thus, a less harmful method for assessing the health of neonates with RDS is needed.
To analyze the relationships between pulmonary ultrasonography and respiratory distress scores, oxygenation index, and chest X-ray grade of neonatal RDS to identify predictors of neonatal RDS severity.
This retrospective study analyzed the medical information of 73 neonates with RDS admitted to the neonatal intensive care unit of Liupanshui Maternal and Child Care Service Center between April and December 2022. The pulmonary ultrasonography score, respiratory distress score, oxygenation index, and chest X-ray grade of each newborn before and after treatment were collected. Spearman correlation analysis was performed to determine the relationships among these values and neonatal RDS severity.
The pulmonary ultrasonography score, respiratory distress score, oxygenation index, and chest X-ray RDS grade of the neonates were significantly lower after treatment than before treatment (P < 0.05). Spearman correlation analysis showed that before and after treatment, the pulmonary ultrasonography score of neonates with RDS was positively correlated with the respiratory distress score, oxygenation index, and chest X-ray grade (ρ = 0.429–0.859, P < 0.05). Receiver operating characteristic curve analysis indicated that pulmonary ultrasonography screening effectively predicted the severity of neonatal RDS (area under the curve = 0.805–1.000, P < 0.05).
The pulmonary ultrasonography score was significantly associated with the neonatal RDS score, oxygenation index, and chest X-ray grade. The pulmonary ultrasonography score was an effective predictor of neonatal RDS severity.
Core Tip: Current diagnostic and therapeutic evaluation methods for neonatal respiratory distress syndrome (RDS) often cause physical harm to neonates. No studies have yet reported the predictive value of pulmonary ultrasonography scores for neonatal RDS. This study evaluated the relationship between changes in the pulmonary ultrasonography score and the respiratory distress score, oxygenation index, and chest X-ray stage before and after treatment of neonatal RDS. Receiver operating characteristic curves showed that the pulmonary ultrasonography score predicted neonatal RDS severity.
- Citation: Yang H, Gao LJ, Lei J, Li Q, Cui L, Li XH, Yin WX, Tian SH. Relationship between neonatal respiratory distress syndrome pulmonary ultrasonography and respiratory distress score, oxygenation index, and chest radiography grading. World J Clin Cases 2024; 12(20): 4154-4165
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4154.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4154
Neonatal respiratory distress syndrome (RDS) is a common clinical disease caused by the progressive collapse of alveoli owing to a lack of pulmonary surfactant (PS). RDS is most often seen in preterm infants with a gestational age of < 35 wk. The typical manifestations of RDS include progressive dyspnea, moaning, cyanosis, and an inspiratory triple concave sign within 4–12 h after birth. In severe cases, RDS can progress to respiratory failure in newborns, which can be life-threatening and is one of the main causes of neonatal death[1,2]. Therefore, conducting a proper evaluation of the severity of RDS can provide a reference for developing treatment plans for newborns with RDS and improve their prognosis. Currently, chest X-ray examination is the primary method for diagnosing and evaluating the efficacy of RDS in clinical practice. For many years, researchers in China and abroad have been exploring new methods to diagnose RDS in newborns because of the cumulative damage caused by repeated exposure to ionizing radiation. Pulmonary ultrasonography has been applied to the diagnosis of RDS[3]. Pulmonary ultrasonography has high sensitivity and specificity for the diagnosis of pulmonary diseases. It is both simple and noninvasive, can be performed at the bedside, and does not emit ionizing radiation, making it increasingly popular in clinical practice[4-6]. However, the usefulness of lung ultrasonography for the diagnosis and assessment of RDS has not been validated by research. This study aimed to analyze the relationships between pulmonary ultrasonography findings and scores, neonatal RDS scores, oxygenation indices, and lung X-ray grades to explore the practical value of pulmonary ultrasonography for diagnosing and assessing RDS severity. The results will be used to improve the accuracy of diagnosing RDS, the subsequent therapeutic efficacy, and the prognosis of affected children.
This was a retrospective study that used quantitative variables and equation 1 was used to calculate the sample size[7]. In the equation, if the test level was α = 0.05, then the statistical quantity was U1 − α = 1.96. Based on pre-experimental results, the RDS neonatal ultrasonography score was S = 4 and the survey error was d = 1. After calculation, the required sample size was n ≥ 62. Considering possible cases with missing data, the sample size was increased by 10%. Finally, the study sample size needed to be ≤ 69 cases.
n = U21-α× S2 ÷ d2 (1)
According to the sample size estimation results, 73 newborns with RDS admitted to the neonatal intensive care unit of Liupanshui Maternal and Child Care Service Center between April and December 2022 were selected from our medical record information system and included in the study. After communicating with their families, authorization and signed informed consent forms were obtained. Of the 73 neonates with RDS, were 50 male and 23 were female. The gestational age was 26.43–41.29 wk, with an average of 32.97 ± 3.09 wk and the body weight was 950–3700 g, with an average of 1985.75 ± 674.03 g. The first ultrasonography was performed between 0.5 h and 12 h, with an average of 1.90 ± 2.01 h. The study was approved by the Medical Ethics Committee and was performed following the ethical principles for medical research involving human subjects of the World Medical Assembly Declaration of Helsinki[8].
Inclusion criteria: Eligible participants (1) Met the diagnostic criteria for RDS of the “European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants”[9]; (2) Symptoms such as progressive dyspnea, inspiratory triple concave sign, and nasal fan appeared within 24 h after birth; and (3) Blood gas analysis showed hypercapnia and hypoxemia.
Exclusion criteria: (1) Pregnancy < 26 wk or > 42 wk; (2) Respiratory distress caused by asphyxia, infection, lung dampness, or other factors during childbirth; (3) Severe congenital heart defect or abnormal lung development; (4) Presence of genetic metabolic disease; (5) Presence of chromosomal abnormalities; (6) Twins or above; (7) Serious adverse reactions during treatment; (8) Pulmonary ultrasonography examination not performed before or within 12 h after treatment; and (9) Missing clinical data were reasons for exclusion.
Dropout criteria: Newborns in whom (1) The condition worsened or the patient was voluntarily withdrawn for other reasons; (2) Serious adverse reactions occurred during treatment; (3) Who did not undergo pulmonary ultrasonography examination before or within 12 h after treatment; and (4) Clinical data were incomplete were. withdrawn.
The neonates were given noninvasive continuous positive airway pressure ventilation. The initial positive expiratory end positive pressure was 6–7 cmH2O. The inhaled oxygen concentration was adjusted to maintain the target oxygen saturation of 90%–95%. When the newborn’s demand for inhaled oxygen concentration exceeded 40% or the chest X-ray was classified as grade III or IV, PS treatment was performed according to the relevant standards in the United Kingdom National Consensus expert consensus on surfactant replacement therapy for respiratory distress syndrome in preterm infants[10].
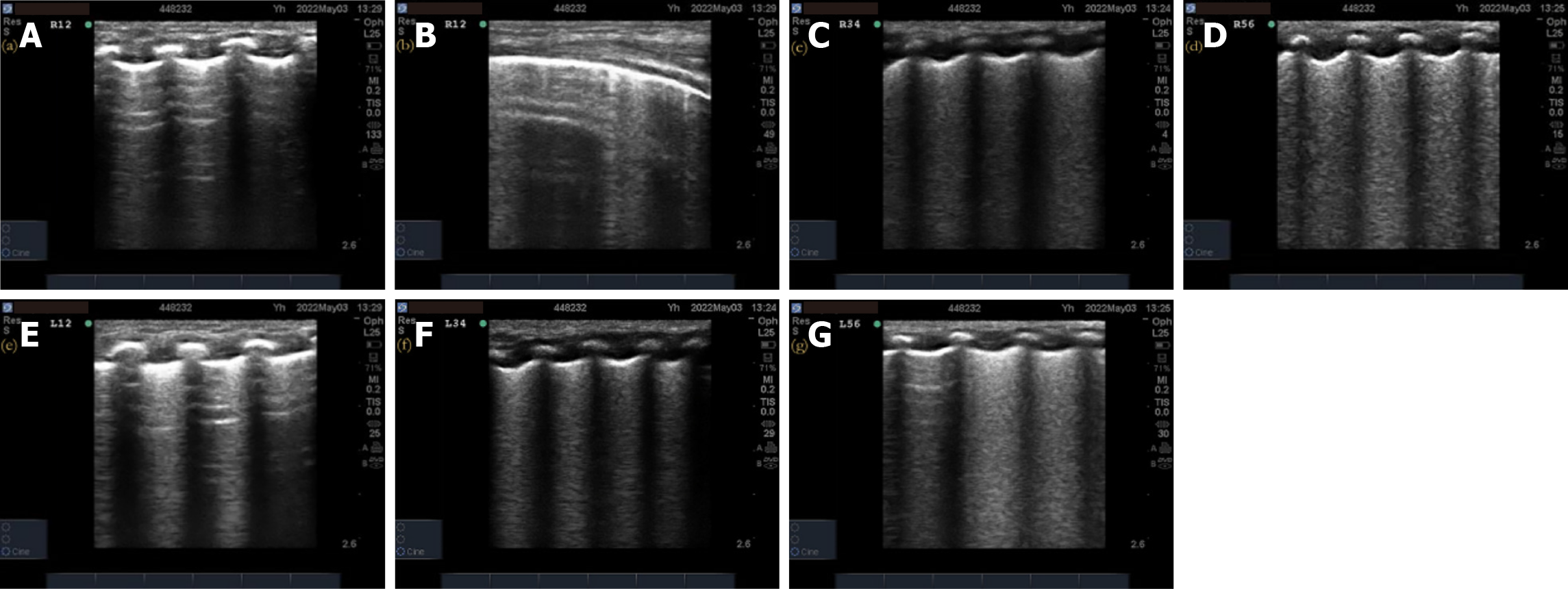
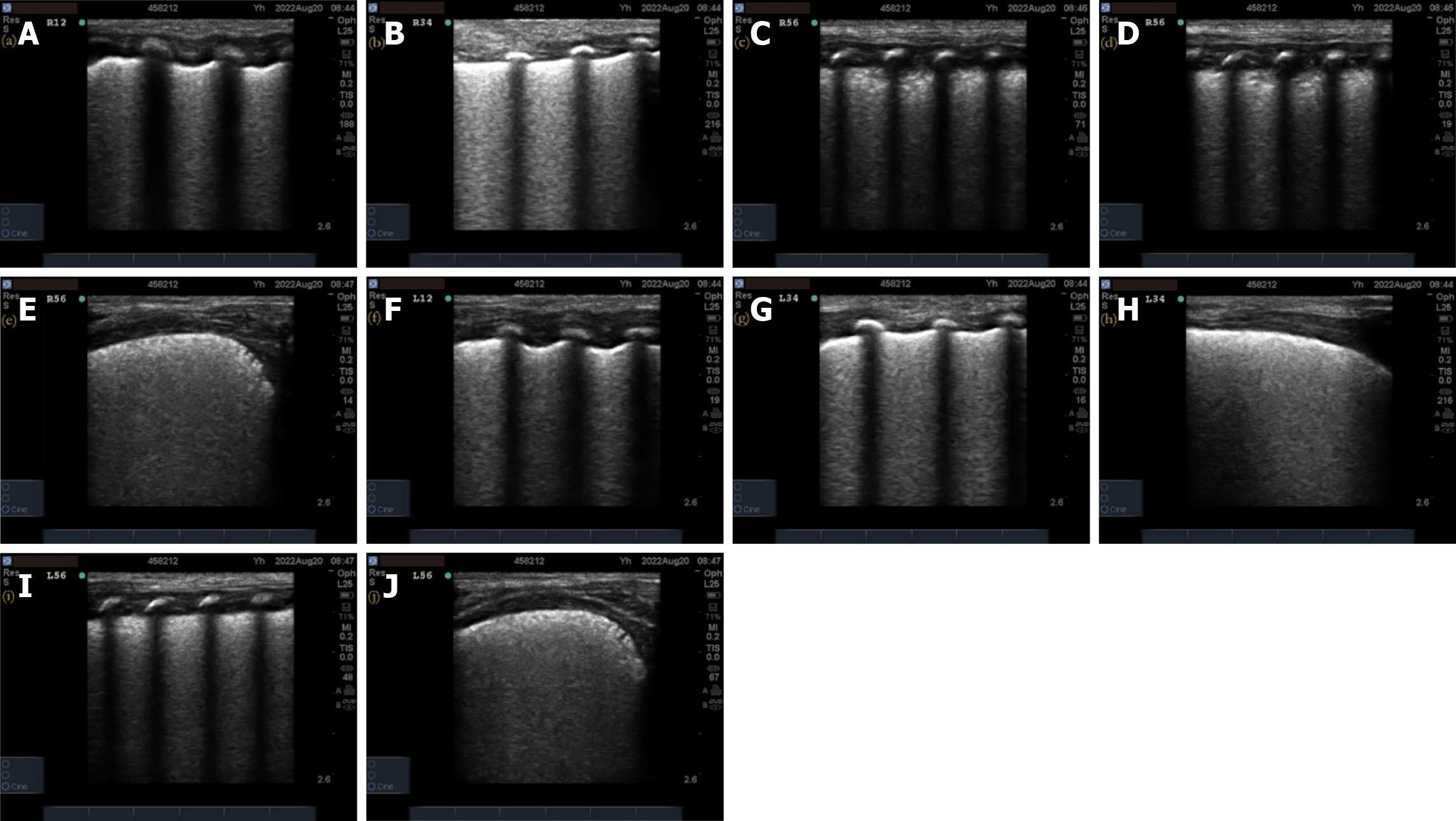
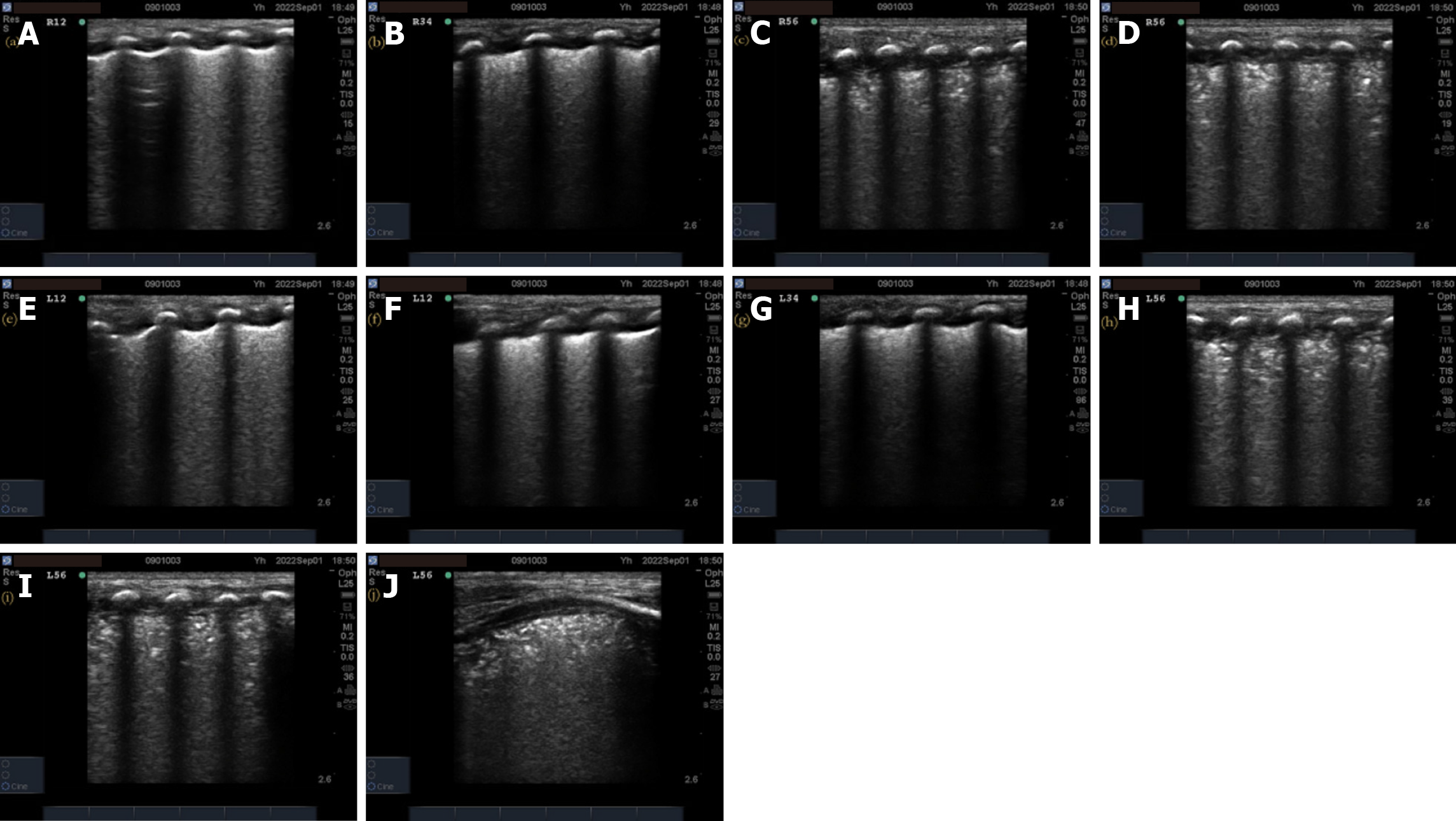
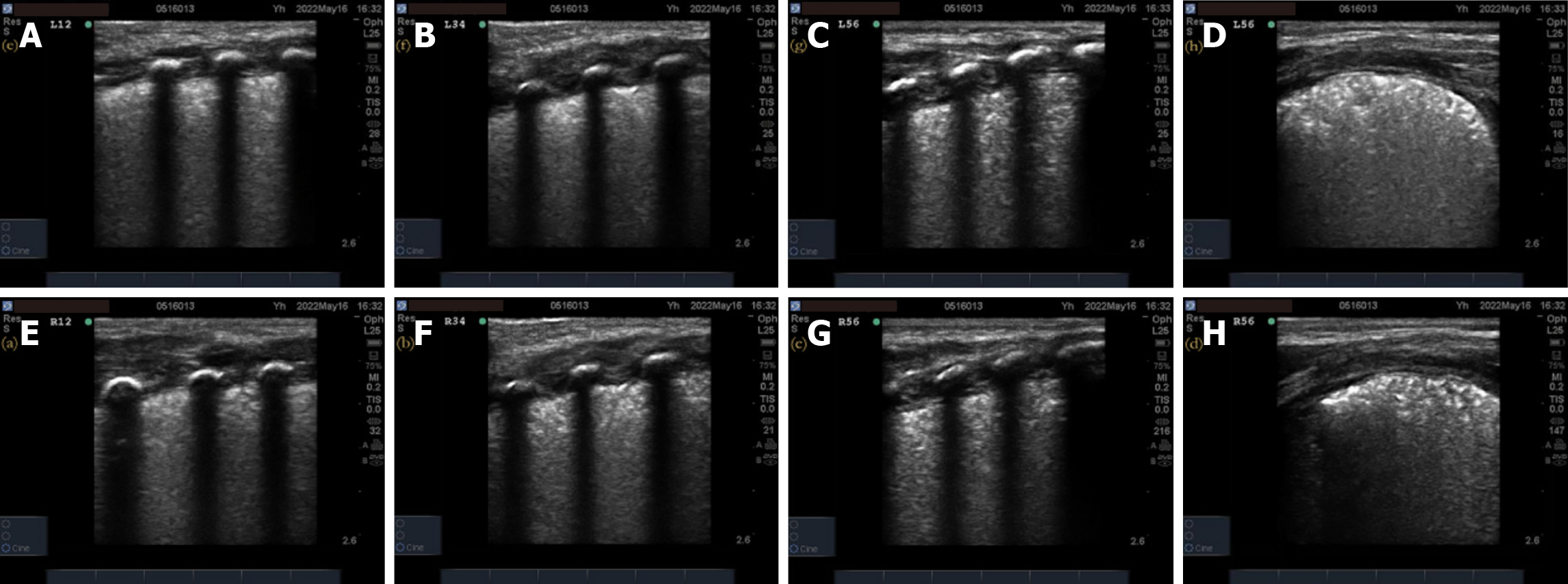
Pulmonary ultrasonography examination and score: (1) The inspection instrument was a SonoSound M-Turbo portable color ultrasonography diagnostic instrument (SonoSound Company, United States) with a linear array probe (frequency 9–13 MHz); (2) The examination method was a. bedside pulmonary ultrasonography examination, requires the newborn to be in a quiet state in supine and lateral positions. The procedure followed the protocol for ultrasonography examination of bilateral lung areas in the “new international guidelines and consensus on the use of lung ultrasonography”[11]. Twelve lung areas in the upper and lower parts of the anterior, posterior, and lateral chest walls of the newborn were evaluated; (3) The pulmonary ultrasonography examination was performed before receiving PS treatment and within 12 h of respiratory support treatment. In addition, a dynamic re-examination of the newborn was conducted according to the condition. The video image data was saved and ultrasonography scoring was performed; and (4) The 12-zone ultrasonography scoring standards were previously described by Bouhemad et al[12] and included b the nature of the B-line and the presence or absence of lung consolidation. A new six-zone ultrasonography scoring system (Table 1) is proposed based on the actual operational structure. The B-line and pulmonary consolidation were assigned scores based on their respective characteristics. The total B-line score was 12 points, the total lung consolidation score was 24 points, and the total overall score was 36 points. Higher scores indicate a more severe neonatal condition.
| Classification | Ultrasonography changes | Ultrasonography scores |
| B-line | There are A-lines and the number of B-lines is < 3 | 0 |
| B-lines appear between two consecutive ribs or more, i.e. B-lines ≥ 3 | 1 | |
| Dense and integrated B-line (without A-line) | 2 | |
| Pulmonary consolidation | No pulmonary consolidation | 0 |
| Involved intercostal space, lung consolidation range ≤ 1/2 of the lung field | 3 | |
| The range of the lung consolidation involving the intercostal space is greater than half of the lung field | 4 |
Neonatal respiratory distress score: The respiratory scoring scale of the Canadian Critical Care Neonatal Care tutorial was used to evaluate the neonatal RDS. The scale consists of six items: respiratory frequency, oxygen demand, oxygen-intake depression, moaning, respiratory sounds, and gestational age. Each item is scored as 0–2 points for a total score of 0–12 points. The higher the score, the worse the respiratory condition of the newborn. Severity was based on the original description by Bouhemad et al[12]. Neonatal respiratory distress was divided into three levels by the score as mild (score of < 5 points), moderate (score = 5–8 points), and severe (score of > 8 points) respiratory distress.
Oxygenation index: Based on the relevant pulmonary ventilation parameters displayed on the ventilator, the oxygenation index was calculated by equations (2) and (3).
MAP = K [(PIP×Ti + PEEP × TE) ÷ Ti + TE] (2)
Oxygenation index= MAP × FiO2 ÷ PaO2 (3)
where MAP is the average airway pressure, K is the pressure waveform coefficient, Ti is the inspiratory time, PEEP is the positive end expiratory pressure, TE is the expiratory time, FiO2 is the inhalation oxygen concentration, and PaO2 is the arterial oxygen partial pressure.
The higher the oxygenation index, the worse the respiratory status of the newborn. Neonatal respiratory distress was divided into three levels by the oxygenation index: mild (oxygenation index = 4.0–7.9), moderate (oxygenation index = 8.0–15.9), and severe (oxygenation index > 16).
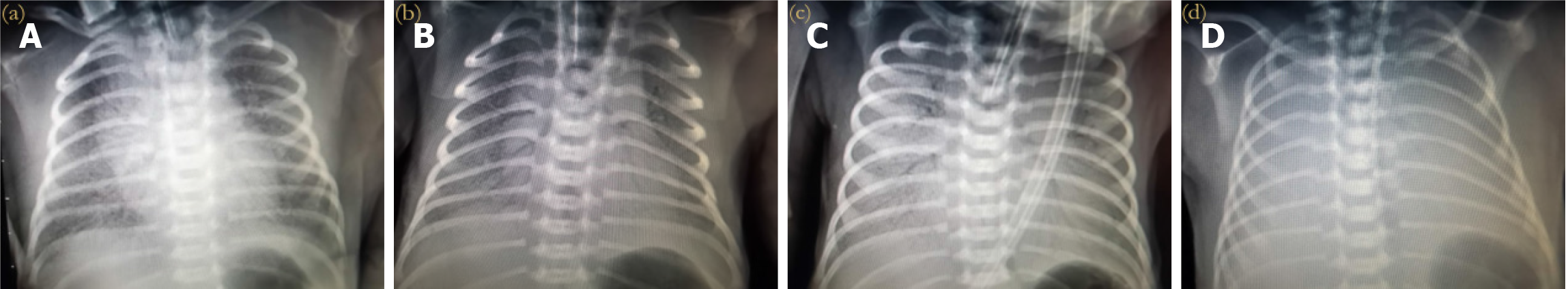
Lung X-ray grading: A Philips MultiDiagnosis X-ray machine was used to perform X-ray examinations on newborns, and their lungs were classified by the imaging findings. The grading standards are shown in Table 2, and the corresponding X-ray imaging manifestations for different X-ray grades are shown in Figure 1. Grades I and II indicate mild RDS, and grades III and IV indicate severe RDS.
| X-ray grading | X-ray imaging manifestations |
| 0 | No image changes |
| I | The transparency of both lung fields is generally reduced, with evenly scattered small particles and reticular shadows visible |
| II | Except for the aggravation of grade I changes, bronchial inflation sign can be seen, extending to the middle and outer zones of the lung field |
| III | The lesion worsens, the transparency of the lung field decreases further, and the cardiac and diaphragmatic margins appear blurry |
| IV | The entire lung field appears as a white lung, with more pronounced bronchial inflation signs resembling bald branches |
The pulmonary ultrasonography score, respiratory distress score, oxygenation index, and pulmonary X-ray grades of all children with RDS were collected before and within 12 h after treatment. Data were collected by two nurses who had received unified professional training. An ultrasonography functional physician and a neonatal physician jointly interpreted the pulmonary ultrasonography for diagnosis and grading.
SPSS 26.0 (IBM Statistics for Windows, IBM Corp., Armonk, NY, United States) was used for data processing and analysis. The Kolmogorov–Smirnov test was performed to verify that the measurement data, reported as means ± SD, were normally distributed. Two categories of baseline data were compared by independent t-tests, and three categories of baseline data were compared by single-factor analysis of variance. Values of clinical indicators before and after treatment were compared with the paired t-test. The Wilcoxon rank sum test was used to compare rank data groups of binary baseline data for the number and percentage (n, %) of use cases of counting data, and the Kruskal–Wallis test was used to compare rank data groups of ternary baseline data. Spearman correlation analysis was performed to assess the relationships of the RDS neonatal pulmonary ultrasonography score, respiratory distress score, oxygenation index, and chest X-ray grade. Graph Prism 9 (GraphPad Software, Boston, MA, United States) was used for plotting. Values of P < 0.05 indicated statistically significant differences.
A total of 73 valid datasets were collected, with a data recovery effectiveness rate of 87.95%. There were no statistically significant differences in the ultrasonography scores, respiratory distress scores, oxygenation index, and chest X-ray grades of RDS newborns among different sexes, gestational weeks, weight, and first ultrasonography time (P > 0.05) before treatment (Table 3). The pulmonary ultrasonography imaging results of one newborn from each of the different chest X-ray grade groups were also analyzed. The details of the pulmonary ultrasonography scores are shown in Figures 2-5.
| Items | n | Ultrasonography score, points | Respiratory distress score, points | Oxygenation index | Chest X-ray grade | |||||
| 0 | I | II | III | IV | ||||||
| Sex | Male | 50 | 26.68 ± 7.41 | 7.60 ± 1.32 | 7.99 ± 2.64 | 0 | 1 | 13 | 17 | 19 |
| Female | 23 | 26.87 ± 7.35 | 7.83 ± 0.98 | 7.76 ± 1.59 | 0 | 1 | 7 | 5 | 10 | |
| t/Z | −0.102 | −0.730 | 0.388 | −0.057 | ||||||
| P value | 0.919 | 0.468 | 0.699 | 0.955 | ||||||
| Pregnancy duration in wk | < 32 | 29 | 25.66 ± 7.83 | 7.69 ± 1.26 | 7.59 ± 2.76 | 0 | 2 | 7 | 10 | 10 |
| 32–36 | 35 | 26.77 ± 6.95 | 7.57 ± 1.29 | 8.01 ± 2.17 | 0 | 0 | 11 | 11 | 13 | |
| > 36 | 9 | 30.11 ± 6.86 | 8.00 ± 0.87 | 8.66 ± 1.40 | 0 | 0 | 2 | 1 | 6 | |
| F/H | 1.277 | 0.436 | 0.744 | 2.073 | ||||||
| P value | 0.285 | 0.648 | 0.479 | 0.355 | ||||||
| Weight in g | < 1500 | 21 | 26.29 ± 8.28 | 7.76 ± 1.22 | 7.87 ± 3.06 | 0 | 1 | 6 | 5 | 9 |
| 1500–2500 | 38 | 26.29 ± 6.85 | 7.53 ± 1.22 | 7.56 ± 2.03 | 0 | 1 | 10 | 14 | 13 | |
| > 2500 | 14 | 28.64 ± 7.39 | 7.93 ± 1.27 | 8.97 ± 1.70 | 0 | 0 | 4 | 3 | 7 | |
| F/H | 0.576 | 0.625 | 1.901 | 0.494 | ||||||
| P value | 0.565 | 0.538 | 0.157 | 0.781 | ||||||
| First ultrasonography time in h | < 1 | 15 | 29.20 ± 6.13 | 8.07 ± 0.80 | 9.02 ± 2.65 | 0 | 0 | 2 | 3 | 10 |
| 1–1.5 | 39 | 25.23 ± 7.68 | 7.59 ± 1.33 | 7.68 ± 2.30 | 0 | 2 | 12 | 13 | 12 | |
| > 1.5 | 19 | 27.89 ± 7.09 | 7.53 ± 1.26 | 7.55 ± 2.06 | 0 | 0 | 6 | 6 | 7 | |
| F/H | 1.954 | 1.000 | 2.135 | 5.689 | ||||||
| P value | 0.149 | 0.373 | 0.126 | 0.058 | ||||||
The RDS neonatal ultrasonography scores, respiratory distress scores, oxygenation indexes, and chest X-ray grades were significantly lower after treatment than before treatment (P < 0.05) (Table 4).
| Items | Before treatment, n = 73 | After treatment, n = 73 | t/Z | P value | |
| Ultrasonography score (points) | 26.74 ± 7.34 | 15.12 ± 6.11 | 17.324 | < 0.001 | |
| Respiratory distress score (points) | 7.67 ± 1.23 | 3.93 ± 1.25 | 47.887 | < 0.001 | |
| Oxygenation index | 7.92 ± 2.35 | 5.84 ± 2.13 | 9.543 | < 0.001 | |
| Chest X-ray grade | 0 | 0 (0.00) | 32 (43.84) | −7.460 | < 0.001 |
| I | 2 (2.74) | 24 (32.88) | |||
| II | 20 (27.40) | 16 (21.92) | |||
| III | 22 (30.14) | 1 (1.37) | |||
| IV | 29 (39.73) | 0 (0.00) | |||
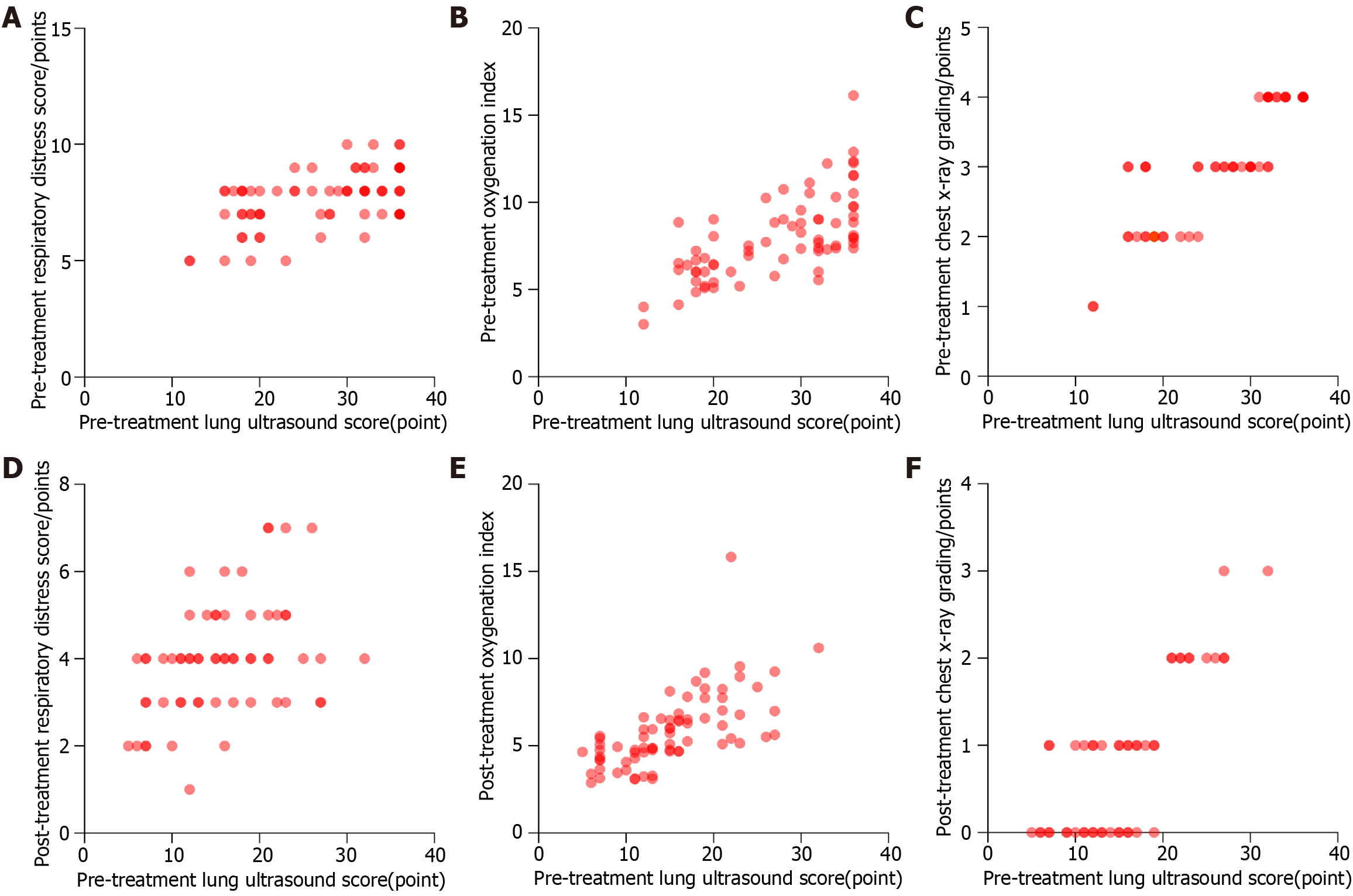
The RDS neonatal ultrasonography scores, respiratory distress scores, oxygenation indexes, and chest X-ray grades showed a significant decrease after treatment compared to before treatment (P < 0.05) (Table 4). Of these, the linear relationship shown in Figure 6A–D was relatively good, but the scatter in Figure 6E–F was relatively broad, with a relatively poor linear relationship. Spearman’s correlation analysis showed that the ultrasonography scores of the RDS newborns before and after treatment were positively correlated with the respiratory distress scores, oxygenation indexes, and chest X-ray grades (< 0.05) (Table 5).
| Comparator | Ultrasonography score | |||
| Before treatment | After treatment | |||
| ρ | P value | ρ | P value | |
| Respiratory distress score | 0.446 | < 0.001 | 0.429 | < 0.001 |
| Oxygenation index | 0.703 | < 0.001 | 0.748 | < 0.001 |
| Chest X-ray grade | 0.859 | < 0.001 | 0.764 | < 0.001 |
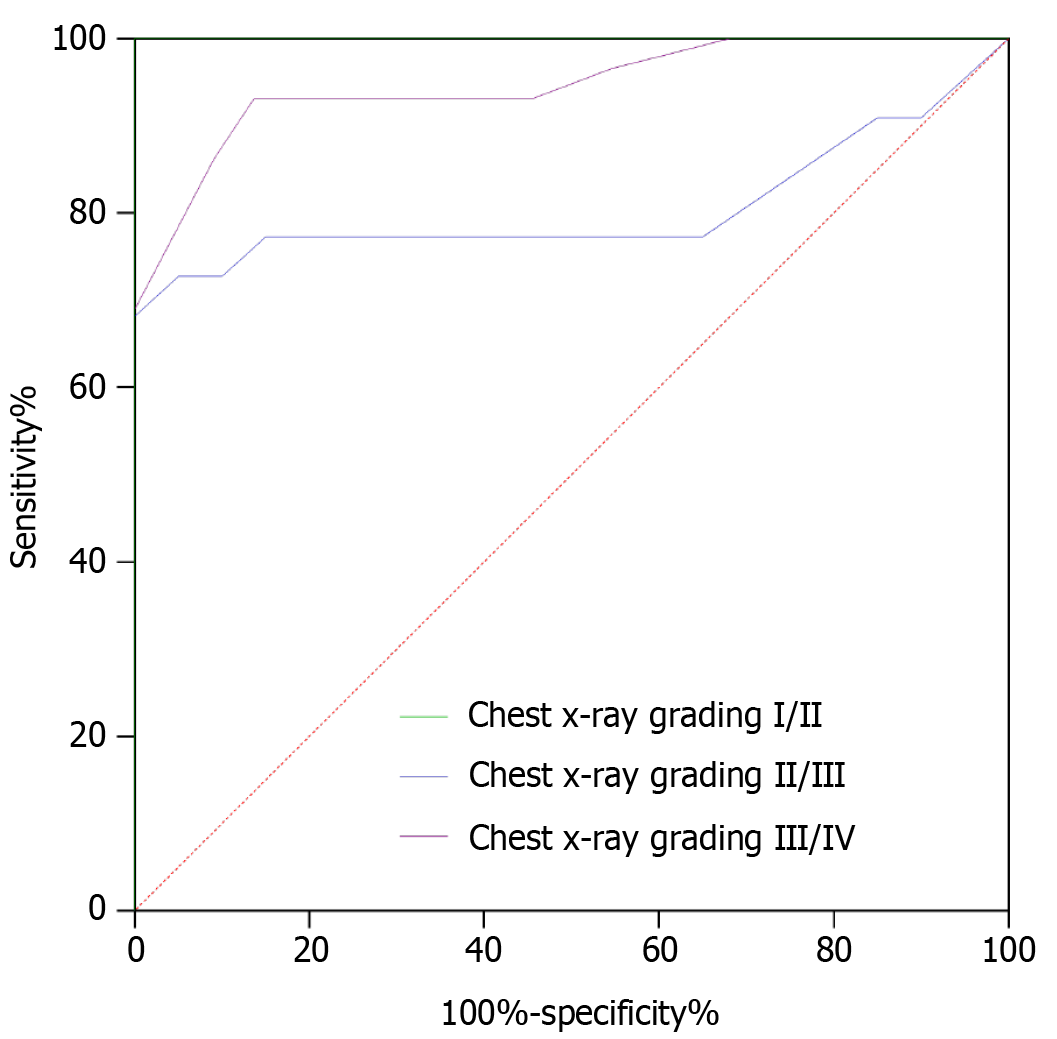
To verify the value of the pulmonary ultrasonography score for predicting neonatal RDS severity, the pulmonary ultrasonography score was used as a variable and the chest X-ray grade as a categorical variable to perform receiver operating characteristic (ROC) analysis. The results showed that the pulmonary ultrasonography score effectively predicted the severity of the RDS condition of newborns. Table 6 shows the ROC-related parameters, and Figure 7 shows the ROC curves.
| Categorical variable | AUC | SE | P value | Youden index | Optimal cutoff value | Sensitivity, % | Specificity, % |
| I–II | 1.000 | 0.000 | < 0.001 | 1.000 | > 12 | 100.00 | 100.00 |
| II–III | 0.805 | 0.077 | < 0.001 | 0.682 | > 24 | 68.18 | 100.00 |
| III–IV | 0.946 | 0.030 | < 0.001 | 0.795 | > 30 | 93.10 | 86.36 |
The theory of pulmonary ultrasonography was first proposed by Lichtenstein in 1992. The total reflection of ultrasonography when encountering gas in the alveoli and the influence of a bony chest led most researchers to believe that pulmonary ultrasonography had limitations that prevented it from becoming a routine auxiliary examination method in the early stages of development. After years of research and clinical practice, it has been found that under pathological conditions, the gas–liquid ratio of pulmonary tissue changes, greatly reducing the effect of gas in the alveoli on imaging. The sensitivity and specificity of pulmonary ultrasonography diagnosis have increased with advancements in ultrasonography technology. Given that this technique is convenient, radiation free, and repeatable, pulmonary ultrasonography technology is widely used in the clinical diagnosis and treatment of various pulmonary diseases. In newborns with RDS, pulmonary ultrasonography can often recognize and distinguish between normal and abnormal pulmonary tissue based on features such as extensive alveolar collapse, pulmonary interstitial edema, and the formation of an eosinophilic transparent membrane. This differentiation is possible because of the formation of ultrasonography reverberation artifacts[13,14]. In this study, the ultrasonographic examination of the lungs of. newborns with RDS was characterized by abnormal pleural lines, abnormal A-lines, the presence of B-lines, manifestations similar to pulmonary adenocarcinoma in situ, and pulmonary consolidation changes accompanied by the bronchial inflation sign, which were consistent with the pulmonary ultrasonography results of RDS newborns in previous studies[15,16].
The neonatal respiratory distress score was derived from the Canadian ACoRN tutorial, which has the characteristics of simplicity, accuracy, and high clinical applicability[17]. The neonatal respiratory distress score objectively and accurately assesses the respiratory status of newborns, and the protocol resulted in dividing RDS newborns into three score levels. Noninvasive continuous positive airway pressure ventilation was provided for newborns with moderate respiratory distress, and invasive ventilator-assisted ventilation was provided for newborns with severe respiratory distress, which is similar to the methods used in clinical practice. This score also provided a good diagnosis and treatment reference for physicians with relatively limited clinical experience. The oxygenation index reflects the ventilation function of the lungs. Some studies have reported that dynamic changes in the oxygenation index can effectively predict the prognosis of patients with RDS[18]. This is because the extravascular lung water index is closely related to damage of the pulmonary vascular endothelium and alveolar epithelial cells. An increase of the extravascular lung water index often indicates aggravated lung injury. However, when the extravascular lung water is more than twice the normal value, gas diffusion and lung function are affected, leading to pulmonary edema, thereby reducing the static compliance of the oxygenation index[19]. In addition, Picano and Pellikka[20] noted that the number of B-lines was related to the extrava
In summary, pulmonary ultrasonography findings and scores had varying degrees of correlation with the neonatal RDS score, oxygenation index, and chest X-ray grade, and were used clinically as predictors of the neonatal RDS condition. However, owing to the small sample size used in this study, the results may be biased. Future studies with larger sample sizes would be useful to further explore the value of pulmonary ultrasonography findings and scores for evaluating neonatal RDS conditions.
| 1. | Altman M, Vanpée M, Cnattingius S, Norman M. Risk factors for acute respiratory morbidity in moderately preterm infants. Paediatr Perinat Epidemiol. 2013;27:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GH, Halliday HL. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology. 2017;111:107-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (2)] |
| 3. | Yang JH, Wang S, Gan YX, Feng XY, Niu BL. Short-term prone positioning for severe acute respiratory distress syndrome after cardiopulmonary bypass: A case report and literature review. World J Clin Cases. 2022;10:13435-13442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Vasovic LV, Littlejohn J, Alqunaibit D, Dillard A, Qiu Y, Rand S, Bronstein M, Gibson CJ, Kelly AG, Lee C, Minneman JA, Narayan M, Shou J, Smith KE, Villegas CV, Winchell RJ, Cushing MM, Barie PS. Rotational thromboelastometry in patients with acute respiratory distress syndrome owing to coronavirus disease 2019: Is there a viscoelastic fingerprint and a role for predicting thrombosis? Surgery. 2022;171:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Staub LJ, Mazzali Biscaro RR, Kaszubowski E, Maurici R. Lung Ultrasound for the Emergency Diagnosis of Pneumonia, Acute Heart Failure, and Exacerbations of Chronic Obstructive Pulmonary Disease/Asthma in Adults: A Systematic Review and Meta-analysis. J Emerg Med. 2019;56:53-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Peixoto AO, Costa RM, Uzun R, Fraga AMA, Ribeiro JD, Marson FAL. Applicability of lung ultrasound in COVID-19 diagnosis and evaluation of the disease progression: A systematic review. Pulmonology. 2021;27:529-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Widowati W, Wargasetia T, Rahardja F, Gunanegara R, Priyandoko D, Gondokesumo M, Afifah E, Wijayanti C, Rizal R. Human Wharton’s jelly mesenchymal stem cells inhibit cytokine storm in acute respiratory distress syndrome in a rat model. Asian Pac J Trop Biomed. 2022;12:343. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Shrestha B, Dunn L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J Nepal Health Res Counc. 2020;17:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 9. | Emeriaud G, López-Fernández YM, Iyer NP, Bembea MM, Agulnik A, Barbaro RP, Baudin F, Bhalla A, Brunow de Carvalho W, Carroll CL, Cheifetz IM, Chisti MJ, Cruces P, Curley MAQ, Dahmer MK, Dalton HJ, Erickson SJ, Essouri S, Fernández A, Flori HR, Grunwell JR, Jouvet P, Killien EY, Kneyber MCJ, Kudchadkar SR, Korang SK, Lee JH, Macrae DJ, Maddux A, Modesto I Alapont V, Morrow BM, Nadkarni VM, Napolitano N, Newth CJL, Pons-Odena M, Quasney MW, Rajapreyar P, Rambaud J, Randolph AG, Rimensberger P, Rowan CM, Sanchez-Pinto LN, Sapru A, Sauthier M, Shein SL, Smith LS, Steffen K, Takeuchi M, Thomas NJ, Tse SM, Valentine S, Ward S, Watson RS, Yehya N, Zimmerman JJ, Khemani RG; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) Group on behalf of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Executive Summary of the Second International Guidelines for the Diagnosis and Management of Pediatric Acute Respiratory Distress Syndrome (PALICC-2). Pediatr Crit Care Med. 2023;24:143-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 139] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 10. | Banerjee S, Fernandez R, Fox GF, Goss KCW, Mactier H, Reynolds P, Sweet DG, Roehr CC. Surfactant replacement therapy for respiratory distress syndrome in preterm infants: United Kingdom national consensus. Pediatr Res. 2019;86:12-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Demi L, Wolfram F, Klersy C, De Silvestri A, Ferretti VV, Muller M, Miller D, Feletti F, Wełnicki M, Buda N, Skoczylas A, Pomiecko A, Damjanovic D, Olszewski R, Kirkpatrick AW, Breitkreutz R, Mathis G, Soldati G, Smargiassi A, Inchingolo R, Perrone T. New International Guidelines and Consensus on the Use of Lung Ultrasound. J Ultrasound Med. 2023;42:309-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 143] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 12. | Bouhemad B, Dransart-Rayé O, Mojoli F, Mongodi S. Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann Transl Med. 2018;6:418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Zanforlin A, Giannuzzi R, Nardini S, Testa A, Soldati G, Copetti R, Marchetti G, Valente S, Inchingolo R, Smargiassi A. The role of chest ultrasonography in the management of respiratory diseases: document I. Multidiscip Respir Med. 2013;8:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Rea G, Sperandeo M, Di Serafino M, Vallone G, Tomà P. Neonatal and pediatric thoracic ultrasonography. J Ultrasound. 2019;22:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Copetti R, Cattarossi L, Macagno F, Violino M, Furlan R. Lung ultrasound in respiratory distress syndrome: a useful tool for early diagnosis. Neonatology. 2008;94:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Liu J, Cao HY, Wang HW, Kong XY. The Role of Lung Ultrasound in Diagnosis of Respiratory Distress Syndrome in Newborn Infants. Iran J Pediatr. 2015;25:e323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Xia B, Shen X, He Y, Pan X, Liu FL, Wang Y, Yang F, Fang S, Wu Y, Duan Z, Zuo X, Xie Z, Jiang X, Xu L, Chi H, Li S, Meng Q, Zhou H, Zhou Y, Cheng X, Xin X, Jin L, Zhang HL, Yu DD, Li MH, Feng XL, Chen J, Jiang H, Xiao G, Zheng YT, Zhang LK, Shen J, Li J, Gao Z. SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Res. 2021;31:847-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 18. | Leow EH, Wong JJ, Mok YH, Hornik CP, Ng YH, Lee JH. Fluid overload in children with pediatric acute respiratory distress syndrome: A retrospective cohort study. Pediatr Pulmonol. 2022;57:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Yang J, Cheng D, Hofer I, Nguyen-Buckley C, Disque A, Wray C, Xia VW. Intraoperative High Tidal Volume Ventilation and Postoperative Acute Respiratory Distress Syndrome in Liver Transplant. Transplant Proc. 2022;54:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J. 2016;37:2097-2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 21. | Tang S, Bao L. Clinical characteristics and prognosis-related factors of neonatal acute respiratory distress syndrome. Disan Junyi Daxue Xuebao. 2019;41:898-902. [DOI] [Full Text] |
| 22. | Offer K, Sara D, Avraham M, Gorfil DM, Nisim I. Severe Acute Respiratory Distress Syndrome in a Patient with Sickle-Cell Anemia Requiring Veno-Venous Extracorporeal Membrane Oxygenation Therapy: Case Report and Review of the Literature. CRCM. 2022;11:499-506. [DOI] [Full Text] |
| 23. | Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, De Luca D. Lung Ultrasonography Score to Evaluate Oxygenation and Surfactant Need in Neonates Treated With Continuous Positive Airway Pressure. JAMA Pediatr. 2015;169:e151797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 312] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 24. | Powell MBF, Rajapreyar P, Yan K, Sirinit J, Mikhailov TA. Nutrition practices and outcomes in patients with pediatric acute respiratory distress syndrome. JPEN J Parenter Enteral Nutr. 2022;46:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |