Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4048
Revised: May 6, 2024
Accepted: May 31, 2024
Published online: July 16, 2024
Processing time: 114 Days and 16 Hours
Post-stroke infection is the most common complication of stroke and poses a huge threat to patients. In addition to prolonging the hospitalization time and increa
To explore the risk factors for post-stroke infection in patients with AIS and to construct a nomogram predictive model.
The clinical data of 206 patients with AIS admitted to our hospital between April 2020 and April 2023 were retrospectively collected. Baseline data and post-stroke infection status of all study subjects were assessed, and the risk factors for post-stroke infection in patients with AIS were analyzed.
Totally, 48 patients with AIS developed stroke, with an infection rate of 23.3%. Age, diabetes, disturbance of consciousness, high National Institutes of Health Stroke Scale (NIHSS) score at admission, invasive operation, and chronic obstructive pulmonary disease (COPD) were risk factors for post-stroke infection in patients with AIS (P < 0.05). A nomogram prediction model was constructed with a C-index of 0.891, reflecting the good potential clinical efficacy of the nomogram prediction model. The calibration curve also showed good consistency between the actual observations and nomogram predictions. The area under the receiver operating characteristic curve was 0.891 (95% confidence interval: 0.839–0.942), showing predictive value for post-stroke infection. When the optimal cutoff value was selected, the sensitivity and specificity were 87.5% and 79.7%, respectively.
Age, diabetes, disturbance of consciousness, NIHSS score at admission, invasive surgery, and COPD are risk factors for post-stroke infection following AIS. The nomogram prediction model established based on these factors exhibits high discrimination and accuracy.
Core Tip: This study analyzed the risk factors for post-stroke infection in patients with acute ischemic stroke and constructed a nomogram prediction model. Age, diabetes, disturbance of consciousness, National Institutes of Health Stroke Scale score at admission, invasive surgery, and chronic obstructive pulmonary disease were identified to be important risk factors for post-stroke infection in patients with acute ischemic stroke. The nomogram prediction model established based on these factors has high discriminative power and accuracy.
- Citation: Liu XC, Chang XJ, Zhao SR, Zhu SS, Tian YY, Zhang J, Li XY. Identification of risk factors and construction of a nomogram predictive model for post-stroke infection in patients with acute ischemic stroke. World J Clin Cases 2024; 12(20): 4048-4056
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4048.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4048
Acute ischemic stroke (AIS) is a type of clinically common cerebrovascular disease with a high incidence rate, disability rate, and mortality rate, posing a great threat to the quality of life and health of patients[1,2]. AIS mostly occurs in middle-aged and elderly individuals[2,3]. After the occurrence of the disease, it can damage the central nervous system to varying degrees, cause abnormal autonomic neuromodulation function, cause pulmonary hypertension, and increase the risk of stroke-related infections, such as pulmonary edema and pulmonary infection[3,4].
In addition, AIS has an acute onset and rapid progression, which can also increase the risk of pulmonary infection and other complications owing to the influence of the disease itself and related invasive procedures[5,6]. Stroke related infections can seriously threaten the prognosis and quality of life of patients[6,7]. Research has shown that most AIS patients die from stroke-related complications, and infection is the most common and threatening type of complication. It not only prolongs hospital stay and increases medical burden but also significantly increases the risk of death[7-10].
Based on the above background, it is of great significance to clarify the risk factors for post-stroke infection in patients with AIS, which can guide clinical prevention and control work as early as possible, minimize the risk of stroke-related infections, and ensure good disease outcomes. Therefore, this study conducted a retrospective analysis of the clinical data of AIS patients admitted to our hospital with the aim of identifying the risk factors for post-stroke infection.
Univariate and multivariate binary logistic regression analyses were used to analyze the clinical data of AIS patients, construct a nomogram model to predict the risk of post-stroke infection in AIS patients, and verify the predictive value of the model.
AIS patients admitted to our hospital between April 2020 and April 2023.
A total of 231 patients were included in this study, including eight patients with malignant tumors, seven patients with immune system disorders, five critically ill patients who underwent AIS surgery or were admitted to the intensive care unit, and five patients with missing clinical data. A total of 206 patients met the inclusion criteria for this study.
Inclusion criteria: The inclusion criteria were: (1) Meeting the diagnostic criteria for AIS; (2) First occurrence of AIS; (3) National Institutes of Health Stroke Scale (NIHSS) score at admission ≥ 7 points; and (4) complete clinical data.
Exclusion criteria: The inclusion criteria were: (1) Local or systemic infections occurring upon admission; (2) individuals with other severe systemic conditions; (3) patients with transient ischemic attacks or hemorrhagic stroke; (4) individuals with immune system or blood system diseases; and (5) individuals with malignant tumors.
Sex, age, hypertension, hyperlipidemia, coronary heart disease, atrial fibrillation, smoking, alcohol consumption, presence or absence of diabetes, disturbance of consciousness, NIHSS score at admission, invasive procedures, and chronic obstructive pulmonary disease (COPD) were evaluated as potential risk factors for post-stroke infection in patients with acute ischemic stroke.
SPSS22.0 and R software version 4.0.0 were used for statistical analyses. Measurement data are expressed as the mean ± SD, and intergroup comparisons were conducted using an independent sample t-test. Counting data are represented by n (%), and the comparison between groups was performed using the chi-square test. A binary logistic regression model was used to analyze the influencing factors of post-stroke infection, and the "rms" package in R was used to construct a nomogram. Receiver operating characteristic (ROC) curve analysis was performed to assess the predictive efficacy of the nomogram. The difference was considered statistically significant at P < 0.05.
A total of 206 patients were included, including 126 males and 80 females. They ranged in age from 38–85 years, with an average age of 61.9 ± 8.5 years. Forty-eight AIS patients developed post-stroke infection, with an incidence rate of 23.3%. The infections occurred in the upper respiratory tract in 15 (31.3%) cases, in the urinary system in 12 (25%), in the lower respiratory tract in 8 (16.7%), 7 cases (14.6%) in the gastrointestinal tract in 7 (14.6%), and in the oral cavity, skin, and other parts in 6 (12.5%).
Single-factor analysis showed that there were significant differences between infected and non-infected individuals in age, whether they had diabetes, consciousness disorder, NIHSS score at admission, whether they had invasive operations, and whether they had COPD (P < 0.05) (Table 1).
| Non-infection (n = 158) | Infection (n = 48) | Z/t/χ2 | P value | |
| Gender | 0.015 | 0.903 | ||
| Male | 97 (61.4) | 29 (60.4) | ||
| Female | 61 (38.6) | 19 (39.6) | ||
| Age (years) | 60.16 ± 7.86 | 67.81 ± 7.88 | -5.902 | 0.000 |
| Hypertension | 2.423 | 0.120 | ||
| No | 56 (35.4) | 23 (47.9) | ||
| Yes | 102 (64.6) | 25 (52.1) | ||
| Hyperlipidemia | 0.043 | 0.836 | ||
| No | 133 (84.2) | 41 (85.4) | ||
| Yes | 25 (15.8) | 7 (14.6) | ||
| Coronary heart disease | 0.020 | 0.887 | ||
| No | 137 (86.7) | 42 (87.5) | ||
| Yes | 21 (13.3) | 6 (12.5) | ||
| Atrial fibrillation | 0.000 | 1.000 | ||
| No | 145 (91.8) | 44 (91.7) | ||
| Yes | 13 (8.2) | 4 (8.3) | ||
| Smoking | 0.087 | 0.768 | ||
| No | 95 (60.1) | 30 (62.5) | ||
| Yes | 63 (39.9) | 18 (37.5) | ||
| Drinking | 0.121 | 0.728 | ||
| No | 57 (36.1) | 16 (33.3) | ||
| Yes | 101 (63.9) | 32 (66.7) | ||
| Diabetes | 24.42 | 0.000 | ||
| No | 127 (80.4) | 21 (43.8) | ||
| Yes | 31 (19.6) | 27 (56.3) | ||
| Disturbance of consciousness | 25.115 | 0.000 | ||
| No | 140 (88.6) | 27 (56.3) | ||
| Yes | 18 (11.4) | 21 (43.8) | ||
| NIHSS score at admission [M (IQR), score] | 9 (8.11) | 10 (9.13) | -2.668 | 0.008 |
| Invasive operation | 11.115 | 0.001 | ||
| No | 119 (75.3) | 24 (50.0) | ||
| Yes | 39 (24.7) | 24 (50.0) | ||
| COPD | 16.544 | 0.000 | ||
| No | 116 (73.4) | 20 (41.7) | ||
| Yes | 42 (26.6) | 28 (58.3) |
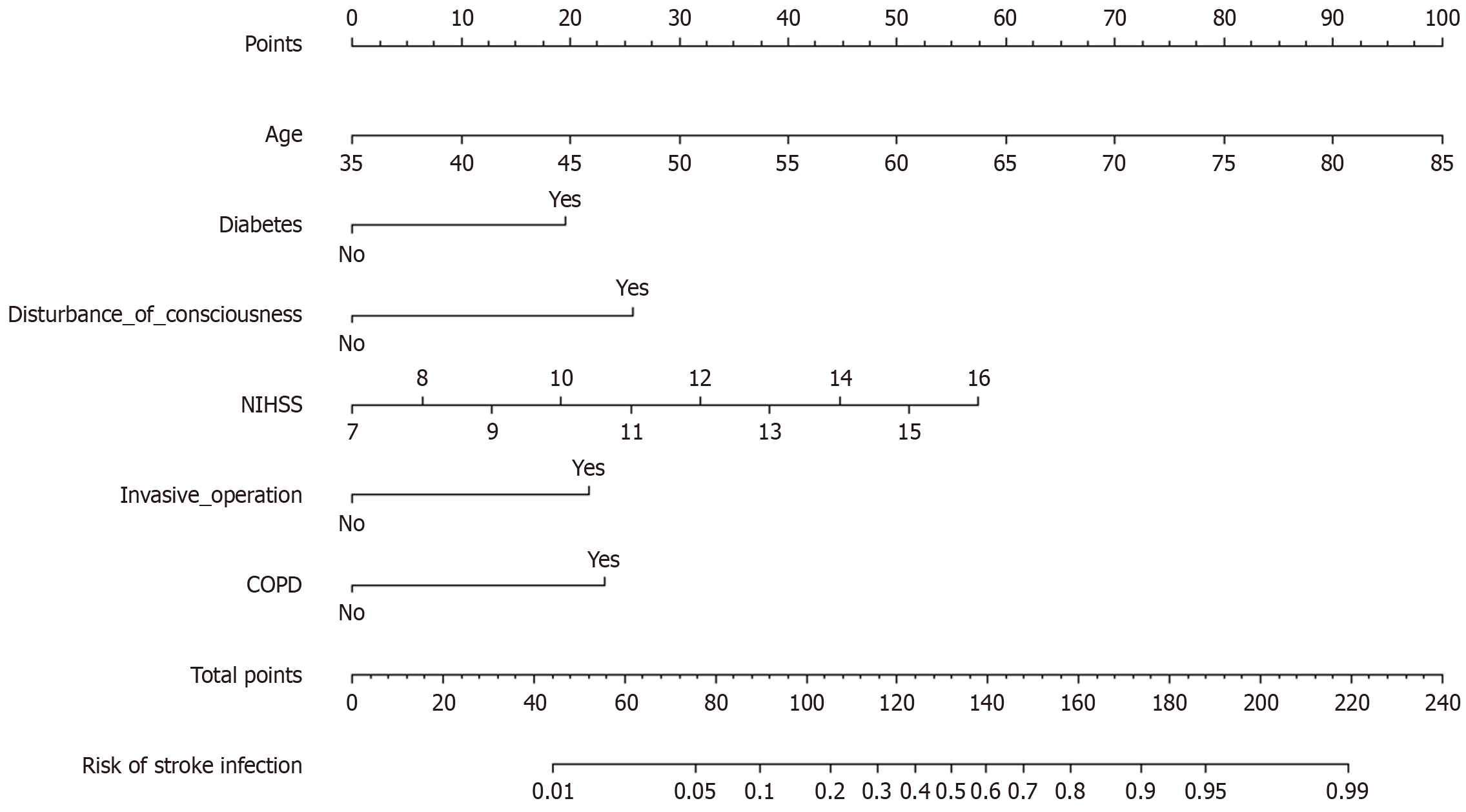
Using post-stroke infection as the dependent variable (non-infection group = 0, infection group = 1), and age, presence of diabetes, disturbance of consciousness, NIHSS score at admission, whether there was invasive operation, and whether there was COPD as independent variables (Table 2), logistic regression analysis demonstrated that age, diabetes, disturbance of consciousness, NIHSS score at admission, invasive operation, and COPD were risk factors for post-stroke infection in AIS patients (P < 0.05) (Table 3).
| Variable | Index | Assigned method |
| X1 | Age | Continuous variable |
| X2 | Diabetes | No = 0; Yes = 1 |
| X3 | Disturbance of consciousness | No = 0; Yes = 1 |
| X4 | NIHSS score at admission | Continuous variable |
| X5 | Invasive operation | No = 0; Yes = 1 |
| X6 | COPD | No = 0; Yes = 1 |
| B | SE | Wald | P value | OR | 95%CI | |
| Age | 0.105 | 0.029 | 13.364 | 0.000 | 1.111 | 1.050-1.175 |
| Diabetes | 1.026 | 0.447 | 5.277 | 0.022 | 2.791 | 1.163-6.701 |
| Disturbance of consciousness | 1.350 | 0.510 | 7.011 | 0.008 | 3.856 | 1.420-10.471 |
| NIHSS score at admission | 0.335 | 0.106 | 10.023 | 0.002 | 1.398 | 1.136-1.719 |
| Invasive operation | 1.139 | 0.452 | 6.362 | 0.012 | 3.123 | 1.289-7.567 |
| COPD | 1.215 | 0.430 | 7.979 | 0.005 | 3.372 | 1.451-7.836 |
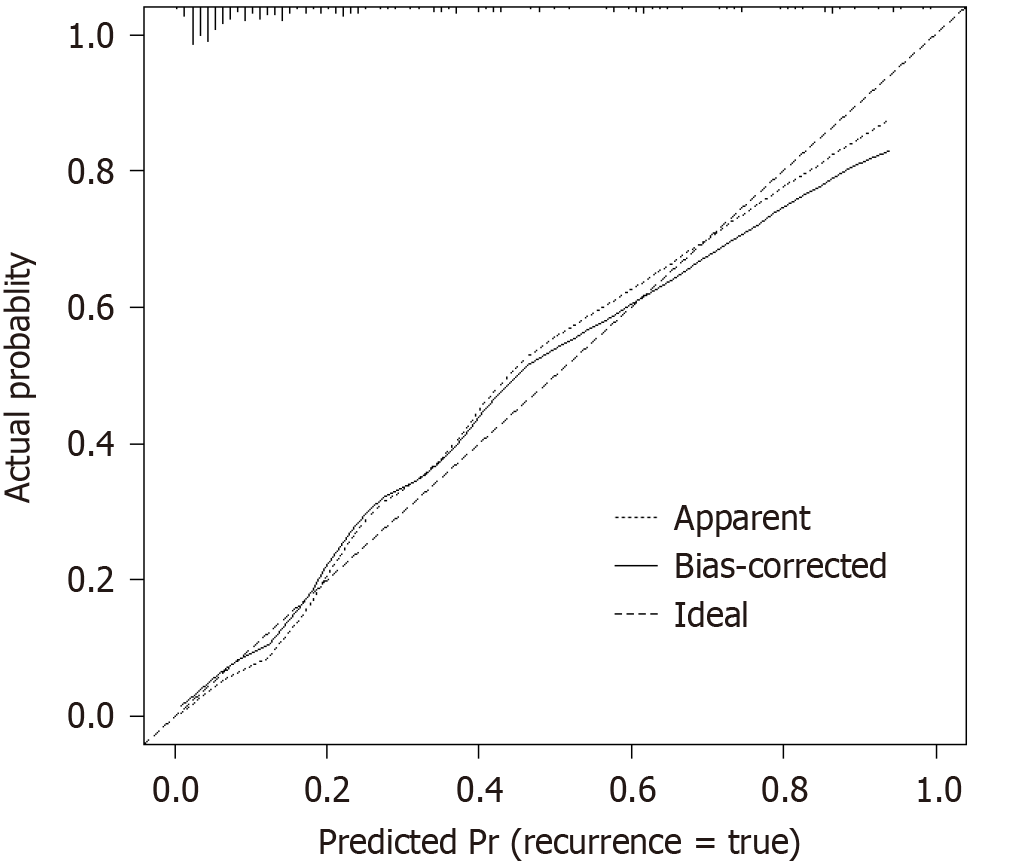
A nomogram was developed using the significant influencing factors in the multivariate logistic regression model, including age, diabetes, consciousness disorder, NIHSS score at admission, invasive operation, and COPD (Figure 1). When using the nomogram to predict post-stroke infection in patients, the C-index of the nomogram prediction model was 0.891, reflecting the good potential clinical efficacy of the prediction model (Figure 2). The calibration curve also showed good consistency between the actual observations and nomogram predictions.
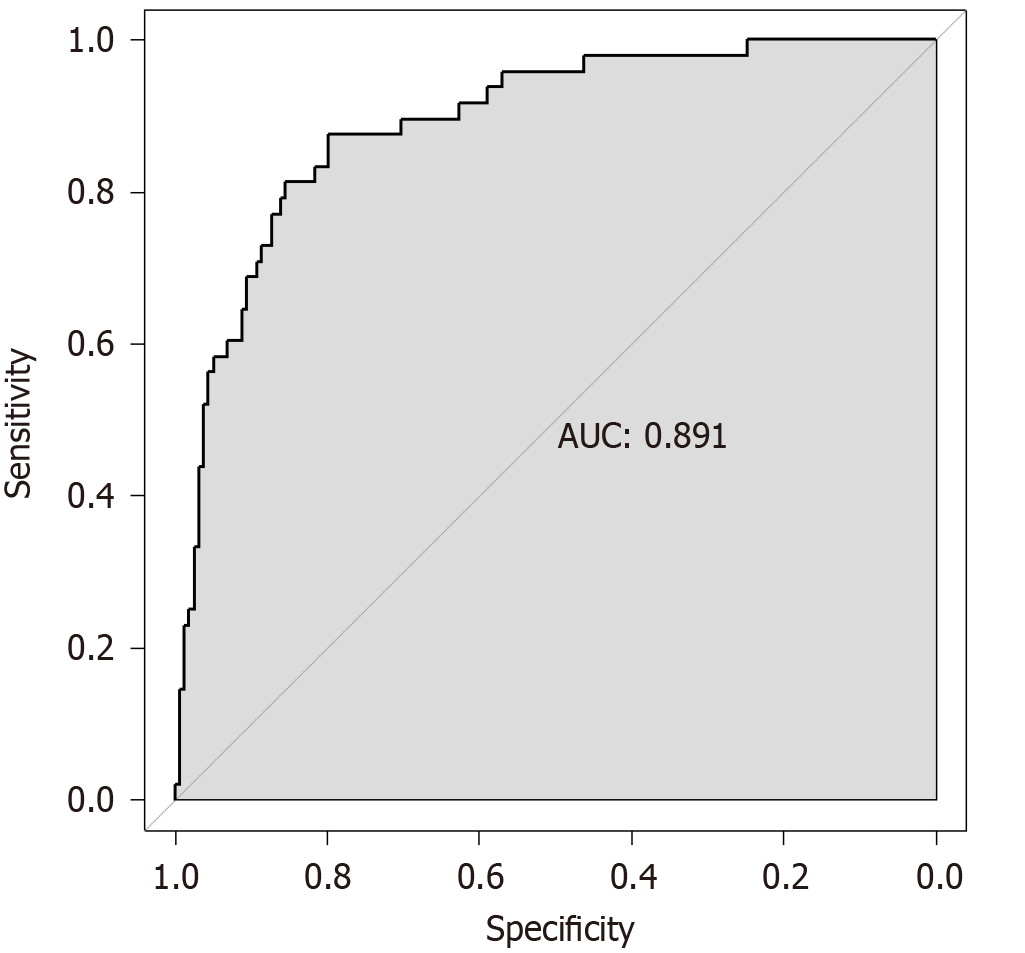
According to the ROC curve analysis, the area under the curve (95% confidence interval) of the nomogram prediction model in predicting the risk of post-stroke infection was 0.891 (0.839-0.942), suggesting that it has appreciated predictive value for post-stroke infection. When the optimal cutoff value was selected, the sensitivity and specificity were 87.5% and 79.7%, respectively, indicating that the nomogram predictive model was effective (Figure 3).
This study found that the incidence of post-stroke infection was 23.3% (48/206), and the main infection sites were the respiratory tract and urinary system. Wang et al[11] found that the infection rate of stroke in AIS patients was 18.5% (128/693). The research results of Huo et al[12] showed that the incidence of post-stroke infection in AIS patients was 15.3% (66/432). The incidence of post-stroke infection in this study was higher than that reported in previous studies. Foreign studies have shown that infection is a common complication after stroke, affecting 15%–30% of patients[13]. Therefore, attention should be actively paid to the factors related to post-stroke infection in the subsequent treatment process and take active and effective preventive measures to reduce the incidence of post-stroke infection.
In this study, we constructed a nomogram predictive model for post-stroke infection in patients with AIS. Age, diabetes, disturbance of consciousness, NIHSS score at admission, invasive operation, and COPD were identified to be risk factors for post-stroke infection in patients with AIS. Age was the primary consideration in this study. The risk of post-stroke infection increases with age, mainly due to varying degrees of immune function decline, organ dysfunction, and comorbidities with different types of underlying diseases in elderly patients with AIS, resulting in a higher risk of infection[14]. Studies have shown that high blood sugar levels are an important risk factor for stroke in AIS patients[15]. The main reason is that high levels of blood sugar can inhibit the phagocytic ability of white blood cells, which is not conducive to antibody formation. Moreover, hyperglycemia can reduce the deformability and oxygen-carrying capacity of red blood cells and affect the body's immune function, thereby increasing the risk of infection[15]. Related studies have shown that COPD can also increase the risk of post-stroke infection in patients with AIS because such patients often have varying degrees of airway function damage, mechanical barrier function damage, decreased bronchial mucosal ciliary swing ability and elastic function, and decreased cough reflex sensitivity, thereby increasing the risk of post-stroke infection[16,17]. In addition, patients with high NIHSS scores at admission are in a serious condition, and most of them are accompanied by different degrees of limb dysfunction, need to stay in bed for a long time, which is very likely to cause hypostatic pneumonia, or need to undergo invasive operations such as an indwelling catheter and gastric tube, thus increasing the risk of post-stroke infection[18,19]. Szylińska et al[19] also confirmed that patients with high NIHSS scores have a more severe condition, and as a result, such patients are often accompanied by varying degrees of swallowing and consciousness disorders, and invasive treatment procedures are required, resulting in a higher risk of post-stroke infection. Invasive procedures such as tracheal intubation are commonly used in AIS treatment and play an important role in saving patients' lives[20]. But it can lead to direct contact between the patient's respiratory tract and the external environment, causing varying degrees of damage to the airway mucosa, affecting the defense ability of the airway mucosa and the discharge of secretions, providing good conditions for the reproduction and growth of bacteria, and increasing the risk of infection[19,20]. Patel et al[21] concluded that patients with AIS receiving nasogastric tubes and invasive mechanical ventilation have a higher incidence of stroke-related pulmonary infections. This is consistent with the fact that invasive procedures were an important risk factor for post-stroke infection in patients with AIS in this study. In addition, swallowing disorders are closely related to post-stroke infection in AIS, mainly due to weakened swallowing reflex, poor cough ability, and high susceptibility to aspiration in AIS patients with consciousness disorders. Moreover, it is difficult to expel secretions from the lungs in a timely manner, which can easily lead to lung infections[19,22]. Beharry et al[22] reported that stroke patients are affected by consciousness disorders, which can lead to swallowing-related muscle paralysis, loss of sensation in the oral and lingual mucosa, and damage to the swallowing center; subsequently, swallowing dysfunction occurs, and gastric reflux, digestive fluid, residue in the oral cavity, and nasal and oropharyngeal secretions are easily inhaled into the respiratory tract, increasing the risk of pulmonary infection. Yuan et al[23] also confirmed that swallowing dysfunction in AIS patients is an important risk factor for post-stroke pneumonia in AIS patients, and it can significantly increase the risk of disease-related death. Shim et al[24] also confirmed that the transformation of bleeding is an important risk factor for pulmonary infection in AIS; Mainly because of the conversion of bleeding into common serious complications after intravenous thrombolysis of AIS, which can cause deterioration of neurological function and exacerbation of the condition, further exacerbating the severity of inflammatory reactions and thereby increasing the risk of pulmonary infection. In addition, Nergiz et al[25] explored the correlation between nutritional status and the risk of infection in patients, and the results indicated that AIS patients with a poor nutritional index have a higher risk of infection, indicating that malnutrition may be an important risk factor for stroke-related infections. In clinical practice, timely nutritional intervention should be performed for patients with a poor nutritional status. In addition, relevant studies have confirmed that the overexpression of inflammatory factors is also an important risk factor for stroke-related infections in patients with AIS[26,27]. However, this study did not observe a correlation between nutritional status and inflammatory factors in stroke patients, and further exploration and research are needed to address this discrepancy.
This study integrated six independent risk factors to establish a visualized nomogram prediction model, which has been verified to have high accuracy, as well as good sensitivity and specificity. The correlation analysis showed that there is no statistically significant difference between the predicted values of the nomogram prediction model and the actual observed values, indicating that the nomogram prediction model has a good calibration ability. This nomogram provides a simple, feasible, and predictive tool for evaluating stroke-related infections in AIS patients. The predictive factors included in the nomogram prediction model are easily collected in clinical practice, making the model have good clinical applicability and operability, and simplifying the evaluation process for medical staff.
This retrospective analysis was conducted in a single center with a small sample size and selection bias. In addition, few observation indicators were included, and the factors affecting post-stroke infection were not comprehensive. Subsequent large-scale, long-term follow-up studies are required to verify the conclusions of this study.
Age, diabetes, disturbance of consciousness, NIHSS score at admission, invasive surgery, and COPD are all important risk factors for post-stroke infection in patients with AIS. The nomogram prediction model established based on these factors exhibits high discrimination and accuracy. In clinical practice, targeted prevention and control measures can be taken based on whether the patient has the aforementioned factors to reduce the risk of stroke and ensure the prognosis of the disease.
| 1. | Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2924] [Cited by in RCA: 3715] [Article Influence: 530.7] [Reference Citation Analysis (0)] |
| 2. | Maheshwari AK, Kumar P, Alam MT, Aurangzeb M, Parkash J, Imran K, Masroor M. Frequency of Hyperthermia in Acute Ischemic Stroke Patients Visiting a Tertiary Care Hospital. J Coll Physicians Surg Pak. 2016;26:490-493. [PubMed] |
| 3. | Kuo YW, Huang YC, Lee M, Lee TH, Lee JD. Risk stratification model for post-stroke pneumonia in patients with acute ischemic stroke. Eur J Cardiovasc Nurs. 2020;19:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Grieten J, Chevalier P, Lesenne A, Ernon L, Vandermeulen E, Panis E, Mesotten D. Hospital-acquired infections after acute ischaemic stroke and its association with healthcare-related costs and functional outcome. Acta Neurol Belg. 2022;122:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Nam KW, Kim TJ, Lee JS, Kwon HM, Lee YS, Ko SB, Yoon BW. High Neutrophil-to-Lymphocyte Ratio Predicts Stroke-Associated Pneumonia. Stroke. 2018;49:1886-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 6. | Boehme AK, Oka M, Cohen B, Elkind MSV, Larson E, Mathema B. Readmission Rates in Stroke Patients with and without Infections: Incidence and Risk Factors. J Stroke Cerebrovasc Dis. 2022;31:106172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Zhu Y, Gao J, Lv Q, Yin Q, Yang D. Risk Factors and Outcomes of Stroke-Associated Pneumonia in Patients with Stroke and Acute Large Artery Occlusion Treated with Endovascular Thrombectomy. J Stroke Cerebrovasc Dis. 2020;29:105223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Li J, Huang J, Pang T, Chen Z, Li J, Wu L, Hu Y, Chen W. Risk Estimation of Infectious and Inflammatory Disorders in Hospitalized Patients With Acute Ischemic Stroke Using Clinical-Lab Nomogram. Front Neurol. 2021;12:710144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Zaid Y, Rajeh A, Hosseini Teshnizi S, Alqarn A, Tarkesh F, Esmaeilinezhad Z, Nikandish R. Epidemiologic features and risk factors of sepsis in ischemic stroke patients admitted to intensive care: A prospective cohort study. J Clin Neurosci. 2019;69:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Mendelson SJ, Prabhakaran S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA. 2021;325:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 432] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 11. | Wang JY, Liu L, Wu W, Li CJ, Bian L, He QL, Qu ZX, Hu HS. [Construction and validation of a stroke related infection risk prediction model for acute ischemic stroke patients]. Zhongguo Ganran Kongzhi Zazhi. 2022;21:837-843. |
| 12. | Huo LJ, Zhang YM, Zhou Q, Gu Q, Li YQ, Ma T, Tie YQ. [Drug resistance, risk factors, and prognosis of stroke related infections in patients with acute ischemic stroke]. Zhongguo Laonianxue Zazhi. 2020;40:257-260. |
| 13. | Vermeij JD, Westendorp WF, van de Beek D, Nederkoorn PJ. Post-stroke infections and preventive antibiotics in stroke: Update of clinical evidence. Int J Stroke. 2018;13:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Boehme AK, Esenwa C, Elkind MS. Stroke Risk Factors, Genetics, and Prevention. Circ Res. 2017;120:472-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 1004] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 15. | Wästfelt M, Cao Y, Ström JO. Predictors of post-stroke fever and infections: a systematic review and meta-analysis. BMC Neurol. 2018;18:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Morgan AD, Sharma C, Rothnie KJ, Potts J, Smeeth L, Quint JK. Chronic Obstructive Pulmonary Disease and the Risk of Stroke. Ann Am Thorac Soc. 2017;14:754-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Badr MY, Elkholy AA, Shoeib SM, Bahey MG, Mohamed EA, Reda AM. Assessment of incidence of cerebral vascular diseases and prediction of stroke risk in chronic obstructive pulmonary disease patients using multimodal biomarkers. Clin Respir J. 2023;17:211-228. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Derbisz J, Nowak K, Wnuk M, Pulyk R, Jagiella J, Slowik J, Dziedzic T, Slowik A. Prognostic Significance of Stroke-Associated Infection and other Readily Available Parameters in Acute Ischemic Stroke Treated by Intravenous Thrombolysis. J Stroke Cerebrovasc Dis. 2021;30:105525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Szylińska A, Bott-Olejnik M, Wańkowicz P, Karoń D, Rotter I, Kotfis K. A Novel Index in the Prediction of Pneumonia Following Acute Ischemic Stroke. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Coleman ER, Moudgal R, Lang K, Hyacinth HI, Awosika OO, Kissela BM, Feng W. Early Rehabilitation After Stroke: a Narrative Review. Curr Atheroscler Rep. 2017;19:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 21. | Patel UK, Kodumuri N, Dave M, Lekshminarayanan A, Khan N, Kavi T, Kothari R, Lunagariya A, Jani V. Stroke-Associated Pneumonia: A Retrospective Study of Risk Factors and Outcomes. Neurologist. 2020;25:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Beharry A, Michel P, Faouzi M, Kuntzer T, Schweizer V, Diserens K. Predictive Factors of Swallowing Disorders and Bronchopneumonia in Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2019;28:2148-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Yuan M, Li Q, Zhang R, Zhang W, Zou N, Qin X, Cai Z. Risk factors for and impact of poststroke pneumonia in patients with acute ischemic stroke. Medicine (Baltimore). 2021;100:e25213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Shim R, Wen SW, Wanrooy BJ, Rank M, Thirugnanachandran T, Ho L, Sepehrizadeh T, de Veer M, Srikanth VK, Ma H, Phan TG, Sobey CG, Wong CHY. Stroke Severity, and Not Cerebral Infarct Location, Increases the Risk of Infection. Transl Stroke Res. 2020;11:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Nergiz S, Ozturk U. The Effect of Prognostic Nutritional Index on Infection in Acute Ischemic Stroke Patients. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 26. | Chen X, Liang X, Zhang J, Chen L, Sun J, Cai X. Serum Calcium Levels and in-Hospital Infection Risk in Patients with Acute Ischemic Stroke. Neuropsychiatr Dis Treat. 2022;18:943-950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Huang L, Zhang R, Ji J, Long F, Wang Y, Lu J, Xu G, Sun Y. Hypersensitive C-reactive protein-albumin ratio is associated with stroke-associated pneumonia and early clinical outcomes in patients with acute ischemic stroke. Brain Behav. 2022;12:e2675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |