Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.451
Peer-review started: November 19, 2023
First decision: November 28, 2023
Revised: December 11, 2023
Accepted: December 21, 2023
Article in press: December 21, 2023
Published online: January 16, 2024
Processing time: 52 Days and 16.6 Hours
Granulomatous mastitis (GM) an inflammatory disease of the breast that usually affects women of childbearing age, occurs very rarely in males.
We present a case study of a 50-year-old male patient with GM. The patient developed a breast lump following the cleaning of a previously embedded dirt-filled nipple. While an initial improvement was noted with antibiotic therapy, a recurrence occurred a year later, showing resistance to the previously effective antibiotics. Subsequently, the lesion was excised. The histopathological exami
GM should be considered a possible diagnosis of male breast masses.
Core Tip: We present the case of a 50-year-old male whose breast lump was excised, confirming the diagnosis of granulomatous mastitis (GM), which occurs in males is very rare. Hormonal imbalances, autoimmunity, and bacterial infections may be associated with GM, for males, hormonal imbalances are most common. A standardized treatment protocol for GM is yet to be established. Male breast cancer and other diseases that cause breast granulomatous should be considered in the differential diagnosis.
- Citation: Cui LY, Sun CP, Li YY, Liu S. Granulomatous mastitis in a 50-year-old male: A case report and review of literature. World J Clin Cases 2024; 12(2): 451-459
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/451.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.451
Granulomatous mastitis (GM) is classified into two primary types based on its causative factors: secondary GM and idiopathic granulomatous mastitis (IGM), with the latter, alternatively referred to as granulomatous lobular mastitis (GLM) because of its pathological characteristics. GLM is defined as an inflammatory response within the breast tissue marked by the formation of non-caseous granulomas in the mammary lobules, in combination with localized infiltrates of multi-nucleated giant cells, epithelioid histiocytes, lymphocytes, and plasma cells. Sometimes it is accompanied by micro-abscess formation and neutrophil infiltration[1].
The clinical features include breast mass with or without pain in the early stage. It subsequently spreads to the areola from the periphery of the breast with swelling, warmth, pain, and nipple retraction, and may include nipple discharge, as well as axillary lymphadenopathy. In more severe instances, abscesses could arise and eventually lead to sinus tracts, fistula, or ulcerated lesions with prolonged healing time. The ultrasonographic and magnetic resonance imaging findings lack specificity and are easily confused with breast cancer, thus requiring a pathological diagnosis for confirmation[1-4].
GLM is predominantly observed in women in the reproductive age range, and the mean age of onset is approximately 35 years[5], rarely occurring in pregnant and lactating women, and even more rarely in men[3]. GLM is primarily prevalent in Asia, the Mediterranean region, and the Middle East including countries such as Turkey, China, and Korea. It mainly affects Hispanic patients in the United States and is less reported in European countries[6,7]. The overall recurrence rate among patients was found to be 17.18%[8].
The etiology remains unclear but potential associations may include autoimmune conditions, bacterial infections, use of oral contraceptives or psychotropic drugs, hyperprolactinemia, smoking habits[9,10], and alpha1-antitrypsin (AAT) deficiency. AAT coded by SERPINA1, is a plasma protease inhibitor that is released into the bloodstream to protect tissues, and its circulating levels are elevated during inflammation, infection, and late pregnancy[11]. Pregnancy (91.4%) and breastfeeding (83.6%) were found to be the main underlying factors for the disease[8]. Diabetes mellitus type 2 and obesity may also be related to GM[5].
Secondary GM is typically observed after the manifestation of a specific disease, including tuberculosis, bacterial infections, parasitic invasions, foreign body granulomas, nodular diseases, and immunological disorders. Regardless of the type, this condition is rare in men.
The patient, a 50-year-old male, sought medical attention due to a painful lump in his right breast accompanied by erythema and edema.
He informed his physician that the lump had initially formed about a year back, following the removal of dirt embedded within his sunken nipple during a shower. He subsequently received treatment after aspiration of pus with intravenous penicillin, following which the erythema and swelling gradually resolved. However, two weeks prior to his current visit, the patient noticed the painful lump in the right breast again without an identifiable trigger. The breast lump was not associated with extra-mammary manifestations such as fever, arthralgia, or erythema nodosum of the lower extremities. The patient received intravenous antibiotics for four days and oral anti-inflammatory medications for six days at the hospital; however, the lump persisted.
The medical history of the patient revealed no instances of breast trauma, tuberculosis, or connective tissue disease. The patient had a history of untreated hepatitis B infection. Noteworthy lifestyle habits included smoking (one pack of cigarettes a day) and occasional alcohol consumption.
The patient denied any family history of malignant tumours.
Upon physical examination, the right breast mass measured approximately 8 cm × 6 cm, with erythematous and edematous skin. No evidence of abscess, ulceration, or fistula formation was observed.
A comprehensive preoperative evaluation was performed before surgery treatment, which included routine blood tests, assessments of C-reactive protein, blood sedimentation rate, coagulation profile, liver, renal, and thyroid functions, screening for infectious diseases (including hepatitis B and C, syphilis, and human immunodeficiency virus), immunological markers (including IgG, IgA, IgM, IgE, IgE2, IgG4, complement 3, complement 4), and rheumatological markers (including rheumatoid factor, anti-streptococcal hemolysin O, transferrin, immunoglobulin kappa light chain, immunoglobulin lambda light chain, kappa/lambda ratio). These tests confirmed active hepatitis B virus replication, normal liver function. Ultrasonography of the liver revealed fatty liver. Notably, the IgE levels were significantly elevated 207.00 IU/mL (reference range 0-100 IU/mL). His progesterone levels were also slightly elevated 0.9 nmol/L (0.159-0.474), whereas estrogen and testosterone levels were within the normal range (Table 1).
| Item | Result | Unit | Reference value |
| Progesterone | 0.9↑ | nmol/L | 0.159-1.474 |
| HBV-LP | (+) 142.18 | AU/mL | < 20 |
| IgE | 207.00↑ | IU/mL | 0-100 |
| HBsAg | (+) 1296.00 | COI | 0-0.9 |
| HBsAb | (-) 2.97 | IU/L | < 10 |
| HBeAg | (-) 0.080 | COI | 0-1 |
| HBeAb | (+) 0.00 | COI | > 1 |
| HBcAb | (+) 0.01 | COI | > 1 |
| HBcAb IgM | (-) 0.05 | COI | 0–1 |
Ultrasound examination revealed a 42 mm × 25 mm heterogeneous echogenic mass located laterally in the right breast. The mass had an ill-defined border and irregular morphology with visible blood flow signals indicating inflammatory changes.
The patient was eventually diagnosed with GM.
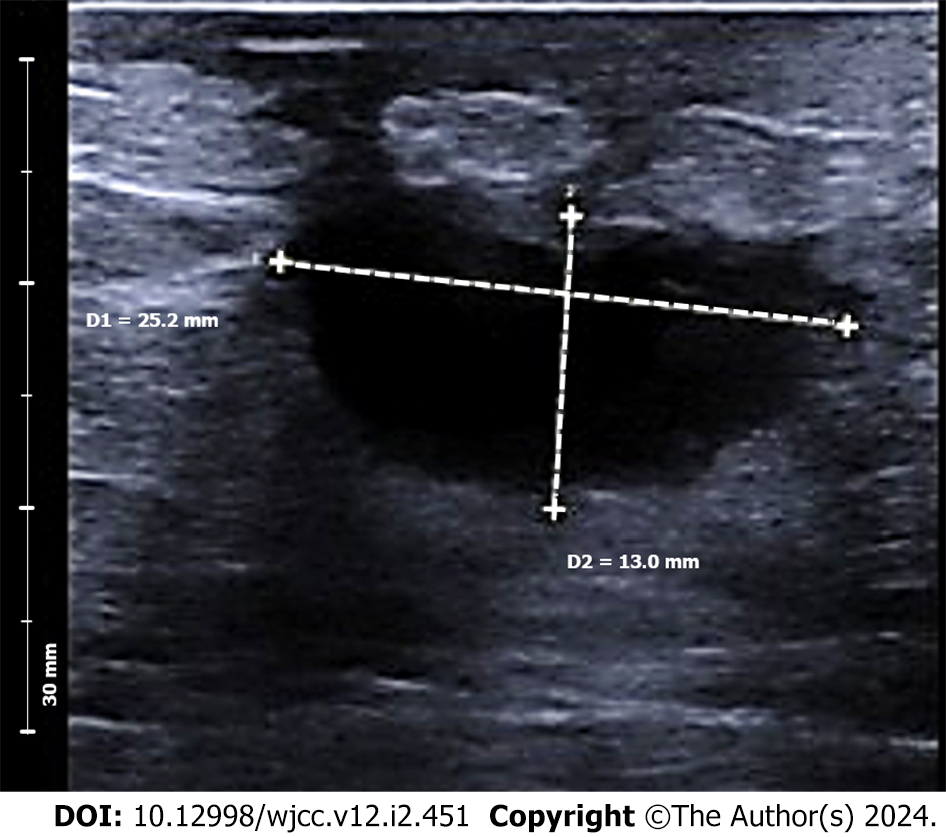
The patient received oral and topical Chinese herbal medicines. After ten days, the lump developed into a suppurative ulcer. The lump size reduced after one month, although some exudates persisted. Ultrasound evaluation after two months (Figure 1) revealed a 25 mm × 13 mm hypoechoic nodule adjacent to the nipple, leading to the decision to proceed with surgery.
Surgery was performed (Figure 2), and intraoperative examination revealed a subareolar lesion invading the nipple. The lesion characteristically had ill-defined borders, lacked a distinct capsule, and contained a reddish-brown necrotic substance. A toothpaste-like discharge was observed in the surrounding ducts, and a partially visible dark brown discharge was observed.
The lesion, along with the surrounding glandular tissue, measuring approximately 5.0 cm × 4.0 cm was excised. The specimen was sent to the pathology department. The excised upper lateral and medial glandular tissues from the right breast were aligned with a skin flap and filled into the remaining cavity. Careful hemostasis was performed around the wound margin until no active bleeding was observed. The incision was sutured layer-by-layer, the inverted nipple was everted, and a purse-string suture was applied. The skin was sutured with interrupted stitches using 4-0 absorbable sutures (Johnson & Johnson).
Surgical specimens were fixed in a 10% neutral formaldehyde solution. Sections, each 4 μm thick, were prepared from paraffin-embedded tissues and were then affixed onto glass slides treated with 3-aminopropyltriethoxysilane. Hematoxylin and eosin and Gram staining were performed and immunohistochemistry was conducted using the EnVision two-step method. The prepared samples were subsequently examined by two experienced pathologists.
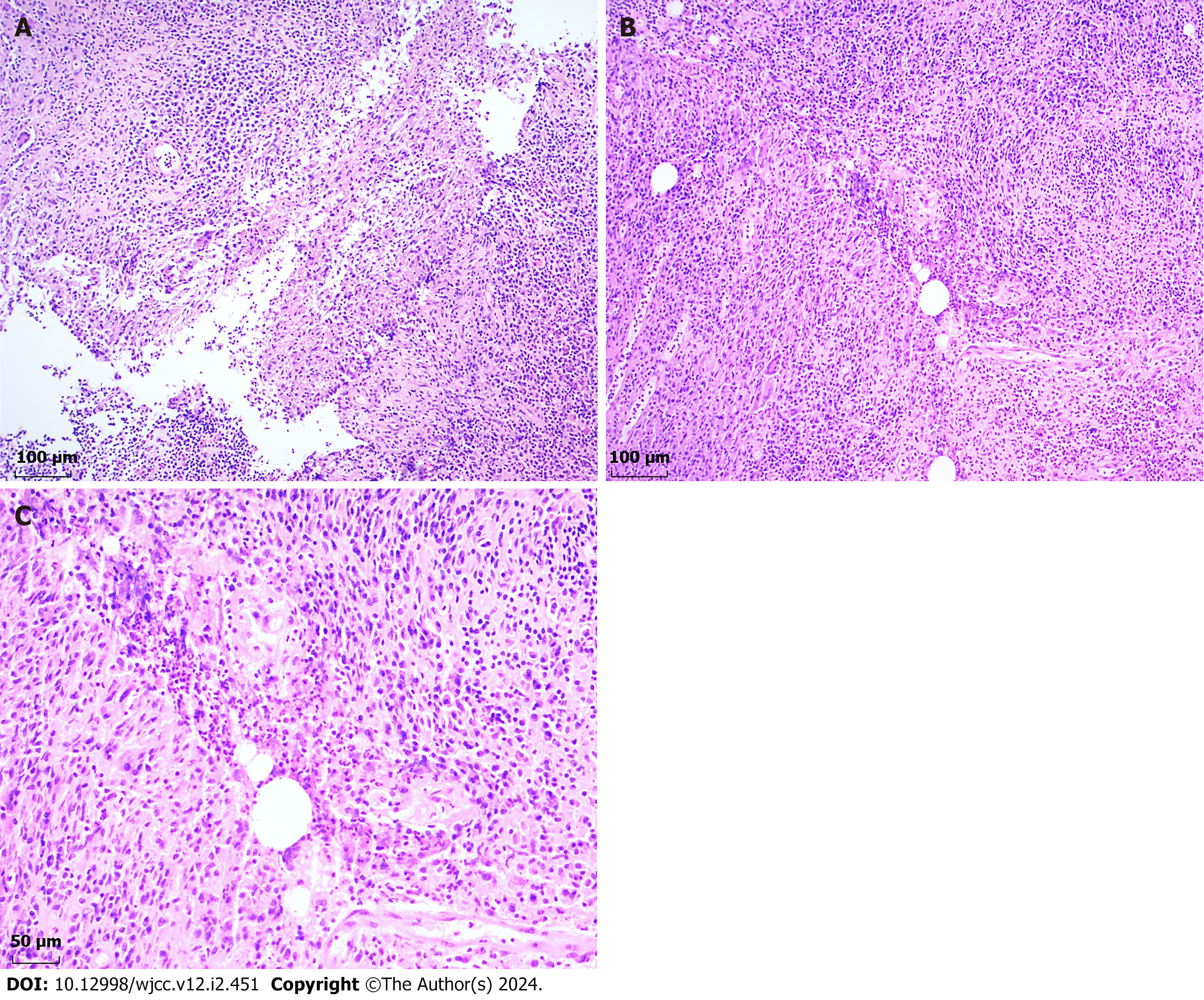
Macroscopic examination revealed the presence of a tissue displaying a grayish-yellowish hue with local grayish-red segments measuring 45 mm × 35 mm × 15 mm situated in the retro-areolar area of the right breast. Pathological investigation revealed a poorly formed non-caseating granuloma surrounded by epithelioid cells, lymphocytes, plasma cells, neutrophils, and multinucleate giant cells (Figure 3A). Parts of the granuloma were centered in a vacuolated space rimmed by neutrophils and surrounded by acute and chronic inflammatory cells (Figure 3B and C). No bacteria were identified by Gram staining. Immunohistochemical analysis revealed the presence of Ki-67 (approximately 15% positive within the granuloma area), CD20 (+), CD4 (+), IgG (+), IgG4 (+), CD3 (+), CD8 (+), CD1a (+), S-100 (+), and Syn (II).
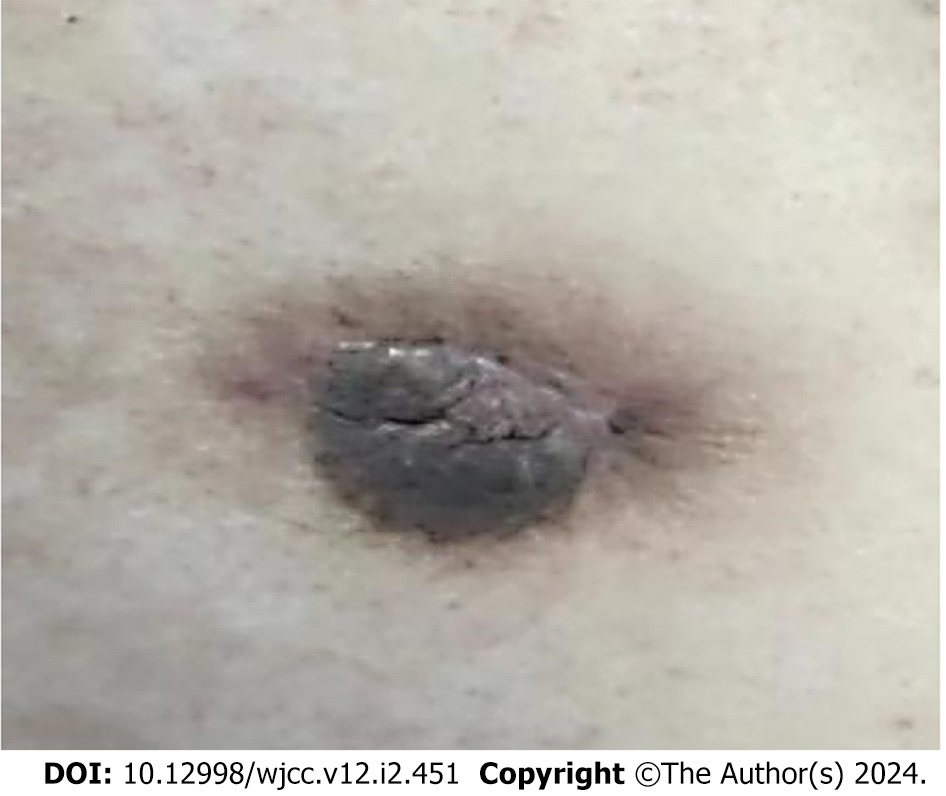
Post-operative follow-up at three months revealed that the surgical incision healed satisfactorily (Figure 4). Ultrasound of the right breast displayed a 19.8 mm × 10.6 mm hypoechoic region characterized by ill-defined borders and irregular morphology. Hyperechoic regions, each measuring 18 mm × 10 mm in the right axilla and 17 mm × 9 mm in the left axilla, were detected along with the lymphatic portals. No significant lymph node enlargement was observed in the bilateral axillae or the supraclavicular areas.
One of the diagnostic challenges in this case was identifying a clear trigger for the onset of the disease, namely the cleaning of the dirty nipples. Given the patient's inverted nipples and ducts filled with secretions, these areas could easily become fertile environments for bacterial growth after immersion in water, which may explain the initial efficacy of the antibiotics. However, without pathological findings, bacterial cultures, or molecular tests, it was impossible to ascertain whether a bacterial infection was present when the patient first discovered the lump. Even assuming an initial bacterial infection, it is challenging to explain the reappearance of the mass a year later in the absence of any identifiable causal factor, failure of antibiotic treatment to resolve the inflammation, and negative Gram staining of the surgical specimen. These elements prompted the decision to diagnose pathologically (as GM) rather than etiologically (as IGM).
Literature on GM in males, particularly GLM, is limited. The characteristic microscopic pathology of GLM reveals non-caseating granulomatous inflammation, composed predominantly of epithelioid histiocytes and associated inflammatory cell infiltration, including lymphocytes, plasma cells, granulocytes, and occasionally multi-nucleated giant cells[12]. Accompanying phenomena include microabscesses and fat necrosis. This condition is typically observed in nonlactating women of reproductive age and is infrequently present during breastfeeding or pregnancy.
The rarity of GLM in males can be attributed to the distinctive diagnostic requirement for GLM, wherein non-caseating granulomas predominantly occur in the breast lobules and may involve the ducts. However, the male breast tissue predominantly comprises fat and stroma, with a paucity of ducts, and lacks lobular structures. In the case under review, despite ultrasound revealing a glandular thickness of approximately 2 cm near the mass, the absence of evidence of lobular presence in the histopathological findings led us to finalize the diagnosis of GM.
Hormonal imbalances, especially hyperprolactinemia and relative or absolute elevations in estrogen levels (increased effective estradiol/testosterone ratio), could potentially contribute to the development of GM in men. Previous reports have associated male patients with GM with concurrent gynecomastia and hyperprolactinemia, along with other sex hormone abnormalities[13,14]. A case report by Sam et al[15] documented a male-to-female transgender patient presenting with bilateral palpable breast masses after six years of estrogen and anti-androgen (spironolactone) therapy. Ultrasound evaluation and pathological examination of the surgically removed right breast mass confirmed GM. Additionally, there are reports where patients show no hormonal abnormalities or gynecomastia[16-18]. Sahin et al[19] presented a case of a male patient with superficial high-grade bladder cancer who developed IGM following intravesical Bacillus Calmette Guerin treatment. Kawashima et al[20] reported the case of a 63-year-old male patient with history of chemotherapy for follicular lymphoma. He was treated with six cycles rituximab in combination with bendamustine, followed by rituximab for 2 years as maintenance therapy. The patient also received lenalidomide and rituximab for recurrence of the lymphoma. However, it is not clear exactly what role these treatments play in the development of GM.
Increased estrogen levels or heightened estrogen sensitivity in the mammary glands can stimulate the growth of male breast ducts and follicles[15]. Elevated estrogen levels are frequently observed in endocrine disorders such as obesity, hypogonadism, adrenal disease, thyroid disease, and diabetes mellitus, and in non-endocrine diseases such as liver cirrhosis, renal failure, and lung tumors[21]. Additionally, certain medications such as gonadotropins and psychotropic drugs can elevate estrogen levels by triggering endocrine dysfunction or by binding to estrogen receptors[22]. Considering our patient's history of hepatitis B infection, it is worth noting that individuals with cirrhosis have an impaired ability to inactivate estrogen, potentially promoting GM development, in addition to gynecomastia. However, in our case, the patient did not display abnormal liver function or ultrasound findings apart from elevated progesterone levels. Consequently, the GM cannot be attributed to hormonal imbalance in this case.
Autoimmunity may play a significant role in granuloma formation. Some patients with GLM display associated nodular erythema of the lower extremities, which often responds favorably to immunomodulators such as corticosteroids and methotrexate (MTX). GLM is considered to be an immune response and a local type IV hypersensitivity reaction triggered by retained milk after childbirth, or a series of immune responses triggered by bacterial infection. However, the precise immune-mediated mechanisms necessitate further elucidation[3,23].
Currently, Corynebacterium, specifically C. kroppenstedtii, is considered to be closely associated with GLM. Corynebacterium is a lipophilic Gram-positive bacterium often found in a fenestrated or "handkerchief-like" arrangement in central lipid vacuoles encircled by neutrophils and an outer cuff of epithelioid histiocytes[24,25]. GM with these pathological features is referred to as cystic neutrophilic GM. A multicenter clinical database study identified cigarette smoke exposure and positive cultures of C. kroppenstedtii in pus/tissue as two independent factors contributing to the recurrence of IGM[26]. Nevertheless, the specific mechanisms underlying Corynebacterium-induced granulomatous inflammation remain unclear. Other potential therapeutic agents include mixed bacterial infections. Bacteria such as Pseudomonas oleovorans, human gammaherpesvirus 4, Acinetobacter baumannii, and Tepidiphilus thermophilus have also been cultured in tissues from GLM[27].
In addition to observation and surgical intervention (excision and drainage), GM can be managed using antibiotics, corticosteroids, immunosuppressants (such as MTX)[28], and traditional Chinese medicine[29]. However, a standardized treatment protocol is yet to be established, and the condition is prone to recurrence. Due to the lipophilic properties of Corynebacterium, lipophilic antibiotics may be more effective in controlling bactericidal activity within the tissue. Identification of bacterial cultures and antimicrobial susceptibility testing can help in the selection of suitable non-penicillin antibiotics, such as clindamycin or vancomycin[30,31]. Corticosteroids are frequently used to decrease lesion size during the acute phase, whereas immunosuppressants are used in resistant cases, both of which require monitoring for potential adverse reactions. traditional Chinese medicine is primarily used in China to manage GLM, especially in patients unresponsive to antibiotics and steroids. Oral and topical herbs are used to shrink lesions and alleviate the symptoms of redness, swelling, and pain, followed by surgical excision to eliminate lesions and minimize the likelihood of recurrence[32].
Once gynecomastia is excluded in male breast lumps, the primary concern in the differential diagnosis becomes male breast cancer, for which surgery is the preferred treatment. Establishing whether the mass is benign or malignant is crucial because breast cancer in men often manifests subtly, resulting in delayed diagnosis and an advanced stage of the disease at presentation[33]. It is essential to differentiate breast granulomas from other conditions that present with features similar to it.
The key pathogens capable of causing granulomatous diseases of the breast include bacteria, fungi, and parasites. Granulomas in mammary tuberculosis are often irregularly distributed, predominantly involve the ducts, and typically exhibit caseous necrosis and Langhans giant cells[34]. A history of tuberculosis, a positive Ziehl-Neelsen stain, and a positive culture can assist in the diagnosis. Fungal identification can be differentiated by silver or Periodic Acid-Schiff staining.
Systemic immune diseases such as Wegener's granulomatosis[35], nodular disease[36], and IgG4-associated diseases[37] can also involve the breast. Although systemic symptoms usually precede breast symptoms, breast involvement can occasionally be the initial symptom.
In summary, we present a rare case of male GM in which the mass initially reduced following antibiotic treatment, but recurred a year later, with antibiotics proving ineffective. A combined approach was found to be beneficial, and the lesion was ultimately removed surgically following herbal treatment. No recurrence was observed at the 12-mo follow-up. GM is exceedingly rare in men, and its pathogenesis requires further investigation. Other diseases causing breast granulomas and breast cancer should be considered in the differential diagnosis.
We thank the patient for providing case information and pictures.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rotondo JC, Italy S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
| 1. | Wolfrum A, Kümmel S, Theuerkauf I, Pelz E, Reinisch M. Granulomatous Mastitis: A Therapeutic and Diagnostic Challenge. Breast Care (Basel). 2018;13:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Bacon DR, Ngeve SM, Jordan SG. Granulomatous mastitis: An underdiagnosed inflammatory disease afflicting minority women. Radiol Case Rep. 2021;16:3990-3994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Yuan QQ, Xiao SY, Farouk O, Du YT, Sheybani F, Tan QT, Akbulut S, Cetin K, Alikhassi A, Yaghan RJ, Durur-Subasi I, Altintoprak F, Eom TI, Alper F, Hasbahceci M, Martínez-Ramos D, Oztekin PS, Kwong A, Pluguez-Turull CW, Brownson KE, Chandanwale S, Habibi M, Lan LY, Zhou R, Zeng XT, Bai J, Bai JW, Chen QR, Chen X, Zha XM, Dai WJ, Dai ZJ, Feng QY, Gao QJ, Gao RF, Han BS, Hou JX, Hou W, Liao HY, Luo H, Liu ZR, Lu JH, Luo B, Ma XP, Qian J, Qin JY, Wei W, Wei G, Xu LY, Xue HC, Yang HW, Yang WG, Zhang CJ, Zhang F, Zhang GX, Zhang SK, Zhang SQ, Zhang YQ, Zhang YP, Zhang SC, Zhao DW, Zheng XM, Zheng LW, Xu GR, Zhou WB, Wu GS. Correction: Management of granulomatous lobular mastitis: an international multidisciplinary consensus (2021 edition). Mil Med Res. 2022;9:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 4. | Martinez-Ramos D, Simon-Monterde L, Suelves-Piqueres C, Queralt-Martin R, Granel-Villach L, Laguna-Sastre JM, Nicolau MJ, Escrig-Sos J. Idiopathic granulomatous mastitis: A systematic review of 3060 patients. Breast J. 2019;25:1245-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Barreto DS, Sedgwick EL, Nagi CS, Benveniste AP. Granulomatous mastitis: etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Res Treat. 2018;171:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Bhattarai P, Srinivasan A, Valenzuela CD, Sulzbach C, Wallack MK, Mariadason JG. Idiopathic granulomatous mastitis: experience at a New York hospital. Ann R Coll Surg Engl. 2022;104:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Altintoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases. 2014;2:852-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (4)] |
| 8. | Fattahi AS, Amini G, Sajedi F, Mehrad-Majd H. Factors Affecting Recurrence of Idiopathic Granulomatous Mastitis: A Systematic Review. Breast J. 2023;2023:9947797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Benson JR, Dumitru D. Idiopathic granulomatous mastitis: presentation, investigation and management. Future Oncol. 2016;12:1381-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Tang X. Progress in the etiology of granulomatous mastitis. Zhongguo Fuyou Baojian. 2018;33:1672-1675. |
| 11. | Rotondo JC, Oton-Gonzalez L, Selvatici R, Rizzo P, Pavasini R, Campo GC, Lanzillotti C, Mazziotta C, De Mattei M, Tognon M, Martini F. SERPINA1 Gene Promoter Is Differentially Methylated in Peripheral Blood Mononuclear Cells of Pregnant Women. Front Cell Dev Biol. 2020;8:550543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Jahanbin B, Anousha K, Azizi M, Soleimani V, Rezvani A, Azmoudeh Ardalan F. Idiopathic Granulomatous Mastitis: Diagnosis and Histopathologic Features, A Review. Arch Breast Cancer. 2022;9:272-278. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Yin L, Agyekum EA, Zhang Q, Wu T, Qian X. Gynecomastia with rare granulomatous lobular mastitis: a case report and literature review. J Int Med Res. 2022;50:3000605221075815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Yaghan RJ, Ayoub NM, Shenawi HM, Yaghan LR. Idiopathic granulomatous mastitis in the male population: A clinical analysis of 13 reported cases. Breast J. 2020;26:1481-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Sam KQ, Severs FJ, Ebuoma LO, Chandandeep NS, Sedgwick EL. Granulomatous Mastitis in a Transgender Patient. J Radiol Case Rep. 2017;11:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Al Manasra AR, Al-Hurani MF. Granulomatous Mastitis: A Rare Cause of Male Breast Lump. Case Rep Oncol. 2016;9:516-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Reddy KM, Meyer CE, Nakdjevani A, Shrotria S. Idiopathic granulomatous mastitis in the male breast. Breast J. 2005;11:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Farrokh D, Alamdaran A, Haddad AS, Kheirollahi M, Abbasi B. Male granulomatous mastitis, a rarely encountered disorder. Breast J. 2019;25:517-518. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Sahin C, Agridag Ucpinar B. Idiopathic granulomatous mastitis in a male breast following intravesical Bacille Calmette-Guerin treatment. J Infect Public Health. 2020;13:830-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Kawashima K, Yamamoto S, Narui K, Fujiwara Y, Adachi S, Sasamoto M, Oshi M, Yamada A, Kumagai E, Otani M, Endo I. Granulomatous mastitis in a male breast: A case report and review of literature. Clin Case Rep. 2023;11:e7048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Sapino A, Kulka J. Breast Pathology (Chinese Edition). Beijing: China Science and Technology Press, 2021: 127-128. |
| 22. | Dickson G. Gynecomastia. Am Fam Physician. 2012;85:716-722. [PubMed] |
| 23. | Yin Y, Liu X, Meng Q, Han X, Zhang H, Lv Y. Idiopathic Granulomatous Mastitis: Etiology, Clinical Manifestation, Diagnosis and Treatment. J Invest Surg. 2022;35:709-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Tariq H, Nazarullah AN. The Emerging Role of Corynebacteria in the Pathogenesis of Granulomatous Lobular Mastitis. Arch Breast Cancer. 2022;9:250-254. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Co M, Cheng VCC, Wei J, Wong SCY, Chan SMS, Shek T, Kwong A. Idiopathic granulomatous mastitis: a 10-year study from a multicentre clinical database. Pathology. 2018;50:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Bi J, Li Z, Lin X, Li F, Xu H, Yu X, Liu L, Liang Y, Xu Z, Wang J, Shao M. Etiology of granulomatous lobular mastitis based on metagenomic next-generation sequencing. Int J Infect Dis. 2021;113:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 28. | Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011;17:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Li ZY, Sun XM, Li JW, Liu XF, Sun ZY, Chen HH, Dong YL, Sun XH. Treatment of bilateral granulomatous lobular mastitis during lactation with traditional Chinese medicine: A case report. World J Clin Cases. 2021;9:8249-8259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 30. | Williams MS, McClintock AH, Bourassa L, Laya MB. Treatment of Granulomatous Mastitis: Is There a Role for Antibiotics? Eur J Breast Health. 2021;17:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Shoyele O, Vidhun R, Dodge J, Cheng Z, Margules R, Nee P, Sieber S. Cystic neutrophilic granulomatous mastitis: A clinicopathologic study of a distinct entity with supporting evidence of a role for Corynebacterium-targeted therapy. Ann Diagn Pathol. 2018;37:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Sawuer R, Wu C, Sun Z, Liu S. The Effectiveness of Traditional Chinese Medicine Combined With Surgery to Treat Granulomatous Mastitis: A Propensity-Matched Analysis. Front Oncol. 2022;12:833742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Preuss C, Wunderle M, Hack CC, Beckmann MW, Wenkel E, Jud S, Heindl F. [Breast cancer in men]. Dtsch Med Wochenschr. 2023;148:301-306. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Ail DA, Bhayekar P, Joshi A, Pandya N, Nasare A, Lengare P, Narkhede KA. Clinical and Cytological Spectrum of Granulomatous Mastitis and Utility of FNAC in Picking up Tubercular Mastitis: An Eight-Year Study. J Clin Diagn Res. 2017;11:EC45-EC49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Goulabchand R, Hafidi A, Van de Perre P, Millet I, Maria ATJ, Morel J, Quellec AL, Perrochia H, Guilpain P. Mastitis in Autoimmune Diseases: Review of the Literature, Diagnostic Pathway, and Pathophysiological Key Players. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Reis J, Boavida J, Bahrami N, Lyngra M, Geitung JT. Breast sarcoidosis: Clinical features, imaging, and histological findings. Breast J. 2021;27:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Erivwo P, Turashvili G. Pathology of IgG4-related sclerosing mastitis. J Clin Pathol. 2021;74:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |