Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.443
Peer-review started: November 8, 2023
First decision: December 5, 2023
Revised: December 17, 2023
Accepted: December 27, 2023
Article in press: December 27, 2023
Published online: January 16, 2024
Processing time: 63 Days and 16.9 Hours
Patients with Parkinson's disease (PD) exhibit symptoms such as antecollis (AC) and camptocormia (CC). The pathology of these two conditions is unclear. Additionally, standard treatment methods have not been established. The article reports the case of a 65-year-old female patient with AC and CC who was treated with central and peripheral interventions to alleviate symptoms.
We present the case of a 65-year-old female PD patient with AC and CC. The course of the disease was 5 years. She was treated with rehabilitation strategies such as sensory tricks and trunk strength training. During the inpatient period, we compared and analyzed the patient's gait, rehabilitation assessment scale score, and angles of her abnormal trunk posture in the first week, the third week, and the fifth week. The patient's stride length increased, indicating that the patient's walking ability was improved. The Unified Parkinson's Disease Scale Part Three score and CC severity score decreased. Furthermore, the score of the other scale increased. In addition, the patient showed significant improvements in AC, upper CC, and lower CC angles.
This case study suggested that sensory tricks and trunk strength training are beneficial and safe for patients with AC and CC.
Core Tip: Sensory tricks and trunk strength training have a strong effect on treating Parkinson's disease patients with antecollis (AC) and camptocormia (CC). However, the diagnosis and the rehabilitation of AC and CC are usually neglected. Therefore, it is especially important to detect these conditions in a timely manner and administer appropriate treatment.
- Citation: Wang JR, Hu Y. Significant improvement after sensory tricks and trunk strength training for Parkinson’s disease with antecollis and camptocormia: A case report. World J Clin Cases 2024; 12(2): 443-450
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/443.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.443
Patients with Parkinson's disease (PD) may have advanced postural abnormalities that can severely affect their activities of daily living[1]. PD-associated sagittal posture abnormalities include antecollis (AC) and camptocormia (CC). PD-associated coronal posture abnormalities include Pisa syndrome and scoliosis[2]. In 1989, Quinn[3] first reported CC in The Lancet, defined as 45° of anterior flexion above C7[4]. The incidence of PD is 5 to 7%[5]. In 1999, Djaldetti et al[6] referred to the phenomenon of the changing position of the spine. He described PD patients with forward flexion of the thoracolumbar segment and disappearance of forward flexion in the supine position as CC. The current diagnostic criterion for upper CC is an upper anterior flexion angle of C7 to T12-L1 greater than 45°. Additionally, the criterion for lower CC is a flexion angle of L1 – to the sacrum, and a hip flexion angle greater than 30°[7]. PD occurs in 7-16% of PD patients[8]. Currently, the pathogenesis of AC and CC is unclear, and the mainstream view is that they are caused by a combination of central and peripheral mechanisms.
This case involved a PD patient with predominantly AC and CC symptoms. This report focused on interventions for treating proprioceptive dysfunction caused by AC and CC and concomitant loss of peripheral trunk muscles. Currently, the treatment of AC relies mainly on adjusting the drug therapy used[9]. For patients with CC, transcranial magnetic stimulation, postural training, botulinum toxin injection, and spinal cord electrical stimulation were used. An expert suggested that nonsurgical intervention be used as first-line therapy[10]. Therefore, in this case, sensory tricks and trunk strength training were used. We aimed to observe whether the patient's sagittal postural abnormalities were alleviated. Thus, we analyzed the patient's gait, Modified Barthel Index (MBI), Lawton's Instrumental Activities of Daily Living (Lawton IADL), Berg Balance Scale (BBS), the Unified Parkinson's Disease Scale Part Three (UPDRS III), CC severity[11] and angles of her abnormal trunk posture in the first week, third week, and fifth week.
A 65-year-old female patient consulted our neurologist and complained of slow movement for more than four years and bending while walking for more than one year.
The disease began in 2017. The initial manifestations of the disease were slow mobility, resting tremors in the right lower extremity, strained face, and shouting while sleep. Additionally, there was no noticeable loss of smell or apparent constipation. She went to the local hospital for treatment. Physical examination showed "face stereotyped, no joint action reduction, forward gait, right lower extremity resting tremor, and muscle tone is indicating lead-pipe rigidity". The electromyography results showed that “the 4-Hz group discharge position could be seen when the right tibial anterior muscle was resting”. Cranial magnetic resonance imaging revealed paraventricular white matter ischemia on both sides, and the patient was diagnosed with PD. The physician administered 375 mg of madopar orally each day. The patient then reported that the effect began 30 min after taking it, lasting 3 h each time. Two years prior during consultation, the right finger flapping test and bilateral leg flexibility test scores were low, and the muscle tone of the right limb increased. Furthermore, the posterior pull test was three steps back, and the Hoehn-Yahr (HY) stage (off-stage) was 2.5. Pramipexole was added to control the symptoms of PD. After six months of regular administration, the above symptoms were not significantly relieved. Selegiline and amantadine were added at the second visit. No obvious side effects were observed during drug administration. The patient reported that the phenomena of dropping her head and bending her waist while walking or standing disappeared when lying down one year ago. During the course of the disease, the duration of the drug effect gradually extended to approximately 1 h. At present, the patient can still walk. However, when she walks, she bends over and drops her head more substantially than before. Before she was admitted to our hospital, she was treated with madopar (500 mg/d), pramipexole (1.5 mg/d), amantadine (0.2 g/d), or selegiline (10 mg/d). Since the onset of the disease, the patient has had a normal mental state and sleeps regularly. She has had no considerable change in weight. Furthermore, she urinates and has bowel movements.
She denied a history of a similar disease. She described lumbar interbody fusion and posterior pedicle screw internal fixation for lumbar spondylolisthesis in 2016 and provided us with a full spine X-ray (see Figure 1).
The patient had no family history of PD.
The patient had clear speech and few facial expressions. Executive neurological function tests were good. We used the Graded Manual Muscle Test (GMMT) to evaluate patient trunk strength. Upper and lower extremity muscle strength were graded as 5, cervical extensor 1+, rectus abdominis 2, abdominal internal and external oblique muscle 3, lumbar back muscle strength 1+, and bilateral gluteus muscle strength 1+. Muscle tone increased in the right extremity, and resting tremor was observed in the right lower extremity. There were no abnormalities in the depth or superficial sensation of either limb. The bilateral finger-nose test and heel-knee-tibia test were stable. The bilateral Babinski sign was negative. The sitting balance was graded as level 3, and the standing balance was graded as level 2. The trunk was bent forward while walking during postural gait examination. The swinging action of both upper limbs was reduced.
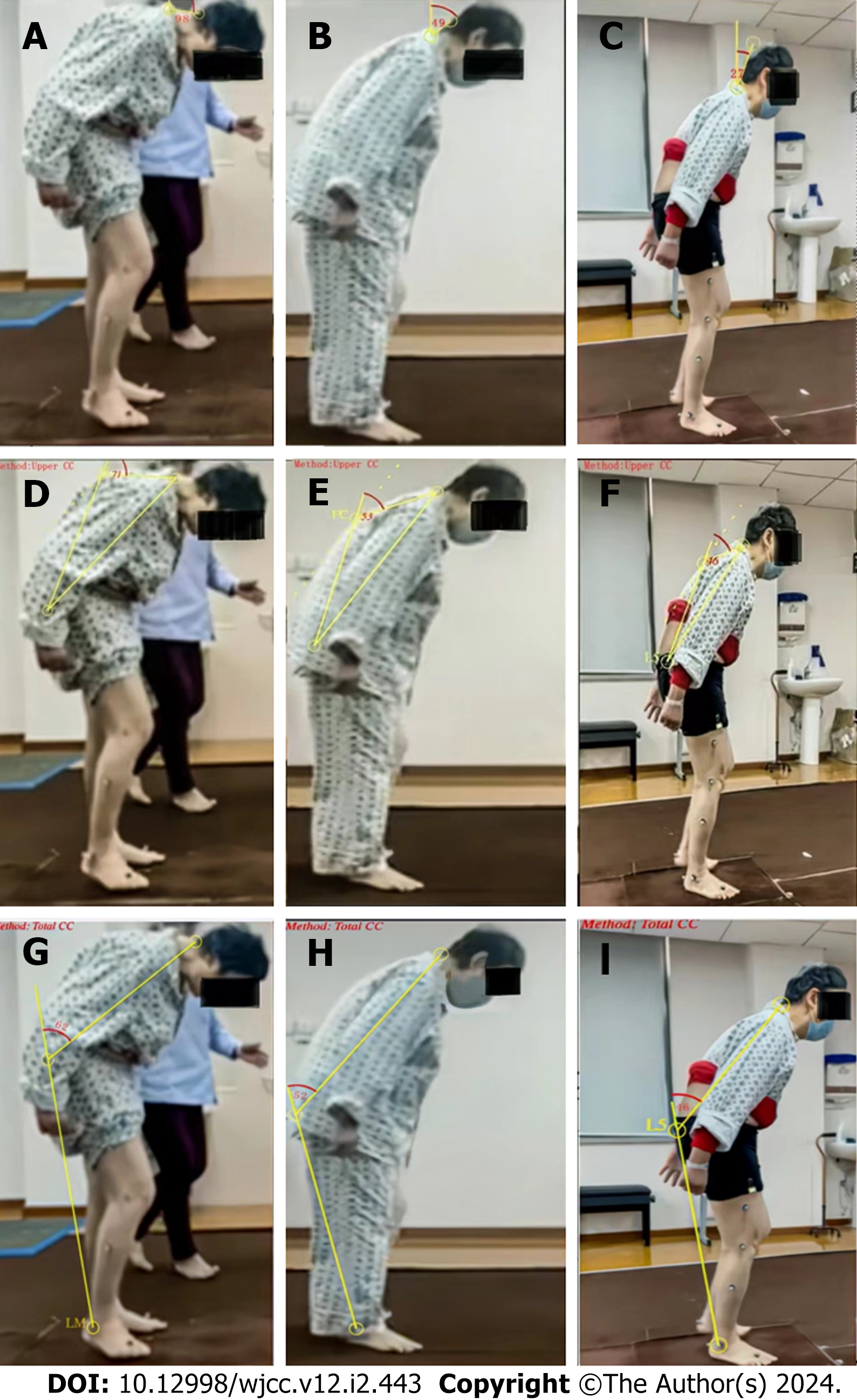
The HY stage was grade 3 (off-stage). The CC severity score was 14/32 points. The BBS was 21/56 points. The MBI score was 89/100 points. The patient needed moderate assistance while walking up and down stairs. The Lawton IADL score was 16/27 points. The rehabilitation physician and physiotherapist evaluated the gait and recorded three periods of step frequency, step length, step width, and stride length. We analyzed the improvements in these parameters through this process and through horizontal comparisons with standard values. We used the Neuro Posture App (http://www.neuroimaging.uni-kiel.de/NeuroPostureApp) to measure the forward flexion angle of the patient's spine according to the methods used in the literature[12].The AC angle was 98.2°, the upper CC was 71°, and the lower CC was 62°. Chasing and scanning in vision function was normal. There was a compensatory saccade in the head-shaking experiment, which showed abnormal vestibular function.
No evident abnormalities were detected.
The anterior-posterior/lateral X-ray findings were unremarkable.
The final diagnosis was PD with AC and CC (HY staging, off-stage 3).
Sensory tricks training is defined as the phenomenon of improving dystonia through a specific event orientation[13]. CC symptoms during forward flexion of the spine disappear while the patient lies and sits against the wall, as indicated by sensory tricks[14]. During the treatment, the author considered whether it was possible to provide assistive devices to achieve the desired effect. A literature search revealed that two sensory tricks methods can be used for patients with CC. One is adding weight to a backpack. In patients with CC, the original muscle position is altered due to forward flexion of the spine, resulting in impaired proprioceptive and sensory integration[15]. We placed clay, air-filled plastic bags, and daily necessities in the backpack ensure that the overall weight was greater than 6 kg. The backpack's weight provides weight to the spine, increasing proprioception. In addition, patients should be able to carry a backpack before discharge and need only minimal assistance to climb up and down two-floor staircases. Another sensory trick is a high-frame walker (HFW), which significantly relieves the forward bending of the trunk when used. This small cart increases the patient's walking safety and assists in shopping. The patient walked for more than 20 min during the treatment each time.
Lumbar muscle strength training: The GMMT of the back muscle was graded 1+, which represents lumbar muscle strength weakness and may affect the patient's standing posture. With the rhythmic auditory stimulation method, patients can stretch their lumbar and paraspinal muscles rhythmically. The patient performed the exercises 5 min every day during the program, which was 6 d per week.
Abdominal muscle strength training: The patient complained of difficulty turning over and getting up. The strength of the rectus abdominis muscle and the internal and external oblique muscles of the abdomen was weak. The abdominal muscles are essential in a patient's recumbent position. As a result, we added abdominal muscle training, such as gluteal bridge support with both legs, gluteal bridge training with one leg, and plank training, to the last 1/3 of the treatment. Abdominal training increased the patient's core strength and helped her maintain the correct posture. The patient spent 10 min at the end of the treatment session every day on this training.
Regarding neck strength training: The patient's cervical extensor strength was weak, and she was unable to control active neck movement. When the patient sat in a wheelchair or stood, the head dropped considerably. This phenomenon adversely affected the patient's aware awareness of things around her and increases her risk of falling. Therefore, the patient was given neck muscle training to help maintain the correct neck posture while in a sitting position. In addition, we used posture correction mirrors to train her in self-stretching to achieve postural reeducation. The patient performed the exercises for 10 min during every day’s program, which was 6 d per week.
The results of the gait analysis of the patient at different time points in the first week, the third week, and the fifth week are shown in Table 1. The gait analysis Videos 1, 2 and 3 were shown in the video part of the publishing system. Her stride length improved. When the UPDRS-III score improved to 19, the CC severity score decreased to 8, the MBI increased to 95, the Lawton IADL score improved to 22, and the BBS increased to 35. A comparison of the scores before and after the intervention is shown in Table 2. The changes in the angles of the AC, upper CC, and lower CC during the different periods are shown in Figure 2, which illustrates the improvements. Figure 2 shows that the patient had better posture control while walking.
| Time | Step frequency (steps/min) | Step length (m) | Step width (m) | Stride length (m) | ||||||||
| Left | Right | Normal value | Left | Right | Normal value | Left | Right | Normal value | Left | Right | Normal value | |
| First week | 93.8 | 90.9 | 98.8 ± 11.6 | 0.28 | 0.36 | 0.59 ± 0.08 | 0.21 | 0.23 | 0.15 ± 0.04 | 0.66 | 0.66 | 1.15 ± 0.04 |
| Third week | 88.2 | 95.2 | 0.32 | 0.35 | 0.28 | 0.27 | 0.67 | 0.68 | ||||
| Fifth week | 90.2 | 93 | 0.33 | 0.44 | 0.24 | 0.23 | 0.76 | 0.77 | ||||
| First week | Third week | Fifth week | |
| UPDRS-III score | 60 | 33 | 19 |
| CC severity score | 14 | / | 8 |
| MBI score | 89 | / | 95 |
| Lawton IADL score | 16 | / | 22 |
| BBS score | 21 | 27 | 35 |
| AC angles | 98.2 | 49 | 22 |
| Upper CC angles | 71 | 53 | 46 |
| Lower CC angles | 62 | 52 | 46 |
Here, we report the case of a PD patient with AC and CC as the primary symptoms. This patient experienced considerable relief in abnormal posture and a great improvement in quality of life after sensory tricks and trunk strength training. Due to a lack of standard treatment, this is a relatively new attempt. In addition, gait analysis was used to objectively assess improvements of balance and walking ability. Gait speed and other gait data measured during gait analysis can be used as indicators of disease severity in PD patients[16]. However, there are challenges in measuring the anterior flexion of the cervical, thoracic, and lumbar spine. The author used the Neuro Posture App method to compare the changes of the corresponding angle before and after treatment.
There are many causative factors for AC and CC, and the mainstream view is that pathogenesis can be divided into central and peripheral mechanisms. The central mechanism is exercise-induced dystonia, a disease of the striatum and its projection to the reticular spinal tract or thalamus in PD patients[17]. In addition, the spinal cord's multisynaptic reflex arch integrates sensory information into motor output. The central mechanism is associated with the progression of PD. Peripheral muscle stiffness due to aging leads to the degeneration of paraspinal muscle groups. Both mechanisms result in characteristic pathological changes in the axial musculature that maintain upright body posture and result in abnormal axial trunk posture[18].
In terms of the central mechanism, we used two sensory tricks methods due to the patient's dystonia while standing or walking. One is the weighted backpack. The backpack generates proprioception and light touch input to the patient, increasing the integration of sensorimotor function[19]. Studies have shown that higher beta waves increase the severity of PD. Thus, effective treatment is needed to reduce beta waves generated by central vibrations[20]. Peripheral sensory input given by inexpensive backpacks may impact the central nervous network, reducing abnormal movements due to dystonia[21]. Second, an HFW is recommended, which can be used as a motor trick to provide continuous proprioception to PD patients while walking. As a behavioral strategy, it can be taught to both the patient and the caregiver[22]. In addition, this HFW, which is a light portable walker, can be used for activities at home and at work[23].
Peripherally, trunk muscle training reduces the risk of falling[24]. Trunk strength can improve because it plays a role in stabilizing the head during dynamic tasks. In back muscle training, we targeted paraspinal muscles and other back extensors, such as the latissimus dorsi. These exercises increase the patient's strength to maintain an upright posture. In abdominal muscle training, training targets the patient's internal and external oblique muscles and can improve forward flexion of the upper trunk. Second, the rectus abdominis and iliopsoas muscles are trained to improve forward flexion of the lower trunk[25]. Training these core muscles simultaneously can improve the ability to transfer from supine to sitting. During the training, the author asked patients to use rhythmic auditory stimulation software (metronome) with visual conduction to enhance visual and proprioceptive input and strengthen the cervical muscle. Adjustment of trunk posture is performed by positive feedback[26]. Trunk stretching is a static stretch that immediately increases tendon length, improving the relationship between muscle tone and tendon length. It alters the viscoelastic function of muscles. Therefore, it can reduce muscle motor neuron hyperexcitability by modulating spinal cord suppressor neurons[27]. As a result, stretching helps the patient improve her stiffness in her trunk muscles.
Notably, we found that the angle of the AC improved even more than the angle of the CC did. In the searched articles, most treatments for AC involved reducing the dose of drugs such as donepezil[28]. Although decreasing pramipexole is also used for patients and is thought to relieve symptoms, this drug is crucial for symptom control in PD patients; therefore, reducing this drug was not considered. In addition, there are no studies on AC rehabilitation, and our study methods may have positive effects on PD patients with AC.
The present study has several limitations. First, Parkinson's medication was not initiated or discontinued during treatment. Thus, the patient was evaluated under the condition of medication. Second, a single case is not universal. We should increase the number of cases. Therefore, further studies are needed to confirm the effectiveness of these therapeutic interventions.
In summary, through sensory tricks and trunk strength training, the PD sagittal posture was improved. In addition, the patient's stride length correspondingly improved, indicating that the patient's walking ability was improved. She improved her ability to perform activities of daily living. She could simulate shopping on the street under supervision and go up and down stairs with minimal assistance, indicating an improved quality of life. The patient still maintained good posture at the 6-month follow-up. The patient was satisfied with the therapy and participated positively in the treatment session and follow-up visit. In the future, we should recruit more patients to conduct a clinical study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rehabilitation
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sotelo J, Mexico S-Editor: Zhang H L-Editor: A P-Editor: Cai YX
| 1. | Mikami K, Shiraishi M, Kamo T. Effect of abnormal posture on quality of life in patients with Parkinson’s disease. Adv Parkinson's Dis. 2016;5:7-14. [DOI] [Full Text] |
| 2. | Doherty KM, van de Warrenburg BP, Peralta MC, Silveira-Moriyama L, Azulay JP, Gershanik OS, Bloem BR. Postural deformities in Parkinson's disease. Lancet Neurol. 2011;10:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 3. | Quinn N. Disproportionate antecollis in multiple system atrophy. Lancet. 1989;1:844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Woo KA, Joo JY, Shin JH, Lee CY, Jeon SH, Lee JY, Kim HJ, Jeon B. Measurement Issue in Antecollis. Tremor Other Hyperkinet Mov (N Y). 2022;12:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Kashihara K, Ohno M, Tomita S. Dropped head syndrome in Parkinson's disease. Mov Disord. 2006;21:1213-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Djaldetti R, Mosberg-Galili R, Sroka H, Merims D, Melamed E. Camptocormia (bent spine) in patients with Parkinson's disease--characterization and possible pathogenesis of an unusual phenomenon. Mov Disord. 1999;14:443-447. [PubMed] [DOI] [Full Text] |
| 7. | Fasano A, Geroin C, Berardelli A, Bloem BR, Espay AJ, Hallett M, Lang AE, Tinazzi M. Diagnostic criteria for camptocormia in Parkinson's disease: A consensus-based proposal. Parkinsonism Relat Disord. 2018;53:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Tiple D, Fabbrini G, Colosimo C, Ottaviani D, Camerota F, Defazio G, Berardelli A. Camptocormia in Parkinson disease: an epidemiological and clinical study. J Neurol Neurosurg Psychiatry. 2009;80:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Iijima M, Osawa M, Uchiyama S, Kitagawa K. Pramipexole-induced antecollis in patients with Parkinson's disease: Two cases and literature review. eNeurologicalSci. 2015;1:21-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Doherty KM, Davagnanam I, Molloy S, Silveira-Moriyama L, Lees AJ. Pisa syndrome in Parkinson's disease: a mobile or fixed deformity? J Neurol Neurosurg Psychiatry. 2013;84:1400-1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Margraf NG, Wrede A, Rohr A, Schulz-Schaeffer WJ, Raethjen J, Eymess A, Volkmann J, Mehdorn MH, Jansen O, Deuschl G. Camptocormia in idiopathic Parkinson's disease: a focal myopathy of the paravertebral muscles. Mov Disord. 2010;25:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Schlenstedt C, Boße K, Gavriliuc O, Wolke R, Granert O, Deuschl G, Margraf NG. Quantitative assessment of posture in healthy controls and patients with Parkinson's disease. Parkinsonism Relat Disord. 2020;76:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Ramos VF, Karp BI, Hallett M. Tricks in dystonia: ordering the complexity. J Neurol Neurosurg Psychiatry. 2014;85:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Zhao J, Wang Q, Sun H, Liu X, Leng B, Wang T. Quetiapine Relieved Pisa Syndrome in Patient With Parkinson Disease. Clin Neuropharmacol. 2020;43:171-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Wolke R, Kuhtz-Buschbeck JP, Deuschl G, Margraf NG. Insufficiency of trunk extension and impaired control of muscle force in Parkinson's disease with camptocormia. Clin Neurophysiol. 2020;131:2621-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Matsumoto H, Shiraishi M, Higashi A, Hino S, Kaburagi M, Mizukami H, Maki F, Yamauchi J, Tanabe K, Sato T, Yamano Y. The Forward and Lateral Tilt Angle of the Neck and Trunk Measured by Three-Dimensional Gait and Motion Analysis as a Candidate for a Severity Index in Patients with Parkinson's Disease. Neurol Int. 2022;14:727-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Djaldetti R, Melamed E. Camptocormia in Parkinson's disease: new insights. J Neurol Neurosurg Psychiatry. 2006;77:1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Schulz-Schaeffer WJ. Camptocormia in Parkinson's Disease: A Muscle Disease Due to Dysregulated Proprioceptive Polysynaptic Reflex Arch. Front Aging Neurosci. 2016;8:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Lena F, Iezzi E, Etoom M, Santilli M, Centonze D, Foti C, Grillea G, Modugno N. Effects of postural exercises in patients with Parkinson's disease and Pisa syndrome: A pilot study. NeuroRehabilitation. 2017;41:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Tan H, Wade C, Brown P. Post-Movement Beta Activity in Sensorimotor Cortex Indexes Confidence in the Estimations from Internal Models. J Neurosci. 2016;36:1516-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Edwards MJ. From Tricks to Treatment-Sensory Input and Dystonic Dyskinesia in Parkinson's Disease. Mov Disord Clin Pract. 2017;4:6-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Moroz A, Edgley SR, Lew HL, Chae J, Lombard LA, Reddy CC, Robinson KM. Rehabilitation interventions in Parkinson disease. PM R. 2009;1:S42-8; quiz S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Nickel E, Hansen A, Pearlman J, Goldish G. A drive system to add standing mobility to a manual standing wheelchair. Assist Technol. 2016;28:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Hubble RP, Naughton GA, Silburn PA, Cole MH. Trunk muscle exercises as a means of improving postural stability in people with Parkinson's disease: a protocol for a randomised controlled trial. BMJ Open. 2014;4:e006095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Srivanitchapoom P, Hallett M. Camptocormia in Parkinson's disease: definition, epidemiology, pathogenesis and treatment modalities. J Neurol Neurosurg Psychiatry. 2016;87:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Aoki R, Shiraishi M, Mikami K, Kamo T. Deterioration of postural deformity in Parkinson's disease patients with punding and hobbyism. J Clin Neurosci. 2019;69:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Guissard N, Duchateau J, Hainaut K. Mechanisms of decreased motoneurone excitation during passive muscle stretching. Exp Brain Res. 2001;137:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Oh YS, Kim JS, Ryu DW, Lee KS. Donepezil induced antecollis in a patient with Parkinson's disease dementia. Neurol Sci. 2013;34:1685-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |