Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.425
Peer-review started: October 23, 2023
First decision: November 21, 2023
Revised: November 25, 2023
Accepted: December 27, 2023
Article in press: December 27, 2023
Published online: January 16, 2024
Processing time: 80 Days and 4.9 Hours
Inflammatory myofibroblastic tumors (IMTs) are exceptionally rare neoplasms with intermediate malignant potential. Surgery is the accepted treatment option, aiming for complete resection with clear margins.
A 39-year-old woman presented with a growing solitary pulmonary nodule measuring 2.0 cm in the right upper lobe (RUL) of the lung. The patient under-went a RUL anterior segmentectomy using uniportal video-assisted thoracoscopy. A preliminary tissue diagnosis indicated malignancy; however, it was later revised to an IMTs. Due to the absence of a minor fissure between the right upper and middle lobes, an alternative resection approach was necessary. Therefore, we utilized indocyanine green injection to aid in delineating the intersegmental plane. Following an uneventful recovery, the patient was discharged on the third postoperative day. Thereafter, annual chest tomography scans were scheduled to monitor for potential local recurrence.
This case underscores the challenges in diagnosing and managing IMTs, showing the importance of accurate pathologic assessments and tailored surgical strategies.
Core Tip: Inflammatory myofibroblastic tumors are rare neoplasms with intermediate malignant potential. Accurate diagnosis and tailored surgical strategies are crucial for successful management. This case highlights the challenges in diagnosing and treating such tumors, emphasizing the importance of precise pathological assessment and individualized surgical approaches.
- Citation: Ahn S, Moon Y. Uniportal video-assisted thoracoscopic fissureless right upper lobe anterior segmentectomy for inflammatory myofibroblastic tumor: A case report. World J Clin Cases 2024; 12(2): 425-430
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/425.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.425
Inflammatory myofibroblastic tumors (IMTs), previously termed “inflammatory pseudotumors” are exceptionally uncommon intermediate neoplasms characterized by relatively high postsurgical recurrence rates and a low propensity for metastasis[1,2]. Predominantly observed in children and young individuals, this tumor has a prevalence of 0.04%-0.7% across sex and racial groups worldwide[3,4]. Of the various primary sites, IMTs in the abdominal cavity show greater prevalence. However, primary lesions of the lungs, pelvis, limbs, skin, and even brain have been documented[2]. Patients typically present with painless masses that often remain asymptomatic until size-related complications arise[5]. The standard treatment for localized IMTs is surgery; however, therapy for advanced disease has not been clearly established[6].
Herein, we describe the case of a 39-year-old woman who underwent uniportal video-assisted thoracoscopic surgery (VATS) for fissureless right upper lobe (RUL) anterior segmentectomy of an asymptomatic IMT. The tumor was deemed malignant upon frozen section examination.
A 39-year-old female outpatient with no history of smoking presented to our department of Thoracic and Cardiovascular Surgery with an enlarging solitary pulmonary nodule (2.0 cm) of the RUL.
A right-lung nodule had been detected 3 years prior and was under surveillance at another medical facility.
The patient had no substantial medical history, aside from a uterine myomectomy at another institution 7 years prior.
The patient reported no relevant personal or family history.
The patient exhibited no apparent signs or symptoms during physical examination.
The results of the preoperative pulmonary tests were within normal ranges.
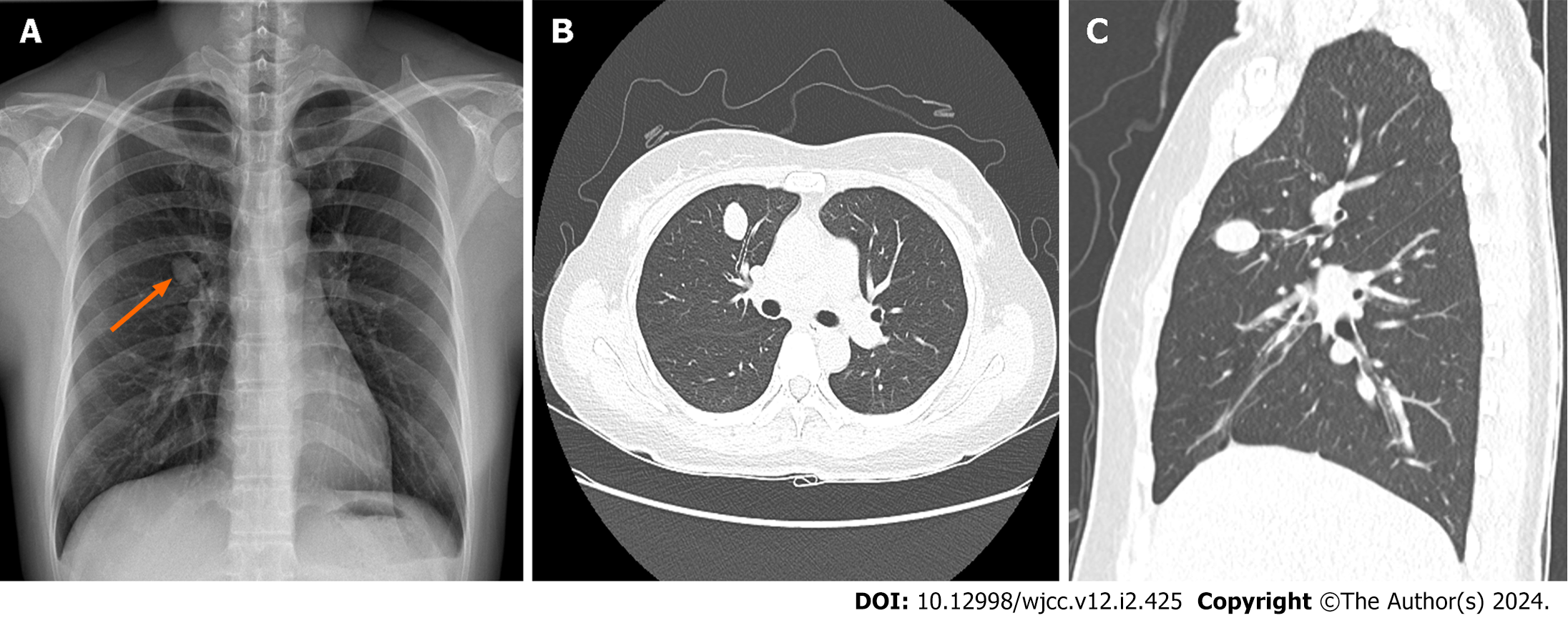
Chest radiography revealed a solitary right hilar nodule (Figure 1A) that measured 2.0 cm × 1.7 cm on computed tomography (CT) of the chest and was situated within the anterior segment of the RUL (Figure 1B and C).
The patient was preoperatively diagnosed with pulmonary hamartoma.
We performed a RUL anterior segmentectomy (rather than wedge resection) using uniportal VATS (Video). This decision was influenced by the nodule’s proximity to the anterior segment bronchus at its origin. Under general anesthesia, the patient was positioned in the left lateral decubitus. Subsequently, a small surgical incision (2.5 cm) was made at the fourth intercostal space along the anterior axillary line. To ensure a well-defined operative field, the working port was equipped with a small wound protector. Thereafter, a 5-mm, 30 scope was positioned at its upper rim by the surgical assistant, with various instruments introduced through the single incision, including a curved suction tip, grasping tools, and articulating endostaplers.
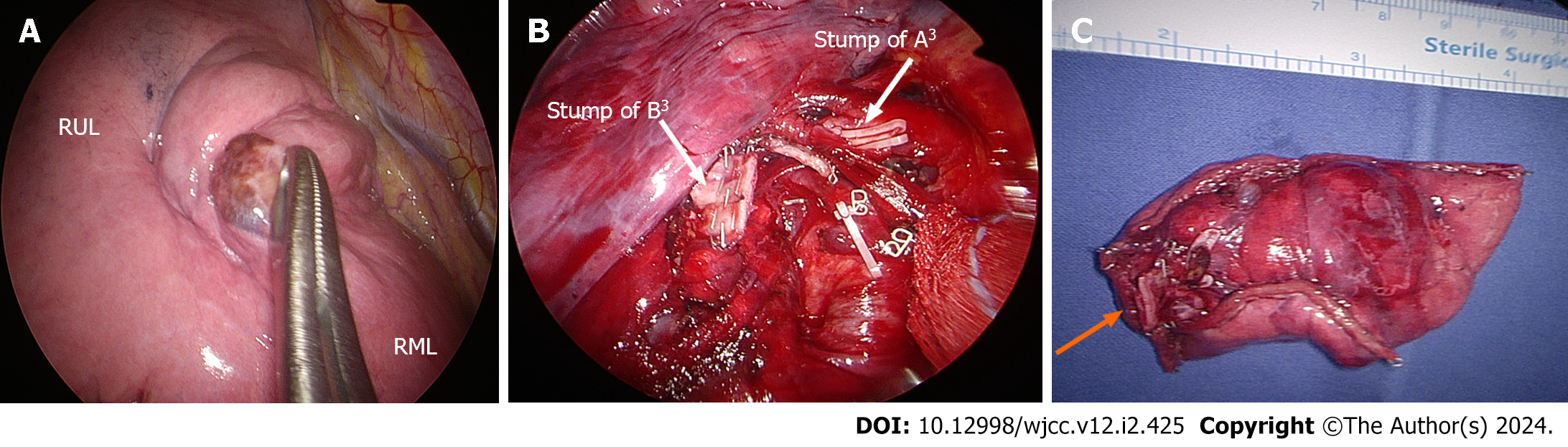
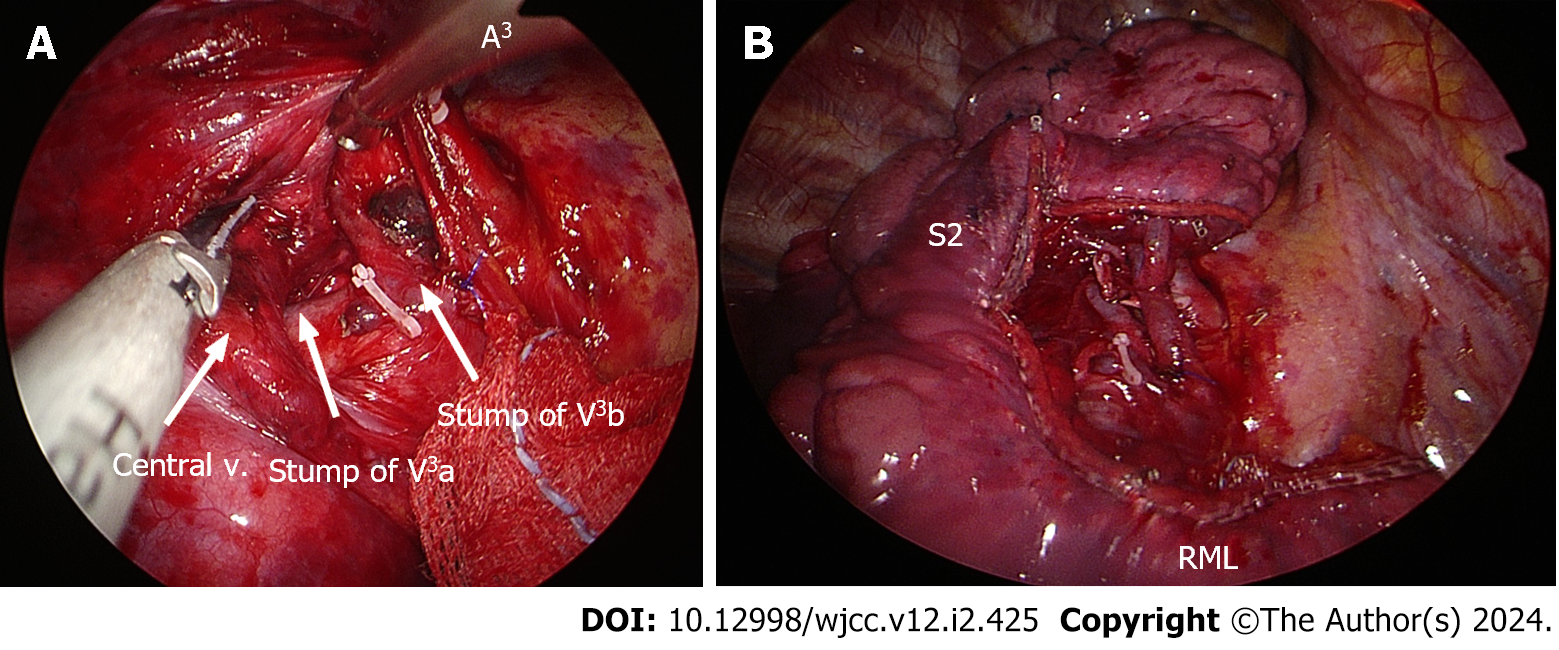
The solitary pulmonary nodule was solid, round, and movable, measuring 2.0 cm. However, when inspecting the right lung, no discernible minor fissure was observed (Figure 2A). To achieve fissureless anterior segmentectomy of the RUL, initial anterior hilar dissection was required to expose branches of the superior pulmonary vein. Subsequently, one of two anterior segmental veins (V3b) was dissected and ligated using a Harmonic scalpel [Ethicon (Johnson and Johnson), Cincinnati, OH, United States], and Prolene sutures (Ethicon) were used to control bleeding. In turn, the other anterior segmental vein (V3a) was clipped (Hem-o-lok; Teleflex, Morrisville, NC, United States) and transected using a Harmonic scalpel (Figure 3A). The anterior segmental artery (A3) was then isolated and ligated using a Hem-o-lok clip (Figure 2B). This was followed by a thorough lymphadenectomy procedure, which was conducted at levels 11–13 and exposed the anterior segmental bronchus.
Before advancing further, we reinstated two-lung ventilation to verify the correct inflation of the RUL-including the apical and posterior segments-and the middle lobes. Subsequently, we proceeded to divide the anterior segmental bronchus using a 45-mm stapler with a purple load. We administered an indocyanine green (ICG) injection to delineate and mark the intersegmental plane (Figure 2B). Once divided along this plane, RUL anterior segmentectomy was completed using four 45-mm staplers (purple cartridges) (Figure 3B).
We secured the resected specimen in an Endo Catch bag (Medtronic, Minneapolis, MN, United States), passing it through the working port for frozen section examination (Figure 2C). Based on a provisional diagnosis of likely malignancy and the solid growth pattern that we observed, systematic lymph node dissection was also undertaken. We applied a water-sealing method to check for and verify the absence of any air leaks. After the irrigation process, we administered an intercostal nerve blockade using 1 mL of bupivacaine at each lower margin of the third to seventh ribs. Subsequently, a 20-Fr chest tube was carefully inserted at the lower incisional edge and the working incision was closed in a layered manner. The chest tube was then connected to a digital drainage system with a suction pressure set at 15 cm H2O. The overall operative time was 130 min, while the duration of anesthesia was 160 min and the estimated blood loss was 50 cc.
In the recovery room, a clear upright anteroposterior chest radiograph was obtained 20 min after completing the procedure (Figure 4A). On the first postoperative day, we removed the chest tube without incident, and the patient was discharged on the third postoperative day.
During the first outpatient visit (1 week postoperatively), a follow-up chest radiograph revealed no abnormalities. Consistent with radiographic findings, the resected lung specimen harbored a solitary pulmonary nodule (2.2 cm × 2.0 cm) in close proximity to the anterior segmental bronchus of the RUL (Figure 4B). Microscopic examination revealed a hypercellular nodule comprising spindle-shaped cells with a fascicular growth pattern (Figure 5A). A high-power view exposed plump spindle cell proliferation intermingled with chronic inflammatory cells (Figure 5B). Notably, nuclear atypia was minimal, with two mitotic figures per 2 mm2. To pathologically diagnose an IMT, multiple immunohistochemical stains were performed to differentiate the tumor from conditions such as pleomorphic carcinoma, benign metastasizing leiomyoma, solitary fibrous tumor, and others. Immunohistochemically, the spindle cells exhibited diffuse and strong positivity for anaplastic lymphoma kinase ventana anti-anaplastic lymphoma kinase, focal positivity for smooth-muscle actin, and negative staining for cluster of differentiation 34, cytokeratin AE1/AE3, desmin, estrogen receptor, S-100 protein, and thyroid transcription factor 1 (Figure 5C). In addition, the tumor exhibited a low Ki-67 proliferative index. These morphological and immunohistochemical findings were indicative of an IMT. Furthermore, the pleural surface appeared smooth and glossy, and all lymph nodes collected from paratracheal (upper, lower), subcarinal, and hilar regions were tumor-free.
In this case, we performed uniportal VATS to achieve fissureless RUL anterior segmentectomy for an asymptomatic IMT. This patient had no obvious symptoms at the time, showing only a solitary, well-circumscribed, and growing RUL pulmonary nodule (2.0 cm). Pulmonary hamartoma was our preoperative diagnosis. In fact, imaging findings of IMTs reflect the chief morphologic characteristics displayed in this case, ranging from ill-defined, infiltrative lesions to well-circumscribed soft tissue masses. The multiplicity of manifestations of this tumor type is attributed to varying inflammatory, stromal, and myofibroblastic tumor components[4,7].
Unfortunately, this morphologic diversity may prove problematic for the histological diagnosis of IMTs[6]. For example, few spindle cells may exist within a largely hyalinized and chronically inflamed background, or an intense myofibroblastic proliferation with atypical features may prevail[6]. The spectrum of phenotypic variability entailed, which offers no distinct immunostaining profile, has thus rendered IMT a diagnosis by exclusion, ruling out other possibilities. The many pertinent differential considerations include local inflammatory processes, idiopathic retroperitoneal fibrosis, and inflammatory fibrosarcoma[6].
Surgical intervention-if feasible-is the usual treatment for localized IMT, aiming for en bloc resection with clear margins[8]. In these cases, both tumor and surrounding normal tissues are removed in continuity to ensure that the excision is complete[6]. In our patient, we fully achieved the desired goal through the use of uniportal VATS, performing fissureless RUL anterior segmentectomy. However, circumstances forced us to abandon our customary approach, which involves tunneling to divide the minor fissure (absent in this case)[9]. Instead, the anterior hilum was initially dissected to expose branches of the superior pulmonary vein, and ICG injection played a valuable role in facilitating clear and unencumbered delineation of the intersegmental plane[9].
The local recurrence rates for IMT widely range from < 2% to 25%, with distant metastasis developing in < 5% of patients[10,11]. Adjuvant therapy is not currently recommended in cases of complete resection[6]; however, recurrences should warrant repeat surgical attempts[6,12,13]. Therefore, our patient will undergo annual chest CT scans to monitor for any signs of local recurrence meriting further intervention.
In this case, fissureless RUL anterior segmentectomy of an asymptomatic IMT was achieved using uniportal VATS. This case underscores the inherent difficulties in diagnosing and managing IMT. Moreover, accurate pathological assessments and tailored surgical strategies are critical to optimize patient outcomes in this setting.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ulasoglu C, Turkey S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 671] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 2. | Siemion K, Reszec-Gielazyn J, Kisluk J, Roszkowiak L, Zak J, Korzynska A. What do we know about inflammatory myofibroblastic tumors? - A systematic review. Adv Med Sci. 2022;67:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Panagiotopoulos N, Patrini D, Gvinianidze L, Woo WL, Borg E, Lawrence D. Inflammatory myofibroblastic tumour of the lung: a reactive lesion or a true neoplasm? J Thorac Dis. 2015;7:908-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 4. | Surabhi VR, Chua S, Patel RP, Takahashi N, Lalwani N, Prasad SR. Inflammatory Myofibroblastic Tumors: Current Update. Radiol Clin North Am. 2016;54:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Choi AH, Bohn OL, Beddow TD, McHenry CR. Inflammatory myofibroblastic tumor of the small bowel mesentery: an unusual cause of abdominal pain and uveitis. J Gastrointest Surg. 2011;15:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Gros L, Dei Tos AP, Jones RL, Digklia A. Inflammatory Myofibroblastic Tumour: State of the Art. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 7. | Rasalkar DD, Chu WC, To KF, Cheng FW, Li CK. Radiological appearance of inflammatory myofibroblastic tumour. Pediatr Blood Cancer. 2010;54:1029-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brennan B, Brodowicz T, Buonadonna A, De Álava E, Del Muro XG, Dufresne A, Eriksson M, Fagioli F, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hecker-Nolting S, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kager L, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Mir O, Montemurro M, Morland B, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss S, Sundby Hall K, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Casali PG, Stacchiotti S; ESMO Guidelines Committee, EURACAN and GENTURIS. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann Oncol. 2021;32:1348-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 582] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 9. | Igai H, Matsuura N, Kamiyoshihara M. Uniportal anterior segmentectomy (S3) of the left upper lobe. Multimed Man Cardiothorac Surg. 2020;2020. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Alaggio R, Cecchetto G, Bisogno G, Gambini C, Calabrò ML, Inserra A, Boldrini R, De Salvo GL, G d'Amore ES, Dall'igna P. Inflammatory myofibroblastic tumors in childhood: a report from the Italian Cooperative Group studies. Cancer. 2010;116:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Sagar AES, Jimenez CA, Shannon VR. Clinical and Histopathologic Correlates and Management Strategies for Inflammatory Myofibroblastic tumor of the lung. A case series and review of the literature. Med Oncol. 2018;35:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Iwai H, Yanagawa N, Deguchi H, Tomoyasu M, Shigeeda W, Kaneko Y, Yoshimura R, Kanno H, Sugai M, Shikanai S, Sugai T, Saito H. Surgical treatment for lung metastasis of inflammatory myofibroblastic tumor of the lung: A case report. Thorac Cancer. 2023;14:1644-1647. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Souid AK, Ziemba MC, Dubansky AS, Mazur M, Oliphant M, Thomas FD, Ratner M, Sadowitz PD. Inflammatory myofibroblastic tumor in children. Cancer. 1993;72:2042-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |