Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.405
Peer-review started: September 29, 2023
First decision: December 18, 2023
Revised: December 18, 2023
Accepted: December 26, 2023
Article in press: December 26, 2023
Published online: January 16, 2024
Processing time: 91 Days and 1.6 Hours
Small cell lung cancer (SCLC) is a common and aggressive subtype of lung cancer. It is characterized by rapid growth and a high mortality rate. Approximately 10% of patients with SCLC present with brain metastases at the time of diagnosis, which is associated with a median survival of 5 mo. This study aimed to summarize the effect of bevacizumab on the progression-free survival (PFS) and overall survival of patients with brain metastasis of SCLC.
A 62-year-old man was referred to our hospital in February 2023 because of dizziness and numbness of the right lower extremity without headache or fever for more than four weeks. The patient was diagnosed with limited-stage SCLC. He received 8 cycles of chemotherapy combined with maintenance bevacizumab therapy and achieved a PFS of over 7 mo.
The combination of bevacizumab and irinotecan effectively alleviated brain metastasis in SCLC and prolonged PFS.
Core Tip: Small cell lung cancer (SCLC) accounts for approximately 13%-15% of all lung cancer patients. A five-year survival rate of less than 7 percent makes it one of the deadliest cancers. Compared to Non-Small Cell Lung Carcinoma, SCLC has a faster doubling time in the early stages and is more likely to spread widely. Therefore, 60%-70% of SCLC is diagnosed as extensive stage at initial diagnosis. SCLC cells also show a high tendency of metastasis to the central nervous system, and 10% of patients have brain metastasis at the first visit. Here, we report a patient with extensive stage-small cell lung cancer (ES-SCLC) and brain metastases who received four lines of treatment. We used bevacizumab in combination with irinotecan as a post-fourth-line therapy in an elderly man and achieved a significant partial response.
- Citation: Yang HY, Xia YQ, Hou YJ, Xue P, Zhu SJ, Lu DR. Chemotherapy combined with bevacizumab for small cell lung cancer with brain metastases: A case report. World J Clin Cases 2024; 12(2): 405-411
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/405.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.405
Small cell lung cancer (SCLC) accounts for 13%-15% of all lung cancer patients. A five-year survival rate of less than 7 percent makes it one of the deadliest cancers[1,2]. Compared to Non-Small Cell Lung Carcinoma (NSCLC), SCLC has a faster doubling time in the early stages and is more likely to spread widely. Therefore, 60%-70% of SCLC is diagnosed as extensive stage at initial diagnosis. Due to the high tendency for SCLC to metastasize to the central nervous system (CNS), 10% of patients have brain metastases on their first visit, which bodes poorly for their prognosis[3]. Here, we report a patient with extensive stage-small cell lung cancer (ES-SCLC) and brain metastases who received four lines of treatment. We used bevacizumab in combination with irinotecan as a post-fourth-line therapy in an elderly man and achieved a significant partial response.
Dizziness for more than four weeks.
Dizziness and numbness of the right lower extremity without headache or fever.
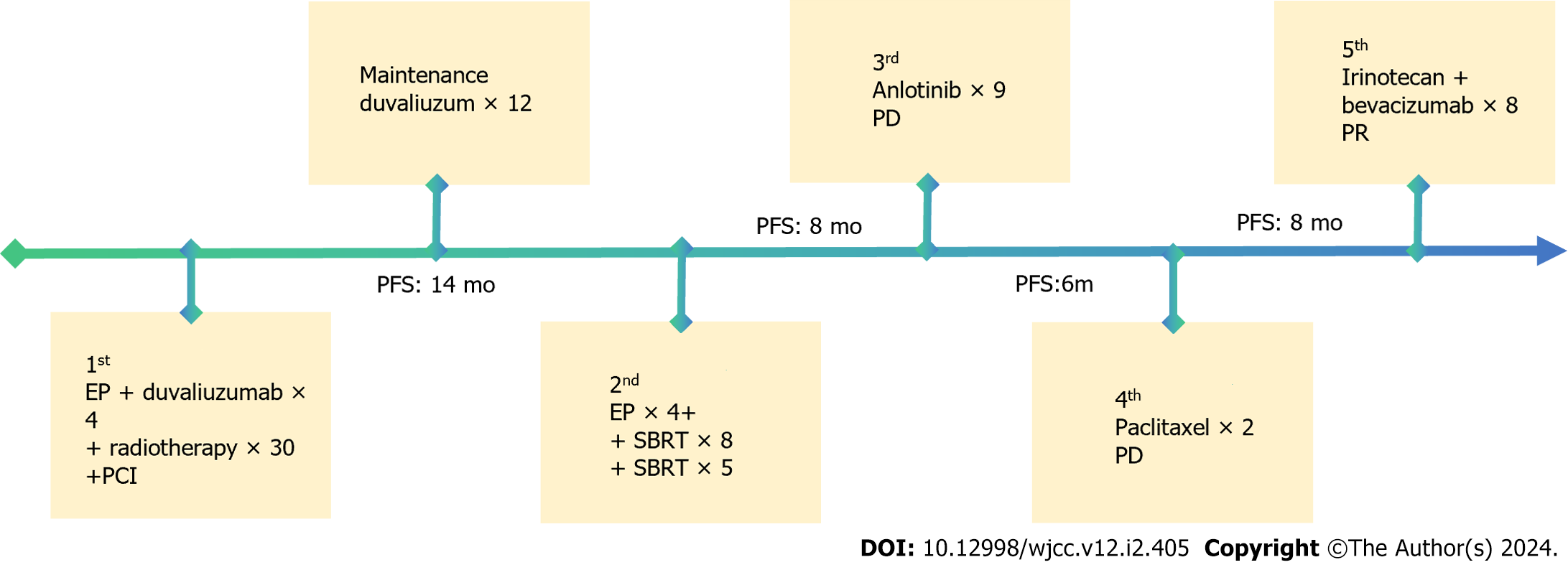
The patient was diagnosed with limited-stage SCLC at Peking University Cancer Hospital on August 1, 2020. With a clear diagnosis, immediately administer four cycles of carboplatin plus etoposide combined with duvalizumab. After chemotherapy, the patient's primary tumor was partially reduced in size, and a total of 30 radiotherapy sessions were administered to the primary lung cancer lesion in the right hilar region. The tumor was significantly reduced in size after chemotherapy and radiotherapy. Subsequently, the patient continued to receive preventive cranial irradiation to prevent brain metastasis. In December 2021, the patient received 12 cycles of immune maintenance therapy until lesion recurrence. The patient underwent the original chemotherapy regimen again for four cycles at Peking University Cancer Hospital from early December 2021 to February 2022, followed by eight rounds of stereotactic radiation therapy for recurrent lesions in March 2022, achieving complete remission. However, brain metastases were found, as was the case with stereotactic radiation therapy. Unfortunately, in July 2022, a new metastatic lesion was found in the right lung, and the patient received treatment with anlotinib, a novel, orally administered multi-targeting tyrosine kinase inhibitor with the main effects of anti-angiogenesis. At the end of October 2022, a brain magnetic resonance imaging (MRI) showed that the brain tumor had shrunk to 4 mm × 2 mm. On January 18, 2023, a brain MRI revealed that the tumor was 10 times larger. Immediately, anlotinib[4] was discontinued, and the patient underwent two cycles of chemotherapy with paclitaxel-albumin.
He denied family history of genetic disease, history of chronic disease, history of cancer, etc.
A detailed general examination of the patient was performed and no obvious positive signs were found.
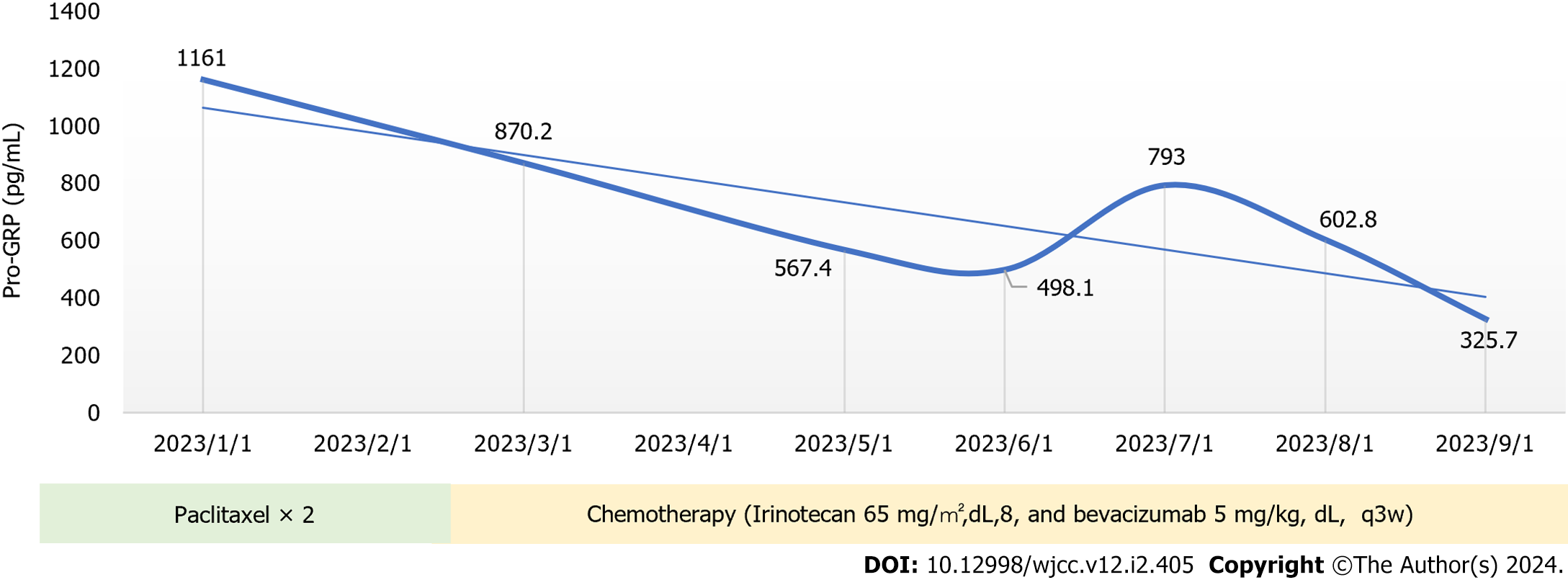
The pro-gastrin-releasing peptide (Pro-GRP) (2023-01-01): 1161 (pg/mL).
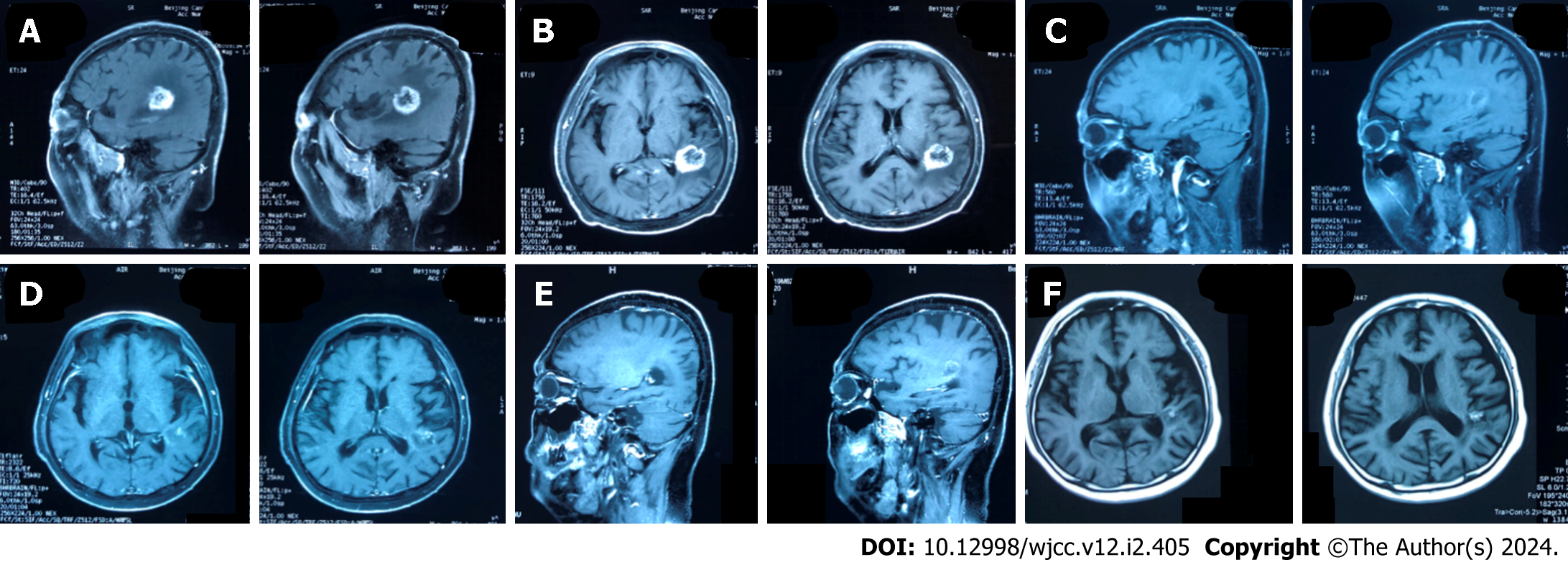
On February 22, 2023, an MRI examination of the head revealed a metastatic tumor in the left temporal lobe, which was larger than previously noted. The tumor was a carcinoma of the right upper lung (Figure 1A and B).
SCLC with brain metastases.
On March 2, 2023, the patient visited our hospital for treatment, and we comprehensively assessed the physical fitness, age, body surface area (weight, 60 kg; height, 168 cm; body surface area 1.67 m2), and previous treatments (Figure 2). Accordingly, the patient started chemotherapy comprising irinotecan (120 mg, intravenous infusion on days 1 and 8) combined with bevacizumab (5 mg/kg, intravenous infusion on day 1), which was repeated every 3 wk. After the second cycle of chemotherapy, dizziness and headache disappeared, and lower limb mobility improved further. The Pro-GRP (pg/mL) serum concentration immediately declined following the initiation of therapy (Figure 3), and a head MRI in other hospitals showed that the intracranial metastases were smaller than before (Figure 1C and D). Notably, the patient had almost no bone marrow suppression or diarrhea; therefore, chemotherapy was initiated on the 8th day of the 8th cycle as maintenance therapy.
Fortunately, the head MRI showed no significant change in the intracranial tumor compared with the previous examination in June 2023 (Figure 1E and F). The patient has had progression-free survival (PFS) for over 7 mo, and we will continue to follow up with the patient and strictly review the changes in their condition.
Patients with ES-SCLC survive only about 1 year, and about 10% of them are diagnosed with brain metastases at the first visit. Patients with brain metastases survive less than 5 mo. Currently, chemotherapy remains an effective combination treatment for patients with SCLC and brain metastases. A review of the literature by Chen et al[5] found that the response rate of brain metastases from SCLC to various chemotherapies ranged from 22% to 85%, with median survival of patients ranging from 3 to 9 mo. However, chemotherapy drugs cannot damage cancer cells in the CNS owing to their inability of chemotherapy drugs to pass through the blood-brain and blood-tumor barriers, which may be the reason for the high mortality of patients with SCLC with brain metastasis[6]. Our patient was treated with fifth-line irinotecan and bevacizumab, which was well tolerated and showed significant responses, particularly regarding brain metastases. This represents a significant improvement in quality of life and extends PFS beyond 7 mo.
Currently, an increasing number of clinical trials have shown that irinotecan plus cisplatin or carboplatin is effective for patients with ES-SCLC, not only with prolonged survival, but also with fewer toxic side effects and good patient tolerability. Small cell carcinomas are sensitive to chemotherapy. Chemotherapy with etoposide plus cisplatin or carboplatin (EP/EC) or irinotecan plus cisplatin or carboplatin (IP/IC) remains the current standard of care. However, most patients relapse within weeks or months of treatment, and survival is only about 10 mo[7].
Initially, the IP protocol showed some advantages in clinical trials. A multicenter, randomized phase 3 study in Japan compared the efficacy of the IP regimen to the EP regimen in patients with ES-SCLC. The study was stopped early due to significant differences in outcomes between the two groups at the interim analysis. The results showed that among these 154 patients, median survival was 12.8 mo in the IP group compared to 9.4 mo in the EP group (P = 0.002), and 2-year survival was 19.5% in the IP group compared to 5.2% in the EP group. The experiment proves that the IP regimen can prolong life better than the EP regimen[7]. Therefore, in this trial, irinotecan in combination with Cisplatin performed well and was a more attractive option for patients with ES-SCLC.
Hermes et al[8] conducted a study comparing IC with oral EC. Patients with SCLC were randomly assigned to receive IC or EC. Of the 209 patients included in the analysis, median survival was 8.5 mo for IC and 7.1 mo for EC. One-year survival was 34% in the IC arm and 24% in the EC arm. The incidence of grade 3 or 4 diarrhea was higher in the IC group. The trial concluded that IC Prolongs Survival in Patients with Small Cell Carcinoma. These two clinical trials demonstrated the efficacy of irinotecan plus cisplatin or carboplatin.
Angiogenesis is an important process in tumorigenesis and progression. Inhibition of angiogenesis has been proven to be an effective strategy in the treatment of several kinds of tumors. Vascular endothelial growth factor (VEGF) is the most important angiogenic protein and is overexpressed in SCLC[9]. As a result, VEGF is elevated in most tumors, including lung cancer. The increased expression of VEGF in SCLC, which means an increased number of new blood vessels, may be one of the reasons for the poor prognosis of SCLC[10,11]. Bevacizumab, the first antiangiogenic drug approved by the United States Food and Drug Administration, is a humanized monoclonal antibody to VEGF. It can bind to VEGF-A isoforms to prevent the interaction between VEGF-A and VEGFR, thereby inhibiting the activation of the VEGF signaling pathway that promotes angiogenesis. Bevacizumab blocks blood vessel growth and promotes the delivery of cytotoxic chemotherapy[10,12-16]. Although bevacizumab monotherapy for SCLC has not shown a significant increase in survival in clinical trials, several studies have shown that bevacizumab in combination with chemotherapy can achieve good results.
A single-arm phase II study enrolled 72 patients with ES-SCLC who received IP in combination with bevacizumab. The results showed higher PFS and overall survival (OS) times compared to results with the same chemotherapy regimen without bevacizumab in the United States: an overall response rate of 75% and a PFS of 7.0 mo, midium OS was 11.6 mo[17].
Another multicenter phase II study of bevacizumab plus IP in previously untreated patients with ES-SCLC showed a median OS of 12.1 mo; 1- and 2-year OS rates were 51% and 14%, respectively. This study shows that IP in combination with bevacizumab can provide better results than chemotherapy alone[18].
A randomized phase II-III study of bevacizumab plus EP in patients with ES-SLCL who had not received antitumor therapy evaluated the efficacy and safety of bevacizumab after induction chemotherapy. The 74 patients were randomly assigned to the EP group and the EP plus bevacizumab group. At the end of the fourth cycle, there was no significant difference between the two groups. Therefore, combination therapy with bevacizumab after chemotherapy does not improve the prognosis of patients with ES-SCLC[19].
In addition, bevacizumab is safe in brain metastases and is approved for the treatment of nonsquamous NSCLC[20,21]. All of the above experiments are demonstrated the efficacy of bevacizumab in combination with chemotherapy.
Although studies using systemic chemotherapy have shown improved response and survival rates in patients with SCLC, most of these studies did not conduct a separate subgroup analysis of patients with brain metastases or the number of enrolled patients with brain metastasis was relatively small. most of these studies did not conduct a separate subgroup analysis of patients with brain metastases or the number of enrolled patients with brain metastasis was relatively small. Chen et al[5] reviewed 8 articles analyzing the effects of chemotherapy on brain metastasis and included 14 patients treated in their study and found that the response of brain metastases ranged from 22% to 85%. Multiple clinical studies have demonstrated that the blood-brain barrier (BBB) is often disrupted by brain metastasis, making it permeable to anticancer drugs. Additionally, contemporary anticancer drugs, such as irinotecan, carboplatin, and bevacizumab, have been shown to penetrate the BBB more effectively and exhibit greater anticancer activity[22].
Currently, chemotherapy remains the standard of care for first- and second-line treatment of SCLC. The above experiments proved that IP regimen may have better therapeutic effect on ES-SCLC. There is also a benefit of combining bevacizumab with chemotherapy. In this case, the combination of irinotecan and bevacizumab, with maintenance treatment, achieved a PFS of over 7 mo. The patient tolerated the treatment well, suggesting that irinotecan combined with bevacizumab may be a promising treatment for brain metastases of SCLC. However, there are few large randomized controlled trials of small-cell carcinoma combined with brain metastases, and each patient has a different tolerability profile. In the clinical setting, systematic evaluation of patients is needed to determine the most appropriate treatment, further clinical trials are needed to confirm its efficacy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kong PZ, China S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 524] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11404] [Article Influence: 3801.3] [Reference Citation Analysis (4)] |
| 3. | Lukas RV, Gondi V, Kamson DO, Kumthekar P, Salgia R. State-of-the-art considerations in small cell lung cancer brain metastases. Oncotarget. 2017;8:71223-71233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Cheng Y, Wang Q, Li K, Shi J, Wu L, Han B, Chen G, He J, Wang J, Qin H, Li X. Anlotinib for patients with small cell lung cancer and baseline liver metastases: A post hoc analysis of the ALTER 1202 trial. Cancer Med. 2022;11:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Chen G, Huynh M, Chen A, Fehrenbacher L, Gandara D, Lau D. Chemotherapy for brain metastases in small-cell lung cancer. Clin Lung Cancer. 2008;9:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Zhu Y, Cui Y, Zheng X, Zhao Y, Sun G. Small-cell lung cancer brain metastasis: From molecular mechanisms to diagnosis and treatment. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 7. | Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N; Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 946] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 8. | Hermes A, Bergman B, Bremnes R, Ek L, Fluge S, Sederholm C, Sundstrøm S, Thaning L, Vilsvik J, Aasebø U, Sörenson S. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol. 2008;26:4261-4267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6944] [Article Influence: 315.6] [Reference Citation Analysis (0)] |
| 10. | Lucchi M, Mussi A, Fontanini G, Faviana P, Ribechini A, Angeletti CA. Small cell lung carcinoma (SCLC): the angiogenic phenomenon. Eur J Cardiothorac Surg. 2002;21:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Stefanou D, Batistatou A, Arkoumani E, Ntzani E, Agnantis NJ. Expression of vascular endothelial growth factor (VEGF) and association with microvessel density in small-cell and non-small-cell lung carcinomas. Histol Histopathol. 2004;19:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 12. | Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4457] [Cited by in RCA: 4446] [Article Influence: 234.0] [Reference Citation Analysis (0)] |
| 13. | Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A, Tonini G, Carlomagno C, Allegrini G, Chiara S, D'Amico M, Granetto C, Cazzaniga M, Boni L, Fontanini G, Falcone A. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 795] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 14. | Ruan G, Ye L, Liu G, An J, Sehouli J, Sun P. The role of bevacizumab in targeted vascular endothelial growth factor therapy for epithelial ovarian cancer: an updated systematic review and meta-analysis. Onco Targets Ther. 2018;11:521-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Horn L, Bernardo P, Sandler A, Wagner H, Levitan N, Levitt ML, Johnson DH. A phase II study of paclitaxel + etoposide + cisplatin + concurrent radiation therapy for previously untreated limited stage small cell lung cancer (E2596): a trial of the Eastern Cooperative Oncology Group. J Thorac Oncol. 2009;4:527-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Spigel DR, Townley PM, Waterhouse DM, Fang L, Adiguzel I, Huang JE, Karlin DA, Faoro L, Scappaticci FA, Socinski MA. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol. 2011;29:2215-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Ready NE, Dudek AZ, Pang HH, Hodgson LD, Graziano SL, Green MR, Vokes EE. Cisplatin, irinotecan, and bevacizumab for untreated extensive-stage small-cell lung cancer: CALGB 30306, a phase II study. J Clin Oncol. 2011;29:4436-4441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Spigel DR, Greco FA, Zubkus JD, Murphy PB, Saez RA, Farley C, Yardley DA, Burris HA 3rd, Hainsworth JD. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol. 2009;4:1555-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Pujol JL, Lavole A, Quoix E, Molinier O, Souquet PJ, Barlesi F, Le Caer H, Moro-Sibilot D, Fournel P, Oster JP, Chatellain P, Barre P, Jeannin G, Mourlanette P, Derollez M, Herman D, Renault A, Dayen C, Lamy PJ, Langlais A, Morin F, Zalcman G; French Cooperative Thoracic Intergroup (IFCT). Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial†. Ann Oncol. 2015;26:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Tiseo M, Boni L, Ambrosio F, Camerini A, Baldini E, Cinieri S, Brighenti M, Zanelli F, Defraia E, Chiari R, Dazzi C, Tibaldi C, Turolla GM, D'Alessandro V, Zilembo N, Trolese AR, Grossi F, Riccardi F, Ardizzoni A. Italian, Multicenter, Phase III, Randomized Study of Cisplatin Plus Etoposide With or Without Bevacizumab as First-Line Treatment in Extensive-Disease Small-Cell Lung Cancer: The GOIRC-AIFA FARM6PMFJM Trial. J Clin Oncol. 2017;35:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 21. | Zustovich F, Ferro A, Lombardi G, Farina P, Zagonel V. Bevacizumab-Based Therapy for Patients with Brain Metastases from Non-Small-Cell Lung Cancer: Preliminary Results. Chemotherapy. 2014;60:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25:2306-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |