Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.399
Peer-review started: September 28, 2023
First decision: October 9, 2023
Revised: October 21, 2023
Accepted: December 25, 2023
Article in press: December 25, 2023
Published online: January 16, 2024
Processing time: 104 Days and 23.6 Hours
Most species of aconite contain highly toxic aconitines, the oral ingestion of which can be fatal, primarily because they cause ventricular arrhythmias. We describe a case of severe aconite poisoning that was successfully treated through veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and in which detailed toxicological analyses of the aconite roots and biological samples were performed using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
A 23-year-old male presented to the emergency room with circulatory collapse and ventricular arrhythmia after ingesting approximately half of a root labeled, “Aconitum japonicum Thunb”. Two hours after arrival, VA-ECMO was initiated as circulatory collapse became refractory to antiarrhythmics and vasopressors. Nine hours after arrival, an electrocardiogram revealed a return to sinus rhythm. The patient was weaned off VA-ECMO and the ventilator on hospital days 3 and 5, respectively. On hospital day 15, he was transferred to a psychiatric hospital. The other half of the root and his biological samples were toxicologically analyzed using LC-MS/MS, revealing 244.3 mg/kg of aconitine and 24.7 mg/kg of mesa
The key to saving the life of a patient with severe aconite poisoning is to introduce VA-ECMO as soon as possible.
Core Tip: Most species of aconite contain highly toxic aconitines, the oral ingestion of which can be fatal, primarily because they cause ventricular arrhythmias. The key to saving the lives of patients with severe aconite poisoning is to introduce veno-arterial extracorporeal membrane oxygenation as soon as possible when circulatory collapse becomes refractory to conventional treatments and to maintain it until the toxins are fully excreted from the body.
- Citation: Kohara S, Kamijo Y, Kyan R, Okada I, Hasegawa E, Yamada S, Imai K, Kaizaki-Mitsumoto A, Numazawa S. Severe aconite poisoning successfully treated with veno-arterial extracorporeal membrane oxygenation: A case report. World J Clin Cases 2024; 12(2): 399-404
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/399.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.399
Aconite is a genus comprising roughly 300 species of flowering plants belonging to the Ranunculaceae family. As most species of aconite contain highly toxic aconitines, oral ingestion can be fatal, mainly due to ventricular arrhythmias. Arrhythmias due to aconite poisoning are often resistant to defibrillation and antiarrhythmic drugs. Prolonged cardiopulmonary resuscitation and cardiopulmonary bypass should be considered as “time-buying” strategies to allow the body to excrete the toxic alkaloids[1].
In this report, we describe a case of severe aconite poisoning that was successfully treated using veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and in which detailed toxicological analyses of the aconite roots and the patient’s biological samples were performed using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
A 23-year-old male was transported by ambulance to the emergency room after a neighbor found him collapsed in the hallway of an apartment building and vomiting.
The patient admitted to the paramedics that he went out to the hallway to seek help because he had experienced nausea and chills after eating aconite root in a suicide attempt. In his room was a cardboard box labeled, “Aconitum japonicum Thunb. subsp. subcuneatum (Nakai) Kodata”, and the residual cooked root (Figure 1).
No previous or family history was available for the patient.
On arrival, his vital signs were as follows: respiratory rate, 15/min; SpO2, impossible to measure; blood pressure, 77/52 mmHg; heart rate, 163 beats/minute; Glasgow Coma Scale, E3V4M6; and body temperature, 35.8 ℃. He was restless and cyanotic throughout his body.
Laboratory analysis revealed lactic acidosis: pH, 7.228; PaCO2, 27.9 mmHg; PaO2, 339.7 mmHg; HCO3-, 11.4 mmol/L; BE, -14.6 mmol/L; and lactate, 71.1 mg/dL. Electrogram showed non-sustained ventricular tachycardia and multifocal ventricular extrasystoles (Figure 2).
Chest and abdominal radiographs showed no abnormal findings. Upper endoscopy showed no aconite remaining in the stomach.
Severe aconite poisoning.
After tracheal intubation, noradrenaline and amiodarone were intravenously infused, and lidocaine was intravenously injected. This allowed for the temporary stabilization of hemodynamics, and his mean blood pressure was maintained above 60 mmHg; the patient was then admitted to the emergency ward. Two hours after arrival, circulatory collapse became refractory to antiarrhythmics and vasopressors, and VA-ECMO was initiated. Approximately nine hours after arrival, an electrocardiogram showed a return to sinus rhythm, and the intravenous infusions of noradrenaline and amiodarone were discontinued. The patient was weaned off VA-ECMO and the ventilator on hospital days 3 and 5, respectively. Later, he confessed that he had purchased the aconites online, soaked one of the roots in hot water, and eaten roughly half of it with mayonnaise.
He was transferred to a psychiatric hospital without physical sequelae on day 15.
Toxicological analyses of the aconite root and biological samples (serum and urine) were conducted as described below.
Calibration standards: Standard stock solutions of aconitine (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), mesaconitine (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), and hypaconitine (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) were prepared in methanol, each at a concentration of 1000 µg/mL. Calibration standards of aconitine, mesaconitine, and hypaconitine were prepared at six spiking levels by diluting the stock solutions with methanol. Peak area was used as the analytical response vs concentration. The correlation coefficient (r) and coefficients of determination (r2) of the calibration curves for aconitine, mesaconitine, and hypaconitine were greater than 0.999 in the 0.001–0.1 µg/mL range.
Apparatus and instruments: The LC-MS/MS system consisted of a TQ-S micro triple quadrupole mass spectrometer (Waters Corporation, Milford, MA, USA) equipped with an ACQUITY UPLC system (Waters Corporation). Chromatographic separation was achieved using a Scherzo-SM C18 column (2 mm × 150 mm, particle size 3 µm; Imtakt Cor
Sample preparation: Exactly 1.0 g of sample was weighed into an extraction tube and homogenized for 3 min with 20 mL methanol. The homogenate was filtered via suction, and the residue was re-homogenized with 20 mL of methanol and filtered again. Combined extracts were adjusted to a total volume of 50 mL with methanol. The extract was diluted with methanol and passed through a 0.20 µm PTFE membrane filter (Advantech Japan, Tokyo, Japan) as the test solution. Finally, 5 µL of this solution was injected into the LC-MS/MS apparatus.
Sample preparation: Extraction from serum and urine samples was performed using the Micro Volume QuEChERS Kit (Shimadzu Corp, Kyoto, Japan). A portion of 100 mg QuEChERS salt was mixed with 300 μL acetonitrile, 200 μL water, 100 μL serum or urine, and 2 μL of 10 ng/mL diazepam-D5 (Sigma-Aldrich Japan, Tokyo, Japan) used as an internal standard. The upper layer was collected through centrifugation at 15000 × g for 10 min. The solvent was evaporated to dryness under a nitrogen stream, and the residue was reconstituted in 100 μL methanol. After centrifugation at 15000 × g for 5 min, the supernatant was analyzed using LC-MS/MS. Calibration curves using authentic standards for aconitine (Sigma-Aldrich Japan, Tokyo, Japan) and mesaconitine (Tokyo Chemical Industry, Tokyo, Japan) were prepared in the range of 1-100 ng/mL for serum and 0.1-250 ng/mL for urine with the appropriate matrices.
LC-MS/MS conditions and detector settings: An LC-MS/MS system consisting of LC-40ADXR and LCMS-8045 (Shimadzu Corp) was used. Chromatographic separation was achieved on a Phenomenex Kinetex XB-C18 column (2.1 mm ID × 100 mm, 2.6 μm; Shimadzu) with an equivalent Phenomenex Security Ultra C18 guard column (2.1 mm ID; Shimadzu). The column temperature was set at 40 ℃. The mobile phases were (A) 10 mmol/L ammonium formate and 0.1% formic acid and (B) methanol containing 10 mmol/L ammonium formate and 0.1% formic acid. The gradient started at 5% B, then increased to 95% over 7.5 min, held for 2.5 min, immediately returned to 5%, and then held for 5 min. The flow rate was set at 0.3 mL/min, and the injection volume was 5 μL. Ionization was performed under ESI positive mode, and the samples were analyzed through multiple reaction monitoring (MRM). Flow rate of the nebulizer gas, drying gas, and heating gas were set at 3 L/min, 10 L/min, and 10 L/min, respectively. The temperatures of the interface, desolvation line, and heat block were set at 300 ℃, 250 ℃, and 400 ℃, respectively. Collision energies for aconitine, mesaconitine, and diazepam-D5 were set at 55, 54, and 34 V, respectively. Monitored ions included the precursor ion of m/z 646.00 to the product ion of m/z 104.95 for aconitine, m/z 632.31 to 105.05 for mesaconitine, and m/z 290.15 to 198.20 for diazepam-D5. The retention times for aconitine, mesaconitine, and diazepam-D5 under conditions of the current LC analysis were 5.83, 5.65, and 6.78 min, respectively. The correlation coefficient (r) and coefficient of determination (r2) of the calibration curves for aconitine and mesaconitine were both ≥ 0.999, indicating good linearity.
We detected 244.3 mg/kg of aconitine and 24.7 mg/kg of mesaconitine in the root. In addition, 10.4 mg/kg of aconitine was detected in the liquid in which the root was soaked.
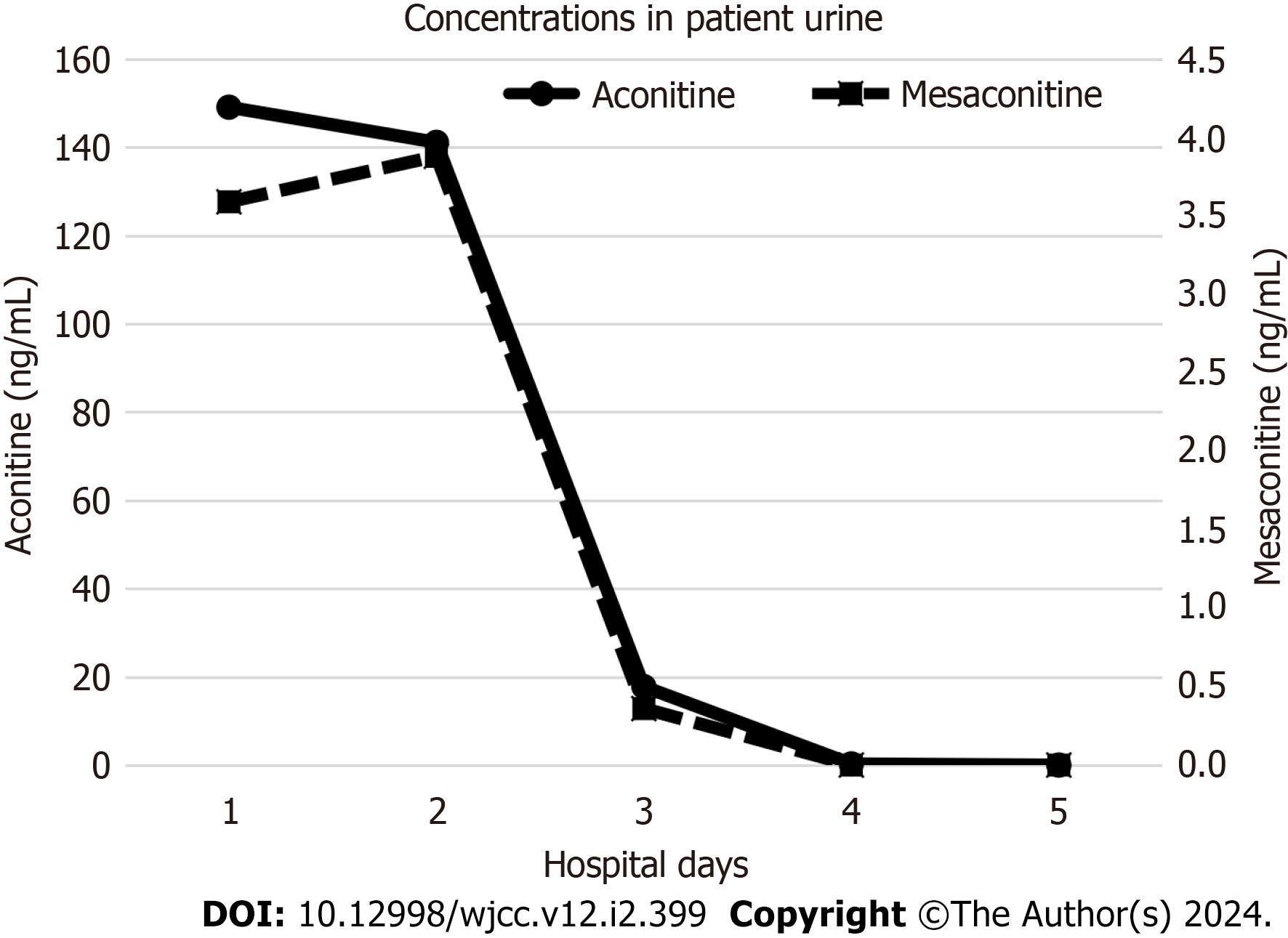
Analysis of serum on admission showed that it contained 1.50 ng/mL of aconitine, with no mesaconitine detected. Neither compound was detected after hospital day 2. We detected 149.09 ng/mL of aconitine and 3.59 ng/mL of mesaconitine in the urine collected on admission, but they rapidly decreased after hospital day 3 (Figure 3).
Most species of aconite contain aconitum alkaloids in all parts of the plant, including the roots, stems, leaves, and flowers. The lethal dose of aconitines in adult humans is 3-6 mg. The average total amount of aconitines in 1 g of root is 2-4 mg, and approximately 1 g of root is considered lethal to adult humans[2]. The primary target of the alkaloids is the fast voltage-gated sodium channel in the myocardium, where they cause sustained activation. Activated sodium channels promote intramyocardial automation of the ventricle, resulting in various types of ventricular arrhythmias[3].
Animal studies have reported that symptoms of aconite poisoning depend on the amount ingested[4]. However, in humans, the relationship between the amount ingested, blood and urine concentrations, and clinical symptoms is less clear. Ventricular arrhythmias may appear at blood aconitine concentrations of 0.12 ng/mL or higher[5]. However, some reports suggest that blood concentrations may not be associated with clinical symptoms[6]. Given the time needed to measure blood or urine concentrations of aconitine, it is difficult to diagnose, evaluate the severity, and determine a treatment strategy based on the results of toxicological analysis. Since aconite can be lethal in small doses, patients who have ingested it in even small amounts should be closely monitored for at least 24 h.
In recent years, it has become possible to measure aconitine in blood and urine using liquid chromatography. Aco
In the present case, toxicological analysis of biological samples was performed at a later time. The serum concentration of aconitine (1.50 ng/mL) was much higher than the concentration required to induce ventricular arrhythmia (0.12 ng/mL); after hospital day 2, aconitine was no longer detected in serum. Urine concentrations of aconitine were high until hospital day 2, but rapidly decreased after hospital day 3, when aconitine was likely to have been fully excreted from the body. A review of the clinical course based on our toxicological results suggests that the key to saving the patient’s life was to introduce VA-ECMO as soon as possible when circulatory collapse become refractory to conventional treatments and to maintain it until the toxins were fully excreted from the body.
Toxicological analysis of the residual root was also performed later. Aconitine and mesaconitine were detected in the root, with far higher levels of the former. The content of each aconitum alkaloid varies depending on the species, where it is collected, and when it develops. In the present case, the patient purchased an ornamental species of aconite online and ingested it in a suicide attempt. Generally, ornamental species are thought to be less toxic than wild species. The results from our analysis suggest that not all ornamental species of aconite should be considered less toxic than wild ones.
VA-ECMO should be initiated as soon as possible when circulatory collapse becomes refractory to conventional treatments in patients with aconite poisoning and must be maintained until the toxins are fully excreted from the body.
We thank the Japanese Society of Clinical & Analytical Toxicology (J’s-CAT) for introducing us to experts in toxicologic analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Toxicology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Han J, China S-Editor: Yan JP L-Editor: A P-Editor: Xu ZH
| 1. | Coulson JM, Caparrotta TM, Thompson JP. The management of ventricular dysrhythmia in aconite poisoning. Clin Toxicol (Phila). 2017;55:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Ito K, Ohyama Y, Hishinuma T, Mizugaki M. Determination of Aconitum alkaloids in the tubers of Aconitum japonicum using gas chromatography/selected ion monitoring. Planta Med. 1996;62:57-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Chan TY. Aconite poisoning. Clin Toxicol (Phila). 2009;47:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 4. | Yakazu SD. Bibliographic studies on the intoxication of aconitine root (Uzu, Bushi) Part II. Jpn J Orient Med. 1958;9:55-64. [DOI] [Full Text] |
| 5. | Yamanouchi S, Arai M, Koyama A, Azuma K, Maki M, Yamamoto S, Shinya F. A case of Aconite poisoning, Survived by Percutaneous Cardio-pulmonary Support. JJAAM. 2004;15:269-73. [DOI] [Full Text] |
| 6. | Jeon SY, Jeong W, Park JS, You Y, Ahn HJ, Kim S, Kim D, Park D, Chang H, Kim SW. Clinical relationship between blood concentration and clinical symptoms in aconitine intoxication. Am J Emerg Med. 2021;40:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Kazunari K, Hirokazu O, Hiroyuki M, Kenji M, Etsuo K, Kiyoshi M, Daiji M, Michinao M. A case of ‘Torikabuto’ intoxication with documented follow-up measurements of Aconitum alkaloids in the urine. Jpn J Toxicol. 1998;6:185-188. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |