Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.335
Peer-review started: October 26, 2023
First decision: November 8, 2023
Revised: November 22, 2023
Accepted: December 21, 2023
Article in press: December 21, 2023
Published online: January 16, 2024
Processing time: 77 Days and 2.1 Hours
Yangxue Qingnao Granules (YXQN) is a Chinese patent medicine that has been commonly used in the clinical treatment of migraine.
To assess the efficacy and safety of YXQN alone for the treatment of migraine.
We searched 10 databases to identify relevant randomized controlled trials (RCTs) published before September 2022. Two review authors independently searched and screened the literature, extracted the data, and assessed the methodological quality of the studies using criteria from ROB 2.0, and analyzed the data using Review Manager 5.4 software.
A total of 12 RCTs including 767 participants with migraine met the selection criteria. We divided these studies into comparisons of YXQN with placebo, routine treatment drugs, and other Chinese patent medicines. The meta-analysis showed the following: (1) Efficacy: The YXQN group outperformed the placebo group [relative risk (RR) = 0.29, 95% confidence interval (95%CI): 0.15–0.43, P < 0.00001], routine treatment group (RR = 0.18, 95%CI: 0.09–0.27, P < 0.0001), and Chinese patent medicine group (RR = 0.27, 95%CI: 0.13–0.41, P < 0.001); (2) frequency of headache: There was a significant difference between YXQN vs placebo [mean difference (MD) =
This study revealed that YXQN is effective and safe for treatment of migraine.
Core Tip: Traditional Chinese medicine is widely used as a treatment option for migraine. Clinical studies have confirmed the effectiveness of Yangxue Qingnao Granules (YXQN) for treatment of migraine. However, there is still insufficient evidence to evaluate the efficacy of YXQN for migraine. Therefore, this meta-analysis aimed to systematically integrate clinical trials to evaluate the efficacy and safety of YXQN alone in the treatment of migraine and provide a basis for its further clinical application and research.
- Citation: Zhou B, Wang GS, Yao YN, Hao T, Li HQ, Cao KG. Efficacy and safety of Yangxue Qingnao Granules in treatment of migraine: A systematic review and meta-analysis. World J Clin Cases 2024; 12(2): 335-345
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/335.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.335
Migraine is a persistent neurological condition ranking among the top 10 global contributors to disease-related disability[1,2]. It is a prevalent ailment marked by severe and pulsating headaches, often accompanied by related symptoms such as sensitivity to light, sensitivity to sound, nausea, vomiting, dizziness, skin hypersensitivity, and cognitive impairment[3-5]. Migraine has become a public health problem that seriously affects the physical health and quality of life[6,7]. Worldwide, migraine stands as a primary factor in neurological disability and significantly impacts society at large[8-10].
Preventing headache episodes can be accomplished through the use of calcium channel blockers, beta-blockers, antidepressants, antiepileptic drugs, and triptans[11]. Nevertheless, the considerable dropout rates observed in numerous clinical trials imply that these medications are often poorly tolerated by patients[12]. Consequently, the exploration of new agents offering a blend of effectiveness and safety is crucial for improving migraine treatment. Traditional Chinese medicine stands out as a promising option due to the observed tolerance and minimal adverse reactions in migraine patients towards supplements and alternative medications[13].
Yangxue Qingnao Granules (YXQN) is a Chinese patent medicine for migraine that consist of > 10 Chinese herbal medicines, including Angelica, Ligusticum, Szechuan Lovage Rhizome, Radix paeoniae alba, Radix rehmanniae, Uncaria, Caulis spatholobi, Prunella vulgaris, Cassia seed, Mother of pearl, Corydalis, and Asarum. Clinical studies have confirmed the effectiveness of YXQN for treatment of migraine. It can improve the overall efficacy, reduce the duration of headache, and increase cerebral blood flow[14]. Adverse reactions include nausea, dizziness, fatigue, and lethargy, which do not affect the treatment. Most adverse reactions resolve without specific treatment, and some are relieved by symptomatic treatment[15].
Nonetheless, the existing evidence remains inadequate in assessing the effectiveness of YXQN for treating migraine. Hence, the primary objective of this meta-analysis was to comprehensively consolidate data from clinical trials, specifically focusing on assessing both the efficacy and safety of YXQN as a standalone treatment for migraine. This analysis also aimed to establish a robust evidence foundation for guiding future clinical applications and research endeavors in this area.
The review protocol was prospectively registered on PROSPERO (CRD42022359662) and our findings were reported according to the PRISMA guidelines. A research librarian conducted a systematic search of the following databases for randomized controlled trials (RCTs) on YXQN alone for treatment of migraine: CNKI (China National Knowledge Infrastructure), CBM (Chinese Biomedicine Database), VIP (Chinese Scientific Journals Database), Wanfang, Web of Science, PubMed, EBSCO, CENTRAL, the Cochrane Library, and Embase (see Supplementary material). The search period was up to September 2022. Citation management was performed using NoteExpress (Clarivate).
The inclusion criteria were: (1) RCTs; (2) migraine patients, all of whom met the relevant diagnostic criteria of the international classification of headache diseases; (3) the treatment group used YXQN treatment along for migraine; the control group used conventional treatment for migraine, such as calcium ion antagonists, calcium channel blockers, antiepileptic drugs, Chinese patent medicine, placebo, etc; and (4) primary outcome was clinical effectiveness (≥ 50% migraine responder rate)[16]. Secondary outcomes included frequency of headache, duration of headache, intensity of headache, and adverse reactions. The exclusion criteria were: (1) No proper control group; (2) migraine was a complication of other diseases and not the main condition studied; (3) special groups, such as pregnant women, adolescents, and children; (4) interventions using YXQN were combined with other therapies or drugs such as acupuncture and psychotherapy that are not conventional treatments; and (5) data were insufficient or in doubt.
Zhou B and Li HQ independently screened the titles and abstracts and then read the full texts to confirm eligibility. Any disagreements were resolved by consensus and by Cao KG. Zhou B and Li HQ independently piloted a data collection form and then independently extracted outcome data. Extracted data were compared by Zhou B and Wang GS, and any discrepancies were resolved through discussion.
Zhou B and Wang GS independently rated the risk of bias of the RCTs using the revised Cochrane risk of bias, version 2 (RoB 2) tool[17]. The assignment or intention to treat was the outcome of interest. Disagreements were resolved by consensus. We contacted authors when information was not reported in the article and/or needed clarification.
Zhou B and Li HQ independently rated the certainty for each comparison and outcome based on the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) method, and consulted with a third reviewer when there were discrepancies. Using the online program GRADEpro (https://gradepro.org/), we assessed the risk of bias; inconsistency, indirectness, and imprecision of the results; and the probability of publication bias with a four-item scale (very low, low, moderate, or high).
Statistical analyses were performed on the collected data using Revman 5.3 statistical software provided by the International Evidence-Based Medicine Collaboration Network. For numerical data, odds ratio or relative risk (RR) was calculated. For continuous variables, if the measurement units and methods were inconsistent, the standardized mean difference (SMD) with 95% confidence interval (95%CI) was used as the effect index. When the measurement units and measurement method were consistent, mean difference (MD) with 95%CI was calculated. When the results showed I2 < 50%, they were considered to be less heterogeneous or nonexistent. When I2 was > 50%, heterogeneity was considered to exist, indicating that the cause of heterogeneity should be analyzed by a sensitivity or subgroup analysis. In addition, sensitivity analyses were performed to identify the robustness of the meta-analysis results by excluding: (1) Studies with high risks of bias; and (2) outliers that were numerically distant from the rest of the data. If > 10 trials were included in the meta-analysis, funnel plots were generated to assess biases such as publication bias.
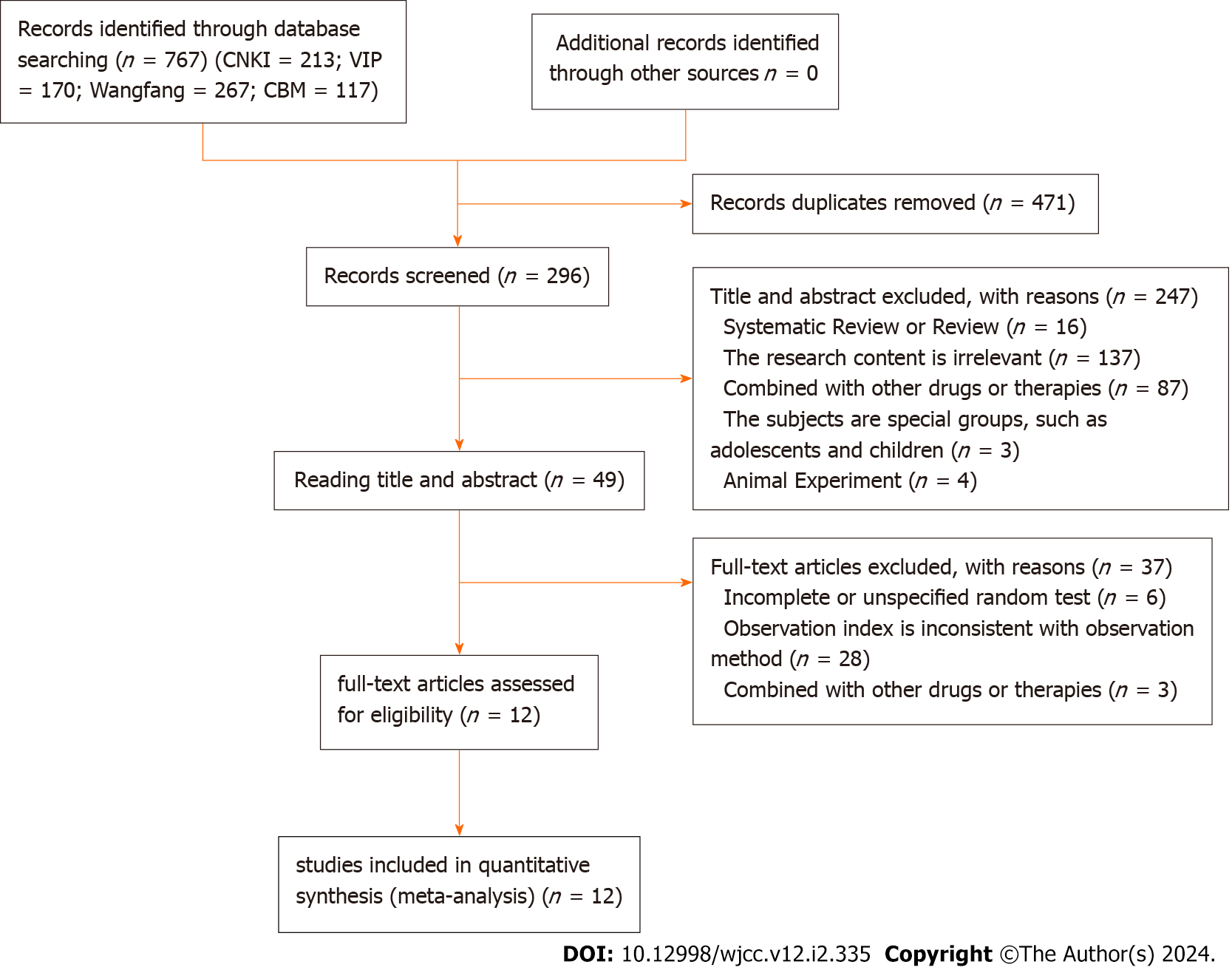
A total of 767 relevant documents were initially searched and imported into NoteExpress. After eliminating duplicates, 296 articles were selected for further evaluation. After reading the titles and abstracts to eliminate duplicates and irrelevant literature, 49 titles were screened for full text evaluation and 12 RCTs were finally included in the meta-analysis, with 1210 participants (607 in the trial group and 603 in the control group). YXQN was compared with sibelium, other Chinese patent medicines, and placebo treatment. All RCTs were conducted in China. Figures 1 and 2 display the flowchart, risk of bias summary, and bias graph, while Table 1 presents the fundamental information of the literature included in this study.
| Ref. | No. of patients [Exp/Con (men/women)] | Course of disease, No. (Exp/Con) | Mean age or range (yr) | Experimental group | Control group | Treatment duration | Other outcomes | Standards for clinical efficacy appraisal | |
| 1 | Luo et al[23], 2001 | Exp 56 (22/34) | Exp 53 | 38.5 ± 8.6 | YXQN | Placebo | 30 d | (1) Clinical efficacy; (2) Frequency of headache; (3) Duration of headache; and (4) Accompanying symptoms | Invalid: No significant improvement in headache, reduction rate < 30% |
| Con 56 (20/36) | Con 54 | 37.6 ± 11 | |||||||
| 2 | Li et al[19], 2003 | Exp 79 (24/55) | Exp 79 | 37.2 ± 14.1 | YXQN | Sibelium capsules | 2 wk | Clinical efficacy | Invalid: No significant improvement in headache, reduction rate < 25% |
| Con 71 (20/51) | Con 71 | 33.8 ± 13.8 | |||||||
| 3 | Li[18], 2003 | Exp 62 (-/-) | Exp 62 | 34 ± 5.2 | YXQN | Quantianma capsule | 1 mo | (1) Clinical efficacy; (2) Frequency of headache; (3) Duration of headache; and (4) Intensity of headache | Invalid: No significant improvement in headache frequency after treatment |
| Con 58 (-/-) | Con 58 | ||||||||
| 4 | Niu 2003[27] | Exp 35 (-/-) | Exp 35 (-/-) | - | YXQN | Sibelium capsules | 30 d | (1) Clinical efficacy; (2) Frequency of headache; and (3) Duration of headache | Invalid: No change in headache severity, < 30% fewer episodes |
| Con 35 (-/-) | Con35 (-/-) | - | |||||||
| 5 | He et al[20], 2005 | Exp 64 (-/-) | Exp 64 (-/-) | 33 ± 4.2 | YXQN | Sibelium capsules/placebo | 30 d | (1) Clinical efficacy; (2) Frequency of headache; (3) Duration of headache; and (4) Intensity of headache | Invalid: No significant improvement in headache frequency after treatment |
| Con 40 (-/-) | Con 40 (-/-) | ||||||||
| Placebo 56 (-/-) | Placebo 56 (-/-) | ||||||||
| 6 | Bai[20], 2011 | Exp 28 (9/19) | Exp 28 (9/19) | 19-42 | YXQN | Sibelium capsules | 8 wk | (1) Clinical efficacy; and (2) Adverse reactions | Invalid: No significant improvement in headache frequency after treatment |
| Con 28 (8/20) | Con 28 (8/20) | 18-42 | |||||||
| 7 | Li and Liu[26], 2012 | Exp 59 (28/31) | Exp 59 (28/31) | 42 ± 6 | YXQN | Sibelium capsules | 1 mo | (1) Clinical efficacy; (2) Frequency of headache; and (3) Duration of headache | Invalid: Headache score decreased < 35% after treatment |
| Con 59 (22/37) | Con 59 (22/37) | 43 ± 5 | |||||||
| 8 | Zhang and Kuang[22], 2015 | Exp 59 (21/19) | Exp 59 (21/19) | 32.8 | YXQN | Rotundinum | 8 wk | (1) Clinical efficacy; and (2) Adversereactions | Invalid: No significant improvement in headache, reduction rate < 50% |
| Con 59 (17/23) | Con 59 (17/23) | 33.5 | |||||||
| 9 | Xie and Peng[24], 2017 | Exp 75 (30/45) | Exp 75 (30/45) | 38.91 ± 7.1 | YXQN | Placebo | 1 mo | (1) Clinical efficacy; (2) Frequency of headache; (3) Duration of headache; (4) Intensity of headache; and (5) Adverse reactions | Invalid: No significant improvement in headache frequency after treatment |
| Con 75 (32/43) | Con 75 (32/43) | 39.33 ± 6.89 | |||||||
| 10 | Jiang[29], 2019 | Exp 34 (13/21) | Exp 34 (13/21) | 52.3 ± 3.2 | YXQN | Sibelium capsules | 30 d | (1) Clinical efficacy; (2) Intensity of headache; (3) Duration of headache | Invalid: No change in headache severity, < 30% fewer episodes |
| Con 26 (10/16) | Con 26 (10/16) | 54.2 ± 2.8 | |||||||
| 11 | Jiang[25], 2020 | Exp 45 (21/24) | Exp 45 (21/24) | 43.5 ± 2.5 | YXQN | Sibelium capsules | 1 mo | (1) Clinical efficacy; (2) Frequency of headache; and (3) Duration of headache | Invalid: No significant improvement in headache frequency after treatment |
| Con 45 (20/25) | Con 45 (20/25) | 44.5 ± 3.5 | |||||||
| 12 | Wang[28], 2021 | Exp 42 (16/26) | 47.3 ± 6.4 | YXQN | Toutongning Capsules | 1 mo | (1) Clinical efficacy; (2) Frequency of headache; (3) Duration of headache; and (4) Intensity of headache | Invalid: No significant improvement in headache frequency after treatment | |
| Con 42 (17/25) | 46.4 ± 6.1 |
According to the analysis using the ROB2.0 bias risk tool, two studies employed the random number table method to generate random sequences, indicating a low risk of bias in random sequence generation. One study was randomly assigned based on time of entry and was therefore identified as high risk. Due to the lack of mention of allocation concealment in the article and the different drugs used in the control group, outcome assessors were aware of the intervention received by the study participants, which was considered to be high risk.
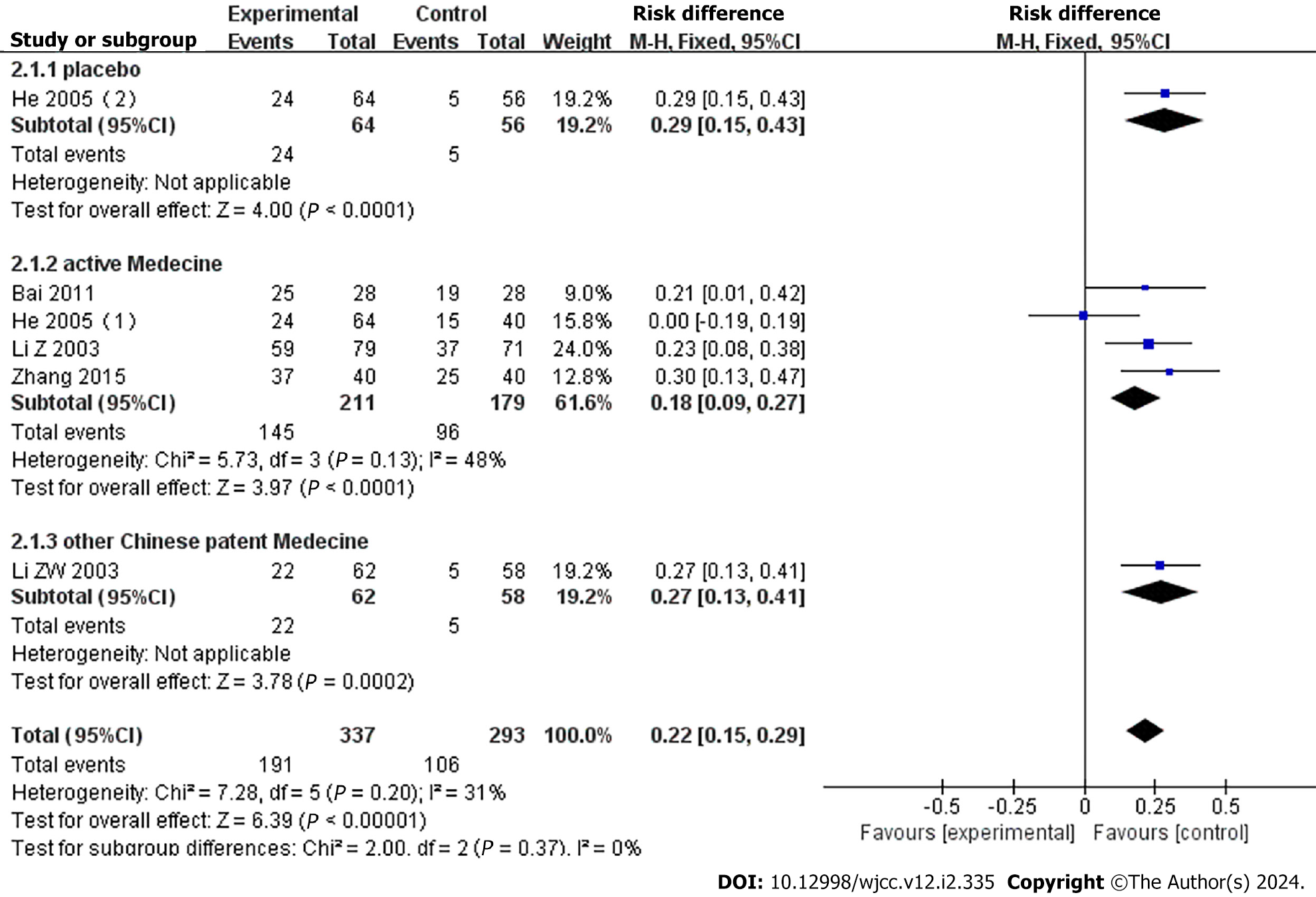
Treatment efficacy was assessed by ≥ 50% migraine responder rate in six studies[18-22]. He et al[20] compared YXQN with sibelium and placebo (vitamin), so we divided this research into two terms for comparison, He 2005(1) and He 2005(2). The YXQN group significantly outperformed the placebo group (RR = 0.29, 95%CI: 0.15–0.43, P < 0.00001), routine treatment group (RR = 0.18, 95%CI: 0.09–0.27, P < 0.0001), and Chinese patent medicine group (RR = 0.27, 95%CI: 0.13–0.41, P < 0.001) (Figure 3).
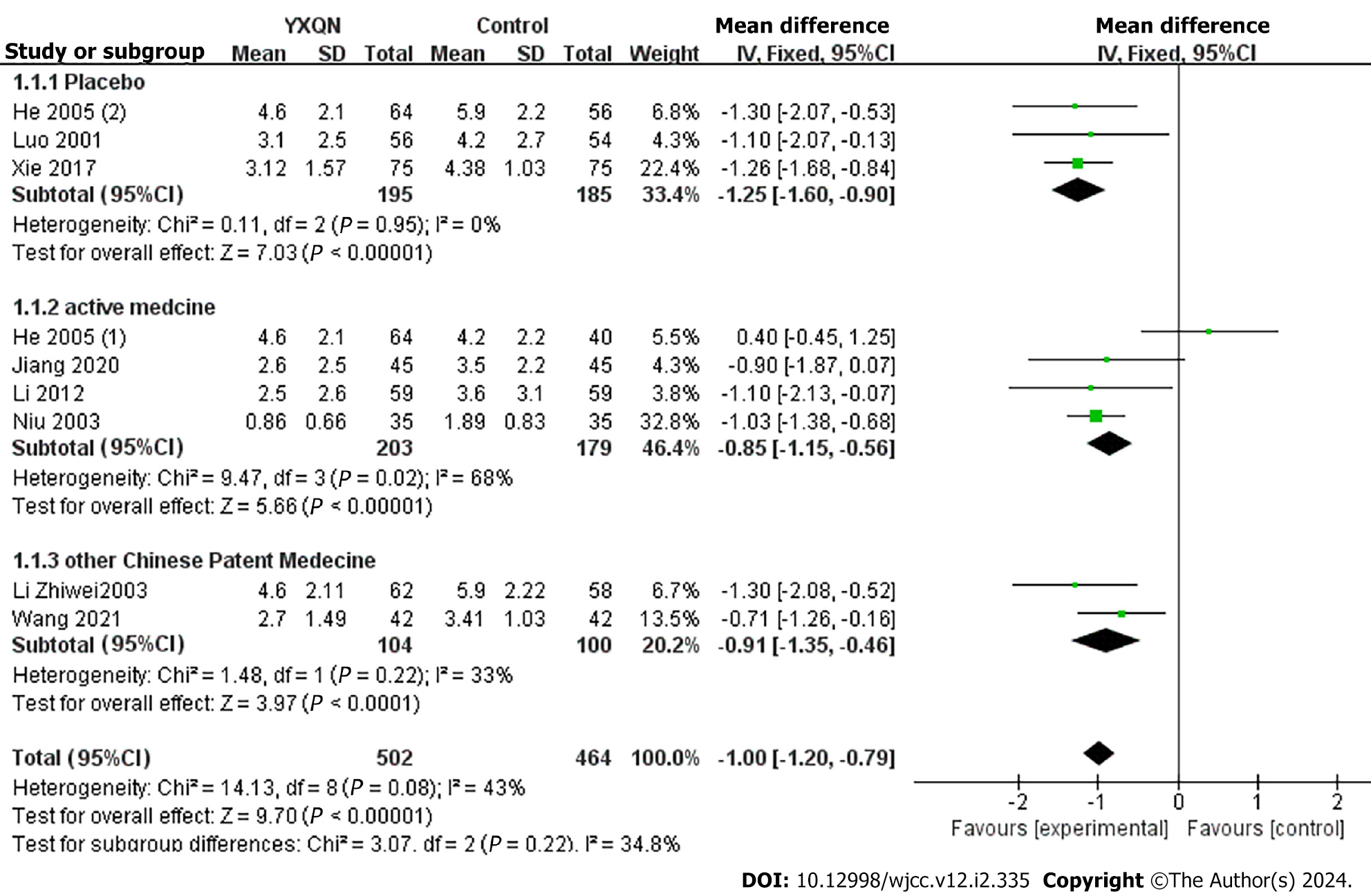
Nine studies reported headache frequency, with subgroup analysis based on control medication[19,20,23-28]. Three studies[20,23-24] compared YXQN vs placebo, and the meta-analysis showed a significant difference in the treatment group vs placebo group (MD = -1.25, 95%CI: -1.60 to -0.90, P < 0.00001). Four studies[20,25-27] compared YXQN vs routine treatment drugs, and the meta-analysis showed high heterogeneity between studies, with significant differences between YXQN and routine treatment drugs (MD = -0.85, 95%CI: -1.15 to -0.56, P < 0.00001). Two studies[19,28] comparing YXQN with other Chinese patent medicines showed that YXQN was more effective in reducing headache frequency (MD = -1.0, 95%CI: -1.20 to -0.79, P < 0.00001) (Figure 4).
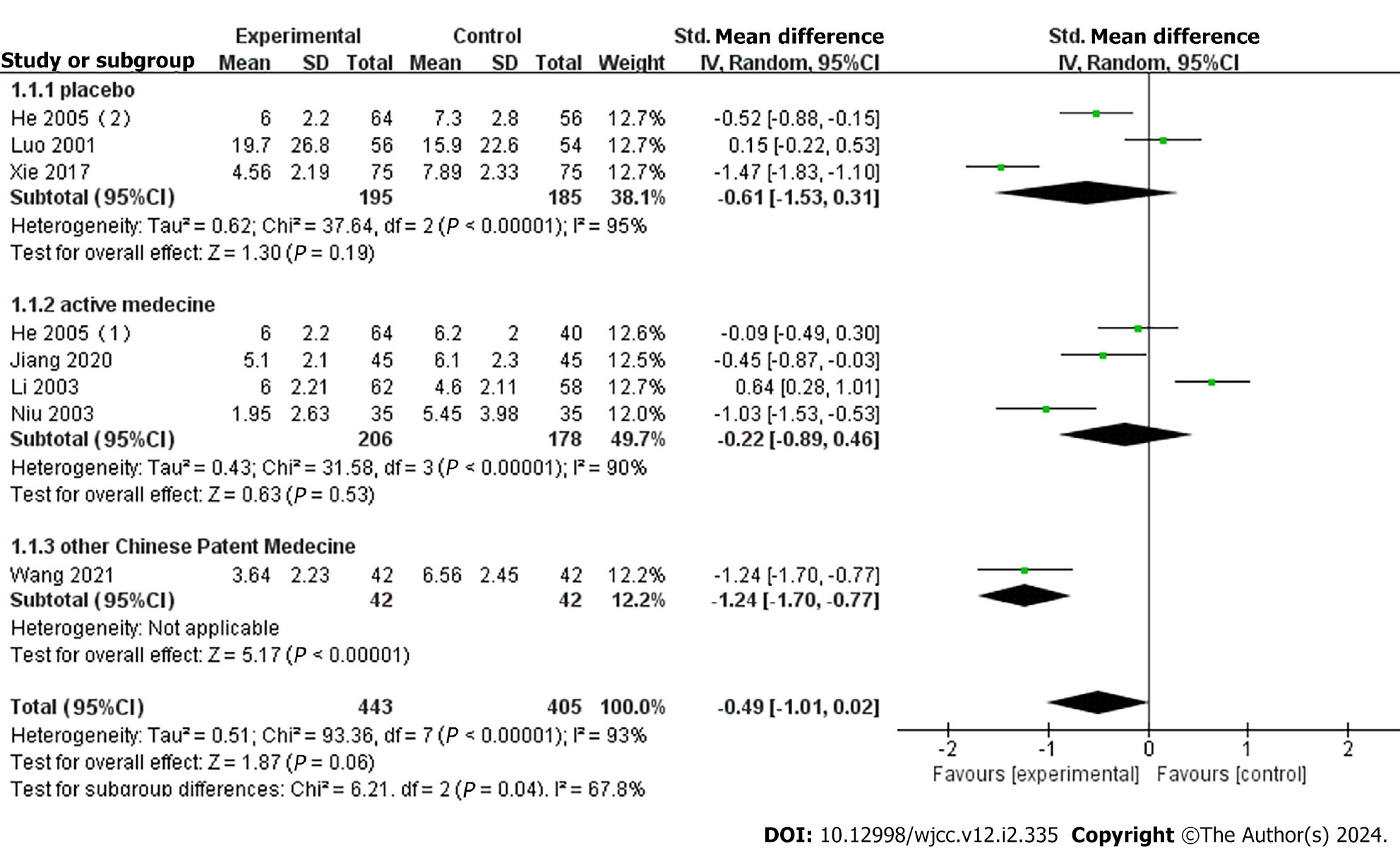
Eight studies reported headache duration, with subgroup analysis based on control medication[18,20,23-25,27-28]. Three studies[20,23-24] compared YXQN vs placebo, with meta-analysis showing a significant difference in the treatment group vs the placebo group (MD = -0.61, 95%CI: -1.53 to -0.31, P = 0.19). Four studies[18,20,25,27] compared YXQN vs routine treatment drugs, and meta-analysis showed high heterogeneity between studies, with no differences between YXQN and routine treatment drugs (MD = -0.22, 95%CI: -0.89 to -0.46, P < 0.53). One study[28] compared YXQN with other Chinese patent medicines and showed that YXQN was more effective in reducing headache duration (MD = -1.24, 95%CI: -1.70 to -0.77, P < 0.00001) (Figure 5).
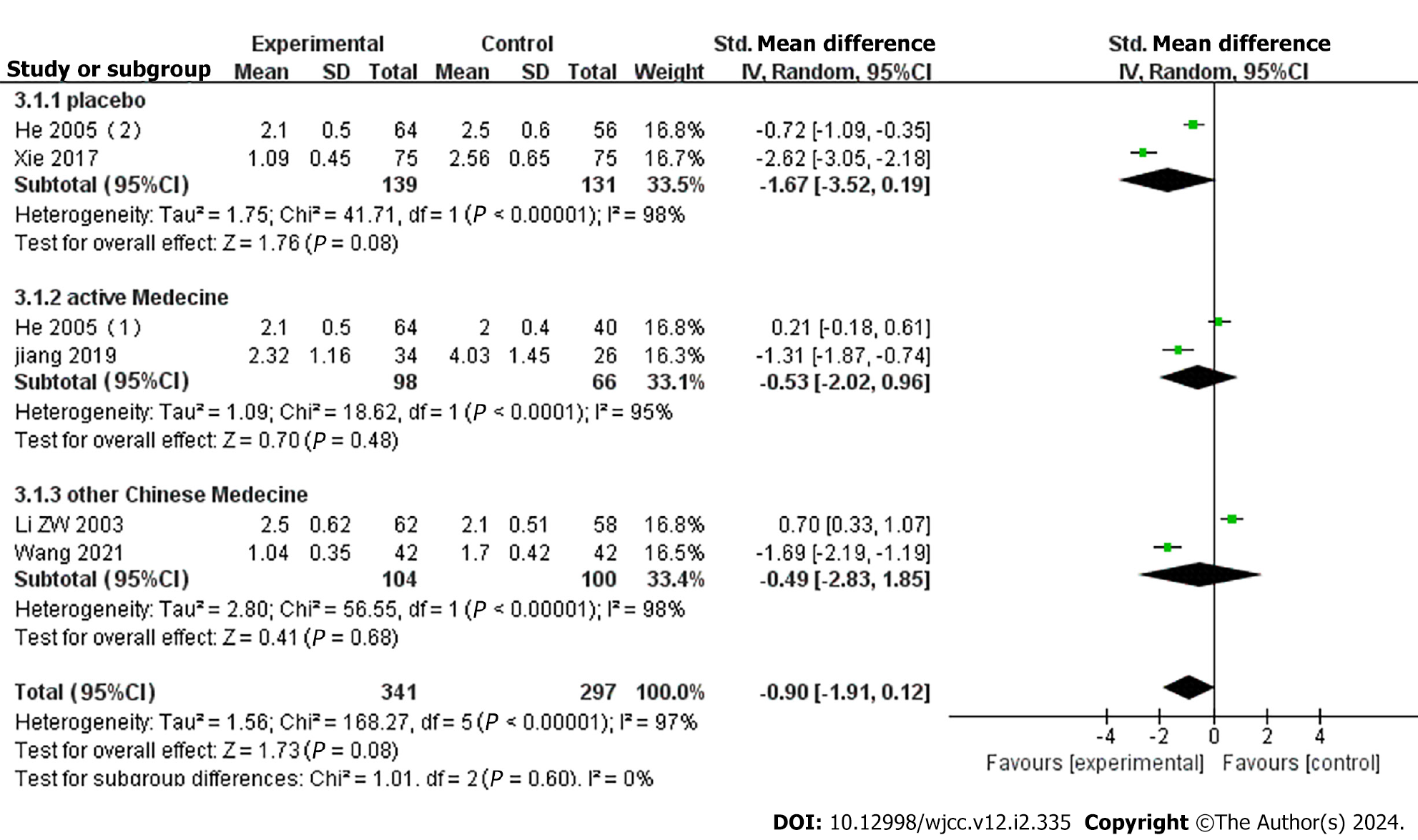
Six studies reported severity of headache, with subgroup analysis based on control medication[18,20,24,28,29]. Two studies[20,24] compared YXQN vs placebo, showing no significant difference between the groups (MD = -1.67, 95%CI: -3.52 to -0.19, P = 0.08). Two studies[20,29] compared YXQN vs routine treatment drugs, and meta-analysis showed high heterogeneity between the studies, with no significant differences between YXQN and routine treatment drugs (MD = -0.53, 95%CI: -2.02 to -0.96, P = 0.68). Two studies[18,28] compared YXQN with other Chinese patent medicines, showing high heterogeneity between the studies, with no significant differences between YXQN and other Chinese patent medicines (MD = -0.49, 95%CI: -2.83 to -1.85, P = 0.68) (Figure 6).
Mild gastrointestinal adverse reactions were reported in three cases.
In recent years, there has been a significant increase in public and medical interest in the use of traditional Chinese medicine for the treatment of migraine, among which YXQN has been proprietary Chinese medicine for the treatment of migraine for many years. Previous research showed that the prophylactic use of YXQN significantly reduced the positive rate of dural mast cell degranulation and significantly decreased the expression level of c-Fos protein in the nucleus of the trigeminal spinalis. The mechanism of action may be related to the stabilization of mast cell membranes to reduce their degranulation and the reduction of c-Fos protein expression in the nucleus of the trigeminal spinal tract[30,31]. YXQN can reduce the blood viscosity of migraine patients and improve the blood rheological indicators such as red blood cell deformation ability, red blood cell aggregation index, low-cut viscosity, high-cut viscosity, and fibrinogen[32]. Adverse reactions are mainly gastrointestinal symptoms, none of which affect the treatment. Most of adverse reactions resolve spontaneously, and some resolve with symptomatic treatment.
There is evidence to support the efficacy of YXQN in the treatment of migraine, which is consistent with the results of our study. To ensure consistency, the clinical efficacy was set at a uniform 50% reduction in the frequency of headache attacks. This study systematically reviewed the Chinese and English literature to determine the effectiveness and safety of YXQN in the treatment of migraine. Twelve RCTs, including 1210 migraine patients, met the inclusion criteria. The main finding of our review was that YXQN appeared to be more effective than controls in the treatment of migraine as assessed by various headache-related measures, including the number and duration of headache attacks.
In terms of clinical efficiency and number of attacks, YXQN was more effective than routine treatment drugs, placebo, and other Chinese patent medicines. Compared with flunarizine and Western medicine, YXQN can reduce the clinical incidence and number of headache attacks, with fewer adverse effects. Compared with placebo, there was no significant advantage for YXQN in terms of duration and degree of headache, which may be related to the inconsistent statistical methods and evaluation criteria used by the investigators. Compared with other Chinese patent medicines, YXQN significantly reduced duration of headache, but there was no significant improvement in the severity of headache. Although the findings appear to be valid, the poor methodological quality and clinical heterogeneity of the included studies limit the evidence supporting the use of YXQN for migraine.
There were some limitations to this study. First, the high heterogeneity may be related to the risk of bias in the included studies. Subgroup analysis did not reduce the heterogeneity, so the accuracy of the results may have been affected. Second, the data analysis used published trials with positive results, indicating that trials with negative results may have been missed, which would have made the true effect different from the estimated effect. Third, the quality of the included studies was evaluated using ROB2.0, but the results varied. Fourth, the small sample sizes of the included studies, the lack of large sample trials, and the small number of included studies affected the reliability of the results. Fifth, the included studies used multiple outcome indicators and even though SMD was used to remove the heterogeneity caused by different outcome indicators, the accuracy of the final conclusions was still weakened. Sixth, some of the included studies did not describe in detail the occurrence of adverse reactions.
The GRADE evidence grading system was used to evaluate the quality of the evidence, and the evidence level for all the outcome indicators was low, except for the frequency of headache attacks in the YXQN group compared with the control group. The main reasons for this were: (1) Limitations, mainly due to the low quality of the original literature, which only mentioned randomization without specifying blinding and allocation concealment; (2) inconsistency, mainly due to the high heterogeneity between studies; (3) imprecision, as the number of included studies was small; and (4) possible publication bias. The results of the present study suggest that the use of YXQN alone is safer and more effective for the treatment of migraine compared with other treatment modalities. However, due to the small number of included studies and the low and very low quality of evidence according to the GRADE system, more high-quality, large-sample, multicenter, double-blind RCTs are needed to validate our findings and make better recommendations for clinical use. Therefore, the following issues should be addressed in future clinical studies: (1) Adopting correct randomization method, allocation concealment, and blinding; (2) conducting large sample clinical trials; (3) strengthening and improving safety studies; and (4) when conducting RCTs, the efficacy assessment of the included studies must be strictly based on uniform requirements and standards.
Migraine patients have the option to use YXQN as a standalone treatment. Despite the supportive evidence in this review regarding the efficacy of YXQN, recommendations for its routine use in migraine treatment are constrained by the inadequate methodological quality and clinical diversity among the studies included. However, this review has highlighted a specific area warranting further investigation. Rigorous RCTs evaluating YXQN are essential for deeper insights and clarification.
Migraine stands as the most prevalent form of neurological disorder, imposing a significant burden on healthcare services. Yangxue Qingnao Granules (YXQN) represents a commonly utilized Chinese patent medicine for managing migraine. Yet, the available evidence remains insufficient to comprehensively assess the efficacy of YXQN for treating migraine.
Traditional Chinese medicine has been extensively employed as a treatment method for migraine. Clinical studies have validated the effectiveness of YXQN in migraine treatment. Nevertheless, there remains insufficient evidence to comprehensively evaluate the efficacy of YXQN for migraine. Consequently, the objective of this meta-analysis was to systematically consolidate data from these clinical trials, aiming to assess both the effectiveness and safety of YXQN as an independent treatment for migraine. This study also intended to establish an evidence-based foundation for guiding its further clinical applications and research endeavors in this field.
To assess the efficacy and safety of YXQN alone for treatment of migraine.
We conducted a comprehensive search across 10 databases to identify pertinent randomized controlled trials (RCTs) published before September 2022. Two review authors independently conducted the literature search and screening and data extraction, and assessed the methodological quality of the studies employing criteria from ROB 2.0. Data analyses were performed using Review Manager 5.4 software.
A total of 12 RCTs including 767 participants with migraine met the selection criteria. We divided these studies into comparisons of YXQN with placebo, routine treatment drugs, and other Chinese patent medicines. The meta-analysis showed the following: (1) Efficacy: The YXQN group outperformed the placebo group [relative risk (RR) = 0.29, 95% confidence interval (95%CI): 0.15–0.43, P < 0.00001], routine treatment group (RR = 0.18, 95%CI: 0.09–0.27, P < 0.0001), and other Chinese patent medicine group (RR = 0.27, 95%CI: 0.13–0.41, P < 0.001); (2) frequency of headache: There was a significant difference between YXQN vs placebo [mean difference (MD) = -1.25, 95%CI: -1.60 to -0.90, P < 0.00001), routine treatment drugs (MD = -0.85, 95%CI: -1.15 to -0.56, P < 0.00001), and other Chinese patent medicines (MD = -0.91, 95%CI: -1.35 to -0.46, P < 0.0001); (3) headache duration: There was great heterogeneity between studies, with no differences between YXQN and placebo (MD = -0.61, 95%CI: -1.53 to -0.31, P = 0.19) and routine treatment drugs (MD = -0.22, 95%CI: -0.89 to 0.46, P < 0.53). YXQN was more effective than other Chinese patent medicines in reducing headache duration (MD = -1.24, 95%CI: -1.70 to -0.77; P < 0.00001); and (4) headache severity: There was no significant difference between YXQN vs placebo (MD = -1.67, 95%CI: -3.52 to 0.19, P = 0.08), routine treatment drugs (MD = -0.53, 95%CI: -2.02 to 0.96, P = 0.68), and other Chinese patent medicines (MD = -0.49, 95%CI: -2.83 to 1.85, P = 0.68). Mild gastrointestinal adverse reactions were reported in three cases.
This study revealed that YXQN is effective and safe for treatment of migraine.
Our meta-analysis indicated that YXQN represents a promising alternative for the treatment of migraines. However, due to the inadequate methodological quality observed in the included studies, further data and extensive investigation are imperative to establish its efficacy definitively. Additionally, there is a necessity for additional research to explore the effectiveness of YXQN for treating migraine in diverse ethnic populations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lanovaz MJ, Canada S-Editor: Lin C L-Editor: Wang TQ P-Editor: Yu HG
| 1. | GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5206] [Cited by in RCA: 4834] [Article Influence: 537.1] [Reference Citation Analysis (0)] |
| 2. | Karikari TK, Charway-Felli A, Höglund K, Blennow K, Zetterberg H. Commentary: Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Front Neurol. 2018;9:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35:6619-6629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 533] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 4. | Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5134] [Cited by in RCA: 5445] [Article Influence: 495.0] [Reference Citation Analysis (0)] |
| 5. | Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-22; quiz 123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 6. | Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 1548] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 7. | Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017;97:553-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1177] [Article Influence: 147.1] [Reference Citation Analysis (0)] |
| 8. | Buse DC, Scher AI, Dodick DW, Reed ML, Fanning KM, Manack Adams A, Lipton RB. Impact of Migraine on the Family: Perspectives of People With Migraine and Their Spouse/Domestic Partner in the CaMEO Study. Mayo Clin Proc. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5428] [Cited by in RCA: 5098] [Article Influence: 637.3] [Reference Citation Analysis (0)] |
| 10. | Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1520] [Cited by in RCA: 1635] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 11. | Nandha R, Singh H. Renin angiotensin system: A novel target for migraine prophylaxis. Indian J Pharmacol. 2012;44:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Sadowsky CH, Farlow MR, Atkinson L, Steadman J, Koumaras B, Chen M, Mirski D. Switching From Donepezil to Rivastigmine Is Well Tolerated: Results of an Open-Label Safety and Tolerability Study. Prim Care Companion J Clin Psychiatry. 2005;7:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Assarzadegan F, Sistanizad M. Tolerability and Efficacy of Memantine as Add on Therapy in Patients with Migraine. Iran J Pharm Res. 2017;16:791-797. [PubMed] |
| 14. | Lin L, Min J, Yun-Ling Z, Yan LU, Xing L, Xiao L, Jing-Jing W, Qian C, Guo-Jing FU. [Systematic review and Meta-analysis on efficacy and safety of Yangxue Qingnao Granules in treatment of migraine]. Zhongguo Zhong Yao Za Zhi. 2020;45:5093-5102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Chen FC, Mu YQ, Peng L, Li P, Qian N, Feng LB. Meta analysis of Yangxue Qingnao Granule combined with Flunarizine hydrochloride in the treatment of migraine. Xiandai Zhongxiyi Jiehe Zazhi. 2013;22:146-148. |
| 16. | Tfelt-Hansen P, Pascual J, Ramadan N, Dahlöf C, D'Amico D, Diener HC, Hansen JM, Lanteri-Minet M, Loder E, McCrory D, Plancade S, Schwedt T; International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia. 2012;32:6-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 17. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15131] [Article Influence: 2521.8] [Reference Citation Analysis (0)] |
| 18. | Li ZW. A Comparative Study on the Treatment of Migraine with Yangxue Qingnao Granules. Chongqing Yixue. 2003;628-629. |
| 19. | Li Z, Li C, Liu K. Yangxue Qingnao Granules for the Treatment of 79 Cases of Migraine. Shiyong Zhongyi Neike Zazhi. 2003;230-231. |
| 20. | He F. A Clinical Study of Yangxue Qingnao Granules in Treatment of Migraine. Luzhou Yixueyuan Xuebao. 2005;05:39-41. |
| 21. | Bai H. Yangxue Qingnao Granules for Treating 28 Cases of Migraine. Zhongguo Xiandai Yaowu Yingyong. 2011;5:67-68. |
| 22. | Zhang Z, Kuang S. Clinical Study of Yangxue Qingnao Granule in the Treatment of Migraine. Xiandai Zhenduan Yu Zhiliao. 2015;26:766-768. |
| 23. | Luo S, Wang X, Kuang P, Jia J, Yang Z, Zhou B, Yu H, Chang S, Ma W. Clinical Study of Yangxue Qingnao Granule in the Prevention and Treatment of Migraine. Zhonghua Shenjing Zazhi. 2001;26:38-41. |
| 24. | Xie Z, Peng H. Clinical Study of Yangxue Qingnao Granule in the Prevention and Treatment of Migraine. Yatai Chuantong Yiyao. 2017;13:127-128. |
| 25. | Jiang DF. A Comparative Study on the Treatment of Migraine with Yangxue Qingnao Granules. Xinli Yuekan. 2020;15:187. [DOI] [Full Text] |
| 26. | Li Y, Liu N. Comparative Study on the Treatment of Migraine with Yangxue Qingnao Granules. Shiyong Yiji Zazhi. 2012;19:1309-1310. |
| 27. | Niu Z, Hou Y, Ren X. Study on the Effect of Yangxue Qingnao Granules on Migraine Patients. Zhongxiyi Jiehe Xinnao Xueguan Zazhi. 2003;06:327-329. |
| 28. | Wang L. Clinical Discussion on the Prevention and Treatment of Yangxue Qingnao Granules. Jiankang Zhiyou. 2021;000:278. |
| 29. | Jiang G. Observation of the Efficacy of Yangxue Qingnao Granules to the Treatment of Migraines. Zhongyi Linchuang Yanjiu. 2019;11:136-138. |
| 30. | Zhao P, Niu Z, Liu P. Effect of Yangxue Qingnao Granule on degranulation of Dura mater Mast cell in migraine rats. Zhongxiyi Jiehe Xinnao Xueguanbing Zazhi. 2013;11:1364-1366. |
| 31. | Lei X, Zhao P, Niu Z. An experimental research on the expression of c ⁃ Fos protein in trigeminal nucleus caudalis of migraine rat model induced by nitroglycerin after the intervention of Yangxueqingnao granules. Zhongguo Xiandai Shenjing Jibing Zazhi. 2014;14:708-716. |
| 32. | Li Y. Effect of Yangxue Qingnao Granule on headache symptoms and Hemorheology in migraine patients. Linchuang Yiyao Wenxian Dianzi Zazhi. 2016;3:11617-11618. |