Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.302
Peer-review started: October 20, 2023
First decision: November 28, 2023
Revised: December 15, 2023
Accepted: December 21, 2023
Article in press: December 21, 2023
Published online: January 16, 2024
Processing time: 82 Days and 20.8 Hours
Akt plays diverse roles in humans. It is involved in the pathogenesis of type 2 diabetes mellitus (T2DM), which is caused by insulin resistance. Akt also plays a vital role in human platelet activation. Furthermore, the hippocampus is closely associated with memory and learning, and a decrease in hippocampal volume is reportedly associated with an insulin-resistant phenotype in T2DM patients without dementia.
To investigate the relationship between Akt phosphorylation in unstimulated platelets and the hippocampal volume in T2DM patients.
Platelet-rich plasma (PRP) was prepared from the venous blood of patients with T2DM or age-matched controls. The pellet lysate of the centrifuged PRP was subjected to western blotting to analyse the phosphorylation of Akt, p38 mitogen-activated protein (MAP) kinase and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Phosphorylation levels were quantified by densitometric analysis. Hippocampal volume was analysed using a voxel-based specific regional analysis system for Alzheimer’s disease on magnetic resonance imaging, which proposes the Z-score as a parameter that reflects hippocampal volume.
The levels of phosphorylated Akt corrected with phosphorylated p38 MAP kinase were inversely correlated with the Z-scores in the T2DM subjects, whereas the levels of phosphorylated Akt corrected with GAPDH were not. However, this relationship was not observed in the control patients.
These results suggest that an inverse relationship may exist between platelet Akt activation and hippocampal atrophy in T2DM patients. Our findings provide insight into the molecular mechanisms underlying T2DM hippocampal atrophy.
Core Tip: Type 2 diabetes mellitus (T2DM) is caused by insulin resistance, and Akt is involved in this process. Akt also plays an important role in human platelet activation. The hippocampus, associated with memory and learning, can decrease in volume in patients with an insulin-resistant phenotype. We investigated the relationship between Akt phosphorylation in unstimulated platelets and hippocampal volume-reflecting Z-score in T2DM patients. The levels of phosphorylated Akt corrected for phosphorylated p38 mitogen-activated protein kinase were inversely correlated with Z-scores in T2DM patients. These results suggest an inverse relationship between Akt activation in platelets and hippocampal atrophy in patients with T2DM.
- Citation: Tokuda H, Hori T, Mizutani D, Hioki T, Kojima K, Onuma T, Enomoto Y, Doi T, Matsushima-Nishiwaki R, Ogura S, Iida H, Iwama T, Sakurai T, Kozawa O. Inverse relationship between platelet Akt activity and hippocampal atrophy: A pilot case-control study in patients with diabetes mellitus. World J Clin Cases 2024; 12(2): 302-313
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/302.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.302
Type 2 diabetes mellitus (T2DM) is a global concern with a significant human health burden and serious economic implications[1]. Patients with T2DM have an elevated risk of vascular complications, such as cardiovascular disease, stroke, and vascular dementia[2-4]. It is well known that platelets play an important role in the pathogenesis of atherosclerosis and thrombosis. Various stimuli, such as thrombin, adenosine diphosphate, collagen, thromboxane A2, shear stress, and hyperglycaemia cause platelet activation[5-7]. Activated platelets perform several functions, including aggregation. We previously reported that T2DM patients exhibit spontaneous microaggregation of platelets and that the P2Y12 receptor plays a pivotal role in this process[8]. Phosphatidylinositol 3-kinase (PI3K) plays a vital role in platelet activation[6,9-14]. Akt, also known as protein kinase B, is the main downstream effector of PI3K[15], and is known to undergo phos
The hippocampus is a vulnerable and elongated structure located deep within the medial temporal lobe of the human brain[18]. The human hippocampus plays a pivotal role in memory and learning because of its intrinsic circuitry; therefore, hippocampal atrophy is frequently observed in memory-disturbed patients, including those with Alzheimer’s disease (AD), which is one of the major causes of cognitive impairment[18,19]. A voxel-based specific regional analysis system for AD (VSRAD) using magnetic resonance imaging (MRI) was developed as an adjunct technique to discriminate early AD patients from controls[20]. The VSRAD presents a Z-score that reflects the volume loss of medial temporal areas, including the hippocampus and entorhinal cortex, and has been approved as a supportive method for AD diagnosis, in Japan[20,21]. In addition, the Z-score of the target volume of interest (VOI) (VSRAD Z-score) is a useful tool for both cross-sectional and longitudinal evaluations of AD patients[22,23]. T2DM is a risk factor for AD[24,25]. In T2DM patients without dementia, hippocampal atrophy evaluated with the VSRAD has been reported to be associated with insulin resistance phenotypes such as abdominal visceral fat, high-sensitivity C-reactive protein, and plasma homocysteine levels[26-28]. However, the mechanisms underlying hippocampal atrophy in T2DM patients remain unclear.
In the present study, we investigated the relationship between Akt activity in unstimulated resting platelets and hippocampal atrophy, as measured using the VSRAD Z-score, in patients with T2DM.
This case-control study investigated the relationship between Akt activity in unstimulated resting platelets and hippocampal atrophy in patients with T2DM.
Phospho-specific Akt (Thr-308) and phospho-specific p38 mitogen-activated protein (MAP) kinase antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Other materials and chemicals were obtained from commercial sources.
Patients who were neither diagnosed with dementia nor impaired instrumental activities of daily living were recruited from those admitted or outpatient at the National Center for Geriatrics and Gerontology. The inclusion criterion for the study group was the presence of T2DM according to the criteria of the World Health Organization. For the control group, we recruited age-matched patients with well-controlled hyperlipidaemia and hyperuricaemia but without diabetes. Patients with a past history of symptomatic cerebrovascular disease; significant cortical damage such as hydrocephalus, brain tumour or cerebral contusion evaluated by MRI; or atrial fibrillation were excluded. Patients with malignancy, infectious diseases, including hepatitis B and hepatitis C, or autoimmune disorders were also excluded. All participants were requested to avoid blood donation or sleep deprivation.
After 15 min of bed rest to maintain steady-state conditions, 10 mL of venous blood was drawn between 8:00 AM and 9:00 AM post overnight starvation, and 14 μmol/L sodium citrate was added immediately as an anticoagulant. Platelet-rich plasma (PRP) was obtained by centrifugation at 155 × g for 12 min at room temperature, and ice-cold ethylenediaminetetraacetic acid (10 mmol/L) solution was immediately added to avoid platelet activation. The mixture was collected and centrifuged at 10000 × g at 4 °C for 2 min. The pellet was washed twice with phosphate-buffered saline (PBS) and lysed by boiling in lysis buffer containing 62.5 mmol/L Tris-HCL (pH 6.8), 2% sodium dodecyl sulphate (SDS), 50 mmol/L dithiothreitol and 10% glycerol for western blot analysis.
Western blotting was performed as previously described[29]. Briefly, SDS-polyacrylamide gel electrophoresis was performed on a 10% polyacrylamide gel, according to Laemmli[30]. The proteins were fractionated and transferred onto a polyvinylidene difluoride (PVDF) membrane, which was then blocked with 5% fat-free dry milk in PBS with 0.1% Tween 20 (PBS-T; 10 mmol/L Na2HPO4, 1.8 mmol/L KH2PO4, pH 7.4, 137 mmol/L NaCl, 2.7 mmol/L KCL and 0.1% Tween 20) for 2 h before incubation with phospho-specific Akt (Thr-308) antibodies, GAPDH antibodies and phospho-specific p38 MAP kinase antibodies as primary antibodies. Peroxidase-labelled anti-rabbit or anti-mouse IgG antibodies were used as the secondary antibodies. Primary and secondary antibodies were diluted to the optimal concentrations using 5% fat-free dry milk in PBS-T. Peroxidase activity on the PVDF membrane was visualised on an X-ray film using an ECL western blotting detection system (GE Healthcare, Buckinghamshire, United Kingdom) as described in the manufacturer’s protocol. The bands were analysed by densitometry using a scanner and imaging software (ImageJ version 1.50; NIH, Bethesda, MD, United States). The quantitative data for each sample were obtained as pixel counts.
MRI was performed using a 1.5-T system (Philips Ingenia, Eindhoven, Netherlands). Three-dimensional volumetric imaging of a T1-weighted gradient echo sequence at 9.3/3.9/1 (repetition time/echo time/excitation) was carried out for voxel-based morphometry analysis with the following parameters: Flip angle, 10 °; acquisition matrix, 256 × 256; field of view, 24.0 cm; and section thickness, 1.00 mm.
A voxel-based specific regional analysis system for AD (VSRAD) (VSRAD advance 2, Eisai Co., Ltd., Tokyo, Japan) segmented MRI images into grey matter, white matter, and cerebrospinal fluid using a unified tissue segmentation procedure after image intensity nonuniformity correction. These linearly transformed and segmented images were then nonlinearly transformed using Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) procedures and modulated to a customised template for DARTEL, followed by smoothing using an 8-mm full width at half-maximum kernel. Each processed segmented image was compared to the mean and standard deviation of the grey matter or white matter images of 80 healthy volunteers using voxel-by-voxel Z-score analysis: Z-score = (control mean - individual value)/(control standard deviation). These Z-score maps were displayed as overlays on tomographic sections of each brain. The VSRAD registers the target VOI in the medial temporal structures, including the hippocampus and entorhinal cortex[20,21], defined by comparing patients with early AD with healthy controls.
Quantitative variables were presented as the mean ± standard deviations. The statistical significance of the correlation between the two variables and linear regression analysis was adopted. All statistical analyses were performed using SPSS version 19.0 (IBM Japan Ltd., Tokyo, Japan). A value of P < 0.05 was statistically significant. The biomedical statistician, Dr. Reo Kawano (National Center for Geriatrics and Gerontology) reviewed the statistical methods used in this study.
The clinical and biochemical characteristics of the participants in the DM (n = 40) and control (n = 15) groups are presented in Table 1. The haemoglobin A1c (HbA1c) levels of all participants in the DM group were higher than the upper limit of the normal range (5.9%). Anthropometric indices were within normal ranges in the Japanese population, and there were no significant changes in metabolic variables. There were no statistically significant differences between the DM and control groups, except for plasma glucose or HbA1c levels. The list and number of antidiabetic medications used by the patients in the DM group are presented in Table 2. The numbers of patients who received metformin, glucagon-like peptide 1 receptor agonist (GLP-1RA) and exogeneous insulins, which can affect Akt activity[31-33], was 3, 2 and 7, respectively.
| Parameters | DM group | Control group |
| Total number (F/M) | 40 (20/20) | 15 (8/7) |
| Age (years) | 75.0 ± 7.6 | 73.4 ± 7.0 |
| DM duration (years) | 13.6 ± 11.3 | None |
| Height (cm) | 156.7 ± 8.8 | 158.5 ± 8.3 |
| Weight (kg) | 57.0 ± 10.5 | 57.8 ± 7.1 |
| BMI | 23.2 ± 3.8 | 23.0 ± 2.6 |
| sBP (mmHg) | 124.5 ± 19.3 | 130.0 ± 15.7 |
| dBP (mmHg) | 67.8 ± 13.6 | 74.4 ± 11.6 |
| HbA1c (%) | 8.1 ± 1.4d | 5.9 ± 0.2 |
| Glu (mg/dL) | 175.0 ± 54.2d | 99.3 ± 13.3 |
| TC (mg/dL) | 185.1 ± 33.3 | 201.6 ± 29.4 |
| TG (mg/dL) | 119.1 ± 53.7 | 110.7 ± 57.0 |
| HDL (mg/dL) | 53.6 ± 14.4 | 61.9 ± 13.7 |
| Plt (× 104/μL) | 21.4 ± 5.9 | 23.8 ± 3.7 |
| VSRAD Z-score | 0.841 ± 0.462 | 0.900 ± 0.489 |
| Agent | Cases |
| Metformin | 3 |
| GLP-1RAs | 2 |
| Insulins | 7 |
| DPP-4 inhibitors | 22 |
| α-Glucosidase inhibitors | 4 |
| SGLT2 inhibitors | 5 |
| Sulfonylureas | 10 |
Individual VSRAD Z-scores and the use of insulin, GLP-1 RA or metformin in the DM group are presented in Table 3. Z-scores for the VSRAD ranged from 0.23 to 1.93. There were no cases with VSRAD Z-scores ≥ 2.0, which primarily indicates significant hippocampal atrophy[20,21]; however, 13 patients presented with scores between 1.0 and 2.0, in the borderline range[20,21]. We investigated the relationship between age and VSRAD Z-scores in the study group; however, no statistically significant relationship (r = 0.181, P = 0.263) was observed. Individual VSRAD Z-scores of the control group are shown in Table 4. Z-scores for the VSRAD ranged from 0.43 to 1.86. There were no cases with VSRAD Z-scores ³ 2.0; however, five patients presented scores between 1.0 and 2.0, in the borderline range.
| Patient | Z-score | Patient | Z-score |
| 1 | 0.53 | 9 | 0.51 |
| 2 | 1.63 | 10 | 0.63 |
| 3 | 1.86 | 11 | 1.27 |
| 4 | 0.50 | 12 | 1.54 |
| 5 | 0.43 | 13 | 0.89 |
| 6 | 0.70 | 14 | 0.48 |
| 7 | 1.32 | 15 | 0.77 |
| 8 | 0.45 |
It is well known that the PI3K-Akt pathway plays a vital role in human platelet activation[6,9-14]. Thus, we first examined the individual levels of Akt phosphorylation corrected by the levels of GAPDH using western blot analysis. The results of western blotting and densitometric analyses are presented in Figure 1A and B for the DM group and Figure 1C and D for the control group. The individual levels of Akt phosphorylation were upregulated in some cases despite the results obtained from unstimulated platelets; however, the levels differed among samples in both groups. At least in part, low levels of Akt-phosphorylation were observed in cases with high VSRAD Z-scores, such as in patient No. 1. However, high levels of phosphorylation were observed in patients with low VSRAD Z-scores, such as in patient No. 2. The individual levels of phosphorylated-Akt/GAPDH against the VSRAD Z-scores were plotted, and a tendency toward an inverse correlation between phosphorylated-Akt/GAPDH levels and VSRAD Z-scores was observed. In contrast, no statistical significance was observed in their relationship (R2 = 0.072, P = 0.094, n = 40) (Figure 2A). In the control group, the individual levels of phosphorylated-Akt/GAPDH had no significant association with VSRAD Z-scores (R2 = 0.003, P = 0.841) (Figure 2B).
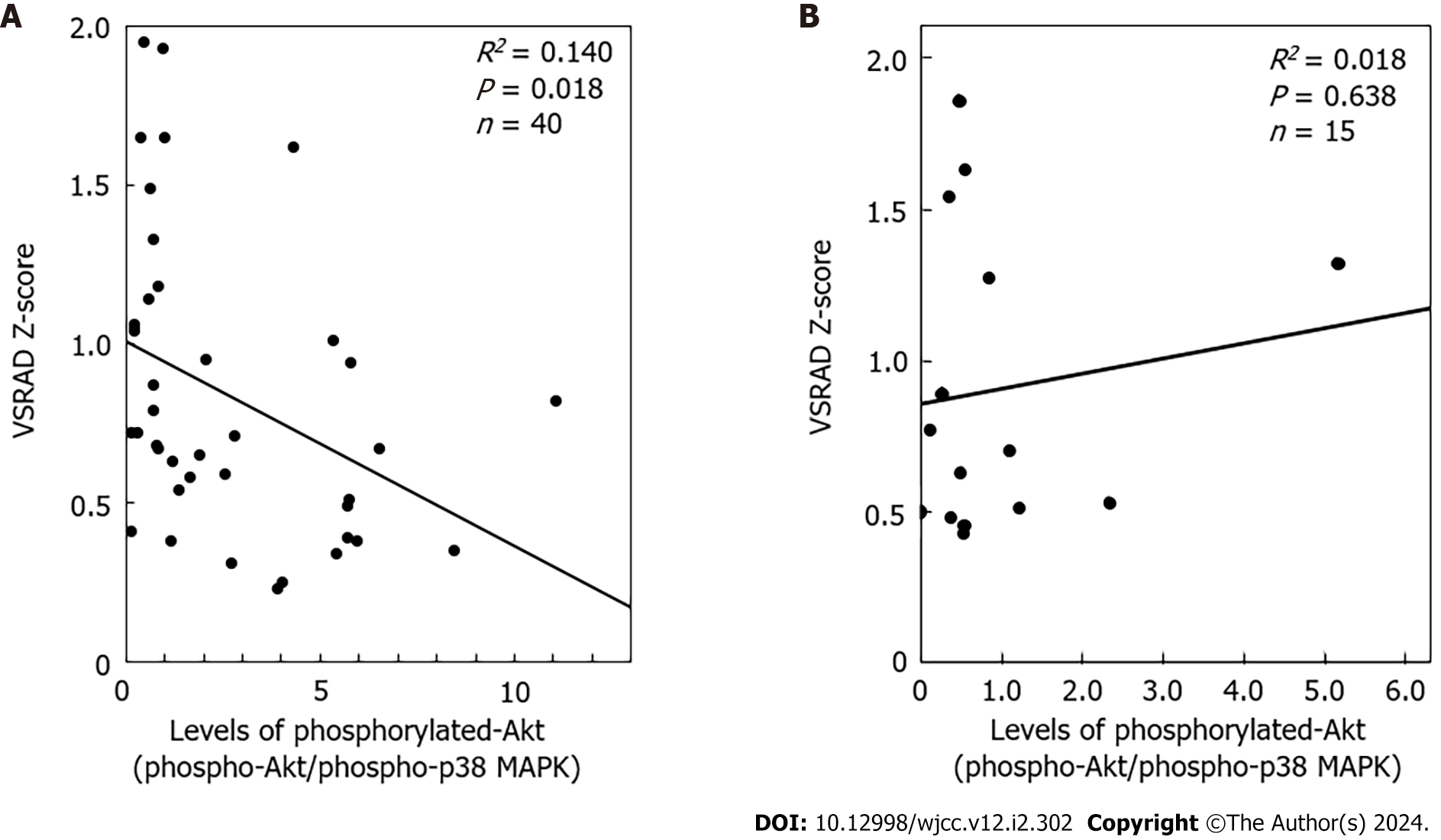
In addition to Akt, it has been reported that p38 MAP kinase is activated not only by environmental stress but also by inflammatory cytokines[34], playing an essential role in platelet activation, including in patients with T2DM[35]. Therefore, we examined individual levels of p38 MAP kinase phosphorylation. The results of western blot analysis of phosphorylated p38 MAP kinase and densitometric analyses for all participants are shown in Figure 3A and B for the DM group, and Figure 3C and D for the control group. Similar to the levels of Akt phosphorylation, the individual levels of p38 MAP kinase phosphorylation were quite different among the samples in both groups, and were significantly upregulated in some cases despite the presence of resting platelets. There was no correlation between the phosphorylation levels of p38 MAP kinase and VSRAD Z-scores (data not shown). For further analysis, we plotted individual phosphorylated Akt/phosphorylated p38 MAP kinase levels against VSRAD Z-scores. In the DM group, the levels of phosphorylated Akt/phosphorylated p38 MAP kinase were inversely correlated with the VSRAD Z-scores (R2 = 0.140, P = 0.018, n = 40) (Figure 4A); however, there was no significant relationship between them in the control group (R2 = 0.018, P = 0.841, n = 15) (Figure 4B).
The present study investigated the possible mechanisms underlying hippocampal atrophy in T2DM patients without cognitive impairment. We evaluated the VSRAD Z-scores mainly reflecting the hippocampus volume[20,21] in the study participants, the DM and control groups, and confirmed that cases defined as significant atrophy were not included. We previously found that spontaneous aggregation occurred, at least partially, in the platelets of T2DM patients[8]. Akt is known to play an important role in human platelet activation[6,9-14], and impairment of Akt signalling is closely related to insulin resistance, a central pathology of T2DM[16,17]. We examined the levels of Akt phosphorylation in the platelets of T2DM patients under unstimulated conditions compared to control subjects with no diabetes. We found that the individual levels of Akt phosphorylation were upregulated in some cases in both groups, but the levels were different. It is likely that Akt in the platelets is spontaneously activated in some T2DM and non-T2DM patients. Thus, we investigated the relationship between the VSRAD Z-scores and Akt phosphorylation levels after adjusting for GAPDH levels. We did not find statistical significance in the relationship between them; however, there was likely a tendency for an inverse correlation only in the DM group. Although ageing is known to impact hippocampal volume significantly, we did not find any significant relationship between age and VSRAD Z-scores. It is unlikely that individual hippocampal volume is closely related to age, at least in the patients with DM in this study.
The activation of environmental stress-related p38 MAP kinase[34], in addition to Akt, plays a role in the excessive activation of platelets in T2DM patients[35]. Therefore, we examined the individual levels of p38 MAP kinase phosphorylation in the unstimulated platelets of the study participants. We found that the levels of p38 MAP kinase phosphorylation were different in each sample and were upregulated in some cases in both groups. It is likely that p38 MAP kinase in platelets could also be spontaneously activated in some T2DM and non-T2DM patients. We confirmed no correlation between the phosphorylation levels of p38 MAP kinase and VSRAD Z-scores in the study participants. However, the individual levels of p38 MAP kinase phosphorylation were quite different from those of Akt phosphorylation and appeared to be contrary in some cases in the DM group.
Finally, we investigated the relationship between the levels of Akt phosphorylation corrected for p38 MAP kinase phosphorylation levels and VSRAD Z-scores in unstimulated platelets of T2DM patients. We found a significant inverse correlation between the levels of phosphorylated Akt/phosphorylated p38 MAP kinase and the VSRAD Z-scores, suggesting that the increased ratio of Akt/p38 MAP kinase activity in unstimulated platelets could negatively contribute to the decline in hippocampal volume in T2DM patients. In contrast, we found no significant relationship between the phosphorylated Akt/phosphorylated p38 MAP kinase ratio and VSRAD Z-scores in the control group. Likely, an inverse relationship between individual VSRAD-Z scores and the levels of Akt phosphorylation to p38 MAP kinase could be observed specifically in T2DM patients. To the best of our knowledge, this is the first report to show a relationship between hippocampal atrophic changes and Akt/p38 MAP kinase activity in unstimulated platelets. The ratio of phosphorylated Akt to phosphorylated p38 MAP kinase indicates the relative activity of individual platelets. The lower the ratio, the greater the decline in hippocampal volume in patients with T2DM. Therefore, the activity of Akt in platelets, in contrast to that of p38 MAP kinase, may have a protective effect against hippocampal atrophy associated with T2DM. Although we evaluated platelet activity only under non-stimulated conditions, the unstimulated activation status of Akt to p38 MAP kinase might be more important in reflecting the pathological platelet status than that observed in ex vivo-stimulated platelets. A limitation of this study is that our analysis probably included biases, such as experimental conditions, randomization, and sampling, which may have affected the observed trends.
A decrease in the hippocampal volume in T2DM patients is observed in comparison with the non-diabetic population[36]. It has been reported that a decline in hippocampal volume could be associated with the insulin resistance phenotype in T2DM patients without dementia[26-28]. Impairment of Akt signalling plays a pivotal role in the pathogenesis of insulin resistance, which is closely associated with the onset of T2DM[16,17]. Conversely, decreased levels of PI3K subunits and blunted Akt kinase phosphorylation have been reported in the brains of patients with AD[37], suggesting that impairment of the PI3K-Akt pathway related to insulin signalling in the brain may play a role in the pathogenesis of AD[37]. Therefore, our present findings regarding the Akt/p38 MAP kinase phosphorylation ratio in platelets may provide a novel indicator of the risk of hippocampal atrophy in T2DM patients. Further research is required to clarify the mechanisms underlying hippocampal atrophy in T2DM patients.
In patients with T2DM, there may be an inverse relationship between Akt activation in platelets and hippocampal atrophy. Our findings provide an important step towards understanding the molecular mechanisms underlying hippocampal atrophy in T2DM.
Type 2 diabetes mellitus (T2DM) is caused by insulin resistance, a condition in which Akt is involved. Akt plays an important role in human platelet activation. A decrease in hippocampal volume is associated with an insulin-resistant phenotype in T2DM patients.
If a relationship exists between Akt phosphorylation in unstimulated platelets and hippocampal volume, Akt phosphorylation could be a risk indicator of hippocampal atrophy in T2DM patients.
Patients with T2DM in the study group and age-matched patients with metabolic disorders without diabetes in the control group were recruited to obtain platelet-rich plasma from venous blood.
Protein phosphorylation in platelets was analysed by western blotting, followed by densitometry. The Z-score by a voxel-based specific regional analysis system for Altzheimer’s disease on magnetic resonance imaging was adopted as a parameter reflecting hippocampal volume.
The levels of phosphorylated Akt corrected for phosphorylated p38 mitogen-activated protein (MAP) kinase were inversely correlated with Z-scores in the study group but not in the control group.
In patients with T2DM, there may be an inverse relationship between Akt activation in platelets and hippocampal atrophy.
The Akt/p38 MAP kinase phosphorylation ratio in platelets may be a novel indicator of the risk of hippocampal atrophy in T2DM patients.
We are very grateful to Prof. Takashi Kato (Department of Radiology, National Center for Geriatrics and Gerontology) and Dr. Reo Kawano (Manager of Data-Management and Biostatistics Unit, Innovation Center for Translational Research, National Center for Geriatrics and Gerontology) for their important comments on the MRI findings and the biostatical review, respectively. We are grateful to Mrs. Yumiko Kurokawa (Department of Pharmacology, Gifu Graduate School of Medicine) and Mrs. Chika Usui (Department of Metabolic Research, Research Institute, National Center for Geriatrics and Gerontology) for their technical assistance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng TH, Taiwan S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Standl E, Khunti K, Hansen TB, Schnell O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur J Prev Cardiol. 2019;26:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 2. | Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1297] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 3. | Goldstein LB, Adams R, Becker K, Furberg CD, Gorelick PB, Hademenos G, Hill M, Howard G, Howard VJ, Jacobs B, Levine SR, Mosca L, Sacco RL, Sherman DG, Wolf PA, del Zoppo GJ. Primary prevention of ischemic stroke: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2001;32:280-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 275] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Hébert R, Lindsay J, Verreault R, Rockwood K, Hill G, Dubois MF. Vascular dementia : incidence and risk factors in the Canadian study of health and aging. Stroke. 2000;31:1487-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1212] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 6. | Woulfe DS. Akt signaling in platelets and thrombosis. Expert Rev Hematol. 2010;3:81-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Sakamoto T, Ogawa H, Kawano H, Hirai N, Miyamoto S, Takazoe K, Soejima H, Kugiyama K, Yoshimura M, Yasue H. Rapid change of platelet aggregability in acute hyperglycemia. Detection by a novel laser-light scattering method. Thromb Haemost. 2000;83:475-479. [PubMed] |
| 8. | Matsuno H, Tokuda H, Ishisaki A, Zhou Y, Kitajima Y, Kozawa O. P2Y12 receptors play a significant role in the development of platelet microaggregation in patients with diabetes. J Clin Endocrinol Metab. 2005;90:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Kovacsovics TJ, Bachelot C, Toker A, Vlahos CJ, Duckworth B, Cantley LC, Hartwig JH. Phosphoinositide 3-kinase inhibition spares actin assembly in activating platelets but reverses platelet aggregation. J Biol Chem. 1995;270:11358-11366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 151] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, Wymann M, Montrucchio G. Resistance to thromboembolism in PI3Kgamma-deficient mice. FASEB J. 2001;15:2019-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Li Z, Zhang G, Le Breton GC, Gao X, Malik AB, Du X. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J Biol Chem. 2003;278:30725-30731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Rittenhouse SE. Phosphoinositide 3-kinase activation and platelet function. Blood. 1996;88:4401-4414. [PubMed] |
| 13. | Canobbio I, Stefanini L, Cipolla L, Ciraolo E, Gruppi C, Balduini C, Hirsch E, Torti M. Genetic evidence for a predominant role of PI3Kbeta catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood. 2009;114:2193-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Kim S, Mangin P, Dangelmaier C, Lillian R, Jackson SP, Daniel JL, Kunapuli SP. Role of phosphoinositide 3-kinase beta in glycoprotein VI-mediated Akt activation in platelets. J Biol Chem. 2009;284:33763-33772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169:381-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1732] [Cited by in RCA: 2635] [Article Influence: 329.4] [Reference Citation Analysis (0)] |
| 16. | Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1393] [Cited by in RCA: 1594] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 17. | Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14:1483-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 1008] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 18. | Knierim JJ. The hippocampus. Curr Biol. 2015;25:R1116-R1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 19. | Jack CR Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 453] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 20. | Hirata Y, Matsuda H, Nemoto K, Ohnishi T, Hirao K, Yamashita F, Asada T, Iwabuchi S, Samejima H. Voxel-based morphometry to discriminate early Alzheimer's disease from controls. Neurosci Lett. 2005;382:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 21. | Waragai M, Hata S, Suzuki T, Ishii R, Fujii C, Tokuda T, Arai H, Ohrui T, Higuchi S, Yoshida M, Igarashi K, Moriya M, Iwai N, Uemura K. Utility of SPM8 plus DARTEL (VSRAD) combined with magnetic resonance spectroscopy as adjunct techniques for screening and predicting dementia due to Alzheimer's disease in clinical practice. J Alzheimers Dis. 2014;41:1207-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Tateno A, Sakayori T, Kawashima Y, Higuchi M, Suhara T, Mizumura S, Mintun MA, Skovronsky DM, Honjo K, Ishihara K, Kumita S, Suzuki H, Okubo Y. Comparison of imaging biomarkers for Alzheimer's disease: amyloid imaging with [18F]florbetapir positron emission tomography and magnetic resonance imaging voxel-based analysis for entorhinal cortex atrophy. Int J Geriatr Psychiatry. 2015;30:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Saji N, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, Niida S, Toba K, Sakurai T. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9:19227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 24. | Alam F, Islam MA, Sasongko TH, Gan SH. Type 2 Diabetes Mellitus and Alzheimer's Disease: Bridging the Pathophysiology and Management. Curr Pharm Des. 2016;22:4430-4442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021;397:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 2642] [Article Influence: 660.5] [Reference Citation Analysis (0)] |
| 26. | Anan F, Masaki T, Shimomura T, Fujiki M, Umeno Y, Eshima N, Saikawa T, Yoshimatsu H. Abdominal visceral fat accumulation is associated with hippocampus volume in non-dementia patients with type 2 diabetes mellitus. Neuroimage. 2010;49:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Anan F, Masaki T, Shimomura T, Fujiki M, Umeno Y, Eshima N, Saikawa T, Yoshimatsu H. High-sensitivity C-reactive protein is associated with hippocampus volume in nondementia patients with type 2 diabetes mellitus. Metabolism. 2011;60:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Shimomura T, Anan F, Masaki T, Umeno Y, Eshima N, Saikawa T, Yoshimatsu H, Fujiki M, Kobayashi H. Homocysteine levels are associated with hippocampus volume in type 2 diabetic patients. Eur J Clin Invest. 2011;41:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Tokuda H, Kuroyanagi G, Tsujimoto M, Enomoto Y, Matsushima-Nishiwaki R, Onuma T, Kojima A, Doi T, Tanabe K, Akamatsu S, Iida H, Ogura S, Otsuka T, Iwama T, Tanikawa T, Ishikawa K, Kojima K, Kozawa O. Release of Phosphorylated HSP27 (HSPB1) from Platelets Is Accompanied with the Acceleration of Aggregation in Diabetic Patients. PLoS One. 2015;10:e0128977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185380] [Cited by in RCA: 188835] [Article Influence: 3433.4] [Reference Citation Analysis (0)] |
| 31. | Zhang Z, Liu H, Liu J. Akt activation: A potential strategy to ameliorate insulin resistance. Diabetes Res Clin Pract. 2019;156:107092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 32. | Yang Y, Fang H, Xu G, Zhen Y, Zhang Y, Tian J, Zhang D, Zhang G, Xu J. Liraglutide improves cognitive impairment via the AMPK and PI3K/Akt signaling pathways in type 2 diabetic rats. Mol Med Rep. 2018;18:2449-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Saltiel AR. Insulin signaling in health and disease. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 242] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 34. | Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420-7426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1750] [Cited by in RCA: 1812] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 35. | Hanai Y, Adachi S, Yasuda I, Takai S, Matsushima-Nishiwaki R, Kato H, Enomoto Y, Akamatsu S, Sakakibara S, Ogura S, Iwama T, Kozawa O, Tokuda H. Collagen-induced p38 MAP kinase activation is a biomarker of platelet hyper-aggregation in patients with diabetes mellitus. Life Sci. 2009;85:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 344] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 37. | Gabbouj S, Ryhänen S, Marttinen M, Wittrahm R, Takalo M, Kemppainen S, Martiskainen H, Tanila H, Haapasalo A, Hiltunen M, Natunen T. Altered Insulin Signaling in Alzheimer's Disease Brain - Special Emphasis on PI3K-Akt Pathway. Front Neurosci. 2019;13:629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |