Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.267
Peer-review started: October 31, 2023
First decision: November 28, 2023
Revised: December 7, 2023
Accepted: December 27, 2023
Article in press: December 27, 2023
Published online: January 16, 2024
Processing time: 71 Days and 22.8 Hours
Currently, there is no standard adjuvant therapy for patients with resected ampulla of Vater (AoV) cancer.
To evaluate the effectiveness of adjuvant concurrent chemoradiotherapy (CCRT) in patients with advanced AoV cancer who underwent curative resection.
This single-centered, retrospective study included 29 patients with advanced AoV cancer who underwent pancreaticoduodenectomy between 2006 and 2018. The impact of CCRT on advanced AoV cancer was analyzed.
The 1-, 3-, and 5-yr recurrence-free survival (RFS) rates for patients with advanced AoV cancer were 82.8%, 48.3%, and 40.8%, respectively, and the overall survival (OS) rates were 89.7%, 62.1%, and 51.7%, respectively. Lymphovas
Adjuvant CCRT did not improve survival outcomes in patients with advanced AoV cancer. These findings contribute to existing knowledge on the effectiveness of CCRT in this patient population and provide important insights for clinical decision-making.
Core Tip: We examined the potential survival benefits of adjuvant concurrent chemoradiotherapy (CCRT). Our findings indicated that adjuvant CCRT may not provide any survival advantage to patients with ampulla of Vater (AoV) cancer who had T3/T4 or lymph node-positive tumors. Therefore, the use of adjuvant CCRT as a standard approach in the treatment of advanced AoV cancer patients should be reconsidered.
- Citation: Kwon CH, Seo HI, Kim DU, Han SY, Kim S, Lee NK, Hong SB, Ahn JH, Park YM, Noh BG. Survival benefit of concurrent chemoradiotherapy for advanced ampulla of Vater cancer. World J Clin Cases 2024; 12(2): 267-275
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/267.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.267
Although ampulla of Vater (AoV) cancer has a relatively good prognosis among periampullary cancers, it still has a 5-yr survival rate of only 37%-51% after resection[1,2]. Advanced stage and lymph node involvement were identified as significant prognostic factors[3,4]. Thus, adjuvant treatment is required for patients at advanced stages with a high risk of recurrence. However, previous studies have suggested that radiotherapy alone has no survival benefit[5,6]. Further, Miura et al[6] reported minor benefits of radiotherapy in patients with lymph node (LN) metastases. Consequently, the most commonly used adjuvant treatment is chemotherapy or concurrent chemoradiotherapy (CCRT).
Several trials have demonstrated that adjuvant chemotherapy is associated with improved survival compared with observation alone, with a benefit greater in patients with advanced T3/T4 lesions or LN metastases[4,7,8]. However, some studies have reported contrasting opinions[9,10]. Despite the controversy surrounding the benefits of adjuvant chemotherapy after resection for AoV cancer and the lack of consensus guidelines, adjuvant chemotherapy tends to be used clinically[11]. Currently, the combination of adjuvant chemotherapy and radiation is the standard approach. However, evidence supporting the use of CCRT in AoV cancer is limited, and further research is needed to determine the best treatment approach. Therefore, this study aimed to retrospectively evaluate the efficacy of adjuvant CCRT in patients with advanced AoV carcinoma who underwent curative-intent resection.
A total of 92 patients with AoV cancer, confirmed by histological examination, underwent pancreaticoduodenectomy between January 2006 and December 2018 at a single tertiary hospital by a single surgeon. Of these, 11 patients with advanced AoV cancer (T3/T4 or LN metastases) who underwent adjuvant CCRT, and 18 patients who did not receive adjuvant therapy and who did not meet the following exclusion criteria were included: exclusive adjuvant chemotherapy or radiotherapy, treatment-associated mortality, significant decline in post-surgery physical strength, variants of adenocarcinoma, neuroendocrine carcinoma, regional LN metastasis beyond LN, total pancreatectomy, incomplete medical records, and cause of death not recurrence but a different medical cause. The clinicopathologic characteristics of the patients are showed in Supplementary Table 1. TNM staging was performed according to the 8th edition of the American Joint Committee Staging System. Demographic, adjuvant treatment, and survival outcome data were retrospectively obtained from the medical records. This study was approved by the Institutional Review Board of Clinical Trial Center in our hospital (IRB No. 2303-007-124).
Adjuvant CCRT was recommended for patients with LN metastasis regardless of the T stage and was administered 4-8 wk after surgery according to the patient's voluntary decision. The chemotherapy regimen consisted of six cycles of fluoropyridine- or gemcitabine-based chemotherapy [5-FU plus leucovorin (FLv) or gemcitabine plus cisplatin (GP)] for 6 cycles, with a radiation dose of 50.4 Gy. One patient died of gastric infarction and was excluded from the study.
Patients underwent tumor marker (CA19-9 and CEA) testing every 3 mo, with additional imaging tests (abdominal and chest computed tomography) performed if abnormalities were detected. If there were no abnormalities, imaging tests were performed every 6 mo. Palliative treatment was administered to patients who experienced recurrence. One patient died from complications due to radiofrequency ablation and was excluded from the study.
The clinical and pathological characteristics of the CCRT and non-CCRT groups were compared and analyzed using the Mann-Whitney U and chi-square tests for continuous and categorical variables, respectively. Univariate and multivariate analyses were performed using Cox proportional hazard models. Statistical analyses were performed using the R software (version 4.2.1). The R packages “moonbook,” “survminer” and “survival,” were used. Statistical significance was set at P < 0.05. The statistical methods of this study were reviewed by Kim JM from Biomedical Research Institute, Pusan National University Hospital.
The 1-, 3-, and 5-yr recurrence-free survival (RFS) rates for advanced AoV cancer were 82.8%, 48.3%, and 40.8%, respectively, and the overall survival (OS) rates were 89.7%, 62.1%, and 51.7%, respectively. The median RFS and OS durations were 34 and 64 mo, respectively (Figure 1). Tables 1 and 2 show the results of the univariate and multivariate analyses for RFS and OS in patients with advanced AoV cancer. Patients with positive lymphovascular invasion (LVI) had a significantly higher risk of RFS [hazard ratio (HR): 2.971, confidence interval (CI): 1.123-7.861, P = 0.028) and OS (HR: 3.35, CI: 1.226-9.153, P = 0.018, respectively). However, other factors such as age; tumor size; T stage; LN metastasis; perineural invasion (PNI); differentiation, and CCRT; and biochemical markers such as, bilirubin, CEA, CA19.9, CRP, albumin, platelet-to-lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR), were not significantly associated with RFS or OS in the univariate analysis. In the multivariate analysis, while LVI did not have a statistically significant impact on RFS or OS, T stage and LN metastasis were significant prognostic factors for OS (HR: 3.015, CI: 0.989-9.095, P = 0.052; HR: 3.702, CI: 1.116-12.283, P = 0.032, respectively).
| Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (male) | 1.988 (0.568-6.955) | 0.282 | ||
| Age | 0.976 (0.939-1.014) | 0.215 | ||
| Size | 0.816 (0.530-1.255) | 0.354 | ||
| T stage | 1.808 (0.485-6.743) | 0.378 | 1.952 (0.499-7.628) | 0.326 |
| LN metastasis (positive) | 2.026 (0.747-5.498) | 0.166 | 1.848 (0.522-6.541) | 0.341 |
| LVI (positive) | 2.971 (1.123-7.861) | 0.028a | 2.002 (0.589-6.806) | 0.266 |
| PNI (positive) | 1.026 (0.395-2.663) | 0.958 | ||
| Differentiation (poor) | 1.366 (0.504-3.706) | 0.540 | ||
| CCRT | 1.543 (0.594-4.006) | 0.373 | ||
| Bilirubin | 0.935 (0.864-1.01) | 0.089 | ||
| CEA (≥ 5 ng/mL) | 0.429 (0.056-3.288) | 0.416 | ||
| CA19.9 (≥ 39 U/L) | 0.635 (0.225-1.796) | 0.392 | ||
| CRP (≥ 0.5 mg/mL) | 1.139 (0.367-3.536) | 0.822 | ||
| Albumin (< 3.3 or > 5.2 mg/dL) | 0.903 (0.119-6.831) | 0.922 | ||
| PLR | 1.001 (0.997-1.005) | 0.661 | ||
| NLR | 0.999 (0.871-1.144) | 0.983 | ||
| Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (male) | 1.752 (0.5667-5.416) | 0.33 | ||
| Age | 0.992 (0.9545-1.031) | 0.693 | ||
| Size | 0.924 (0.6395-1.334) | 0.672 | ||
| T stage | 2.102 (0.6376-6.927) | 0.222 | 3.015 (0.989-9.159) | 0.052 |
| LN metastasis (positive) | 2.596 (0.967-6.968) | 0.058 | 3.702 (1.116-12.283) | 0.032a |
| LVI (positive) | 3.35 (1.226-9.153) | 0.018a | 1.806 (0.549-5.941) | 0.33 |
| PNI (positive) | 1.344 (0.5331-3.39) | 0.531 | ||
| Differentiation (poor) | 1.398 (0.5406-3.617) | 0.489 | ||
| CCRT | 1.073 (0.423-2.724) | 0.881 | ||
| Bilirubin | 0.966 (0.8975-1.04) | 0.359 | ||
| CEA (≥ 5 ng/mL) | 0.423 (0.0555-3.226) | 0.407 | ||
| CA19.9 (≥ 39 U/L) | 1.372 (0.475-3.963) | 0.559 | ||
| CRP (≥ 0.5 mg/mL) | 3.503 (0.7991-15.35) | 0.096 | ||
| Albumin (< 3.3 or > 5.2 mg/dL) | 3.088 (0.6807-14.01) | 0.144 | ||
| PLR | 1.003 (0.9991-1.007) | 0.128 | ||
| NLR | 1.061 (0.9529-1.18) | 0.282 | ||
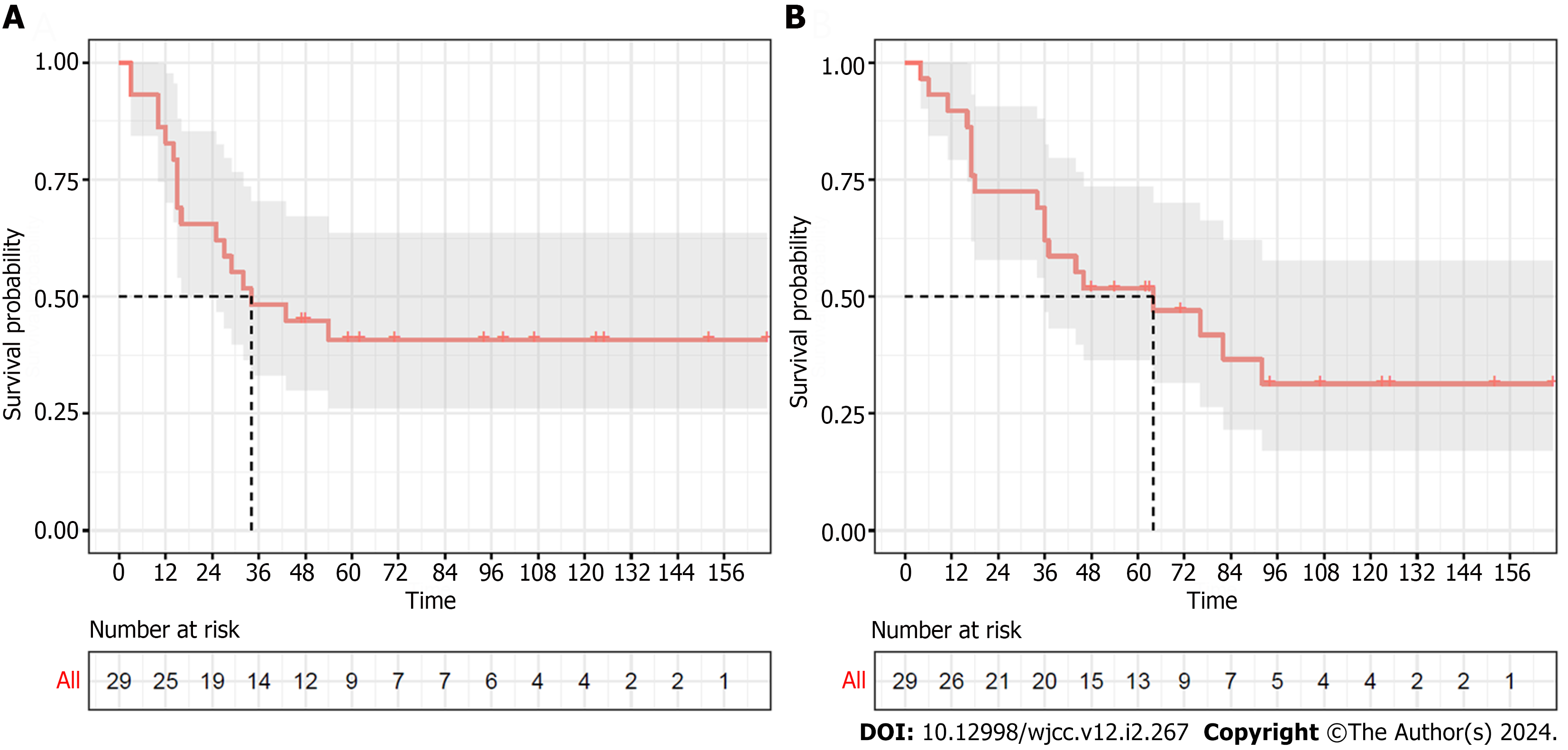
Patients with advanced AoV cancer were divided into two groups according to whether they received CCRT: patients who received adjuvant CCRT (CCRT, n = 11) and those who did not (non-CCRT, n = 18). The clinical features were then compared between these two groups. As shown in Figure 2A, the 3-yr and 5-yr RFS rates were 55.6% and 50.0%, respectively, in the non-CCRT group, and 36.4% and 27.3%, respectively, in the CCRT group. The median RFS in the non-CCRT and CCRT groups was 43 and 32 mo, respectively. The 3-yr and 5-yr OS rates were 55.6% and 55.6% in the non-CCRT group and 72.7% and 45.5% in the CCRT group, respectively. The median OS durations of the non-CCRT and CCRT groups were 76 and 46 mo, respectively (Figure 2B). The patients who had received CCRT showed worse RFS and OS than those who did not receive CCRT, but the difference was not statistically significant (P = 0.37, P = 0.89, respectively). Table 3 shows the patient characteristic in the CCRT and non-CCRT groups. The average of age was significantly lower in the CCRT group than in the non-CCRT group (59.0 ± 8.3 vs 71.22 ± 12.17, P = 0.007). Additionally, the PLR was significantly higher in the CCRT group (P = 0.044). However, there were no significant differences in sex, tumor size, LN metastasis, LVI, PNI, CEA, CA19.9, CRP, albumin, NLR, American Society of Anesthesiologists grade, or transfusion between the two groups.
| Characteristics | CCRT (n = 11) | Non-CCRT (n = 18) | Total (n = 29) | P value |
| Sex (%) | 0.647 | |||
| Male | 9 (81.8) | 12 (66.7) | 21 (72.4) | |
| Female | 2 (18.2) | 6 (33.3) | 8 (27.6) | |
| Age, median (IQR) | 60 (52; 63) | 75.5 (63.3; 79.5) | 65 (60; 76) | 0.007b |
| Size, median (IQR) | 2.9 (1.9; 3.8) | 2.7 (2.2; 3.0) | 2.7 (2.1; 3.2) | 0.928 |
| T stage (%) | 0.200 | |||
| 2 | 3 (27.3) | 2 (11.1) | 5 (17.2) | |
| 3 | 7 (63.6) | 16 (88.9) | 23 (79.3) | |
| 4 | 1 (9.1) | 0 (0.0) | 1 (3.4) | |
| LN metastasis (%) | 1.000 | |||
| Negative | 5 (45.5) | 9 (50.0) | 14 (48.3) | |
| Positive | 6 (54.5) | 9 (50.0) | 15 (51.7) | |
| LVI (%) | 1.000 | |||
| Negative | 7 (63.6) | 12 (66.7) | 19 (65.5) | |
| Positive | 4 (36.4) | 6 (33.3) | 10 (34.5) | |
| PNI (%) | 0.661 | |||
| Negative | 5 (45.5) | 11 (61.1) | 16 (55.2) | |
| Positive | 6 (54.5) | 7 (38.9) | 13 (44.8) | |
| Differentiation (%) | 0.076 | |||
| Well-to-moderate | 4 (36.4) | 14 (77.8) | 18 (62.1) | |
| Poor | 6 (54.5) | 3 (16.7) | 9 (31.0) | |
| Unknown | 1 (9.1) | 1 (5.6) | 2 (6.9) | |
| Bilirubin | 4.6 (1.3; 11.9) | 2.8 (0.5; 13.7) | 4.1 (0.9; 12.9) | 0.515 |
| CEA (%) | 1.000 | |||
| < 5 ng/mL | 8 (88.9) | 14 (87.5) | 22 (88.0) | |
| ≥ 5 ng/mL | 1 (11.1) | 2 (12.5) | 3 (12.0) | |
| CA19.9 (%) | 0.866 | |||
| < 39 U/L | 3 (30.0) | 7 (41.2) | 10 (37.0) | |
| ≥ 39 U/L | 7 (70.0) | 10 (58.8) | 17 (63.0) | |
| CRP (%) | 0.297 | |||
| < 0.5 mg/dL | 1 (11.1) | 7 (38.9) | 8 (29.6) | |
| ≥ 0.5 mg/dL | 8 (88.9) | 11 (61.1) | 19 (70.4) | |
| Albumin (%) | 1.000 | |||
| 3.3-5.2 g/dL | 10 (90.9) | 17 (94.4) | 27 (93.1) | |
| < 3.3 or > 5.2 g/dL | 1 (9.1) | 1 (5.6) | 2 (6.9) | |
| PLR, median (IQR) | 277.0 (215.1; 291.7) | 159.8 (124.1; 237.9) | 202.0 (133.9; 287.5) | 0.044a |
| NLR, median (IQR) | 4.1 (3.3; 4.5) | 2.7 (2.2; 4.7) | 3.6 (2.3; 4.6) | 0.334 |
| ASA | 0.105 | |||
| I | 3 (27.3) | 0 (0.0) | 3 (10.3) | |
| II | 6 (54.5) | 11 (61.1) | 17 (58.6) | |
| III | 2 (18.2) | 6 (33.3) | 8 (27.6) | |
| IV | 0 (0.0) | 1 (5.6) | 1 (3.4) |
After tumor resection, the prognosis of patients with AoV cancer is generally better than that of patients with pancreatic cancer; however, poor survival rates have been observed in patients with advanced-stage cancer. Adjuvant treatment is often used in these cases, and this study evaluated the effectiveness of adjuvant CCRT in patients with AoV cancer with poor prognostic factors, such as high T stage or LN metastases. However, our findings suggest that adjuvant CCRT may not provide any survival benefits in patients with advanced AoV cancer.
While some studies suggest that adjuvant chemotherapy (ACT) provides a survival benefit for patients with advanced AoV cancer, studies such as the ESPAC-3[7] and Al Abbas et al[12] have some limitations, including heterogeneity in the study population and variations in the chemotherapy drugs used. To date, most studies have used 5-FU- or gemcitabine-based chemotherapy as ACT. In this study, FLv or GP regimens were used, but no difference in the survival benefit was observed. Some studies have compared the efficacy of 5-FU- and gemcitabine-based regimens. In a subgroup analysis of the ESPAC-3 study population, the patients who received gemcitabine showed a survival benefit, whereas no benefit was observed among those who received 5-FU with folinic acid[7]. Conversely, in a study by Al Abbas et al[12], 5-FU led to better survival rates than gemcitabine in patients with advanced-stage disease. Therefore, an optimal treatment regimen for patients with AoV cancer is yet to be established.
Recent studies have evaluated the different effects of ACT regimens on survival outcomes based on histological subtypes of AoV cancer[9,12,13]. While 5-FU- and gemcitabine-based regimens may be useful for certain histological subtypes, Al Abbas et al[12] reported that 5-FU-based ACT improved survival regardless of the subtype. However, Ecker et al[9] found no survival benefit of ACT regardless of the histological type or advanced stage. The lack of an association between chemotherapeutic agents and histological type presents a challenge in evaluating the efficacy of ACT. Although the benefits of different chemotherapy regimens according to histologic type have been suggested, the small number of patients in each subset and poor reproducibility of histologic typing are considerable limitations. In this study, most of the patients had not undergone phenotypic classification when receiving adjuvant therapy; therefore, treatment decisions were not based on histological subtypes.
The effectiveness of CCRT in patients with advanced-stage AoV cancer is controversial, despite some studies demonstrating its benefits. The Mayo Clinic reported improved disease relapse and survival rates following CCRT[4]. In addition, Nassour et al[11] showed that CCRT was associated with improved survival compared with observation only. In contrast, studies by Ecker et al[9] and Kim et al[10,14] found no significant differences in survival or recurrence rates, regardless of the addition of radiotherapy. Therefore, the use of CCRT in clinical practice should be carefully considered, as this study highlights that CCRT does not have survival benefits for patients with advanced AoV cancer.
Our study found that age and PLR were correlated with the administration of adjuvant CCRT, with older patients and those with a low PLR being less likely to receive this treatment. These findings are consistent with those reported by the Mayo Clinic regarding age. However, these factors should not be used as the criteria for selecting patients for CCRT, as our study results did not show a significant difference in the survival outcomes between the CCRT and non-CCRT groups.
This study had limitations. First, this was a single-center, retrospective, non-randomized study with a small sample size, which should be taken into consideration when interpreting the results. The limited sample size is attributed to the specificity of the disease, and particularly in advanced stages, the target group inevitably diminishes. Therefore, further confirmation of the findings is warranted through multi-institutional research. Second, the chemotherapy regimens used were not standardized and the histological type was not evaluated. Third, a large proportion of the patient population received palliative treatment, which may have influenced the OS outcomes.
In conclusion, our study provides some evidence that CCRT may not provide a survival benefit to patients with advanced AoV cancer. Therefore, further research is required to address the limitations of the current study and provide definitive answers regarding the role of CCRT in the treatment of advanced AoV cancer.
Although ampulla of Vater (AoV) cancer has a relatively favorable prognosis among periampullary cancers, the 5-yr survival rate after resection remains poor. The benefits of adjuvant treatment for AoV cancer is still controversial, leading to the prevalent use of chemotherapy or concurrent chemoradiotherapy (CCRT).
Despite clinical use of adjuvant treatment, there are no consensus guidelines for patients with AoV carcinoma. This study aims to contribute valuable insights into the survival benefits of CCRT in patients with advanced AoV cancer, providing evidence to guide treatment decisions.
The study aims to retrospectively assess the efficacy of adjuvant CCRT in patients with advanced AoV carcinoma who underwent curative resection.
Eleven patients with advanced AoV cancer [T3/T4 or lymph node (LN) metastases] who underwent adjuvant CCRT, and 18 patients who did not receive adjuvant therapy were retrospectively reviewed.
The study found 1-, 3-, and 5-yr RFS rates of 82.8%, 48.3%, and 40.8%, and OS rates of 89.7%, 62.1%, and 51.7%, respectively, for advanced AoV cancer. T stage and LN metastasis were significantly associated with OS in the multivariate analysis. However, CCRT did not show a statistically significant survival advantage.
This study suggests that adjuvant CCRT may not provide survival benefits for patients with advanced AoV cancer.
Additional multi-institutional studies with larger sample sizes, standardized regimens, and histological evaluations are recommended to identify the optimal options for patients with advanced AoV cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Wang K, China; Yang F, China S-Editor: Gao CC L-Editor: A P-Editor: Cai YX
| 1. | Berberat PO, Künzli BM, Gulbinas A, Ramanauskas T, Kleeff J, Müller MW, Wagner M, Friess H, Büchler MW. An audit of outcomes of a series of periampullary carcinomas. Eur J Surg Oncol. 2009;35:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | O'Connell JB, Maggard MA, Manunga J Jr, Tomlinson JS, Reber HA, Ko CY, Hines OJ. Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol. 2008;15:1820-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Sakata J, Shirai Y, Wakai T, Yokoyama N, Sakata E, Akazawa K, Hatakeyama K. Number of positive lymph nodes independently affects long-term survival after resection in patients with ampullary carcinoma. Eur J Surg Oncol. 2007;33:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Jin Z, Hartgers ML, Sanhueza CT, Shubert CR, Alberts SR, Truty MJ, Muppa P, Nagorney DM, Smyrk TC, Hassan M, Mahipal A. Prognostic factors and benefits of adjuvant therapy after pancreatoduodenectomy for ampullary adenocarcinoma: Mayo Clinic experience. Eur J Surg Oncol. 2018;44:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Zhao W, Wang B, Zhao A, Tian Q, Zhang L, Wang L, Zhao X, Yang J, Dong D. The role of radiotherapy in patients with resected ampullary carcinoma: findings based on the SEER database. HPB (Oxford). 2019;21:1535-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Miura JT, Jayakrishnan TT, Amini A, Johnston FM, Tsai S, Erickson B, Quebbeman EJ, Christians KK, Evans DB, Gamblin TC, Turaga KK. Defining the role of adjuvant external beam radiotherapy on resected adenocarcinoma of the ampulla of vater. J Gastrointest Surg. 2014;18:2003-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, Carter R, Tebbutt NC, Dervenis C, Smith D, Glimelius B, Charnley RM, Lacaine F, Scarfe AG, Middleton MR, Anthoney A, Ghaneh P, Halloran CM, Lerch MM, Oláh A, Rawcliffe CL, Verbeke CS, Campbell F, Büchler MW; European Study Group for Pancreatic Cancer. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 463] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 8. | Kwon J, Kim BH, Kim K, Chie EK, Ha SW. Survival Benefit of Adjuvant Chemoradiotherapy in Patients With Ampulla of Vater Cancer: A Systematic Review and Meta-analysis. Ann Surg. 2015;262:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Ecker BL, Vollmer CM Jr, Behrman SW, Allegrini V, Aversa J, Ball CG, Barrows CE, Berger AC, Cagigas MN, Christein JD, Dixon E, Fisher WE, Freedman-Weiss M, Guzman-Pruneda F, Hollis RH, House MG, Kent TS, Kowalsky SJ, Malleo G, Salem RR, Salvia R, Schmidt CR, Seykora TF, Zheng R, Zureikat AH, Dickson PV. Role of Adjuvant Multimodality Therapy After Curative-Intent Resection of Ampullary Carcinoma. JAMA Surg. 2019;154:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Kim HS, Jang JY, Yoon YS, Park SJ, Kwon W, Kim SW, Han HS, Han SS, Park JS, Yoon DS. Does adjuvant treatment improve prognosis after curative resection of ampulla of Vater carcinoma? A multicenter retrospective study. J Hepatobiliary Pancreat Sci. 2020;27:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Nassour I, Hynan LS, Christie A, Minter RM, Yopp AC, Choti MA, Mansour JC, Porembka MR, Wang SC. Association of Adjuvant Therapy with Improved Survival in Ampullary Cancer: A National Cohort Study. J Gastrointest Surg. 2018;22:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Al Abbas AI, Falvello V, Zenati M, Mani A, Hogg ME, Zeh HJ 3rd, Singhi A, Bahary N, Zureikat AH. Impact of adjuvant chemotherapy regimen on survival outcomes in immunohistochemical subtypes of ampullary carcinoma. J Surg Oncol. 2020;121:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Chang DK, Jamieson NB, Johns AL, Scarlett CJ, Pajic M, Chou A, Pinese M, Humphris JL, Jones MD, Toon C, Nagrial AM, Chantrill LA, Chin VT, Pinho AV, Rooman I, Cowley MJ, Wu J, Mead RS, Colvin EK, Samra JS, Corbo V, Bassi C, Falconi M, Lawlor RT, Crippa S, Sperandio N, Bersani S, Dickson EJ, Mohamed MA, Oien KA, Foulis AK, Musgrove EA, Sutherland RL, Kench JG, Carter CR, Gill AJ, Scarpa A, McKay CJ, Biankin AV. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Kim JH, Jeong JH, Ryoo BY, Kim KP, Chang HM, Oh D, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH, Park Y, Kwon JW, Hwang DW, Lee JH, Lee W, Kim SC, Yoo C, Song KB. Adjuvant Chemotherapy for Resected Ampulla of Vater Carcinoma: Retrospective Analysis of 646 Patients. Cancer Res Treat. 2021;53:424-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |