Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.256
Peer-review started: November 7, 2023
First decision: November 16, 2023
Revised: November 24, 2023
Accepted: December 25, 2023
Article in press: December 25, 2023
Published online: January 16, 2024
Processing time: 64 Days and 18.9 Hours
Myocardial infarction is a high-risk condition prevalent among the elderly popu
To evaluate the effect of individualized cardiac rehabilitation on blood pressure variability (BPV) and baroreflex sensitivity (BRS) in elderly patients with myo
A cohort of 74 elderly patients diagnosed with myocardial infarction and ad
Of 24 h diastolic BPV, 24 h systolic BPV, carbon dioxide ventilation equivalent slope of the research group were lower than those of the control group at T1 and T2, BRS, peak heart rate and systolic blood pressure product, 1 min heart rate recovery were higher than those of the control group, and the incidence of adverse events in the research group was lower than that of the control group, the difference was statistically significant (P < 0.05).
In this study, we found that after individualized cardiac rehabilitation in elderly patients with myocardial infarction, BPV and BRS can be effectively improved, cardiac function is significantly enhanced, and a better prognosis is obtained.
Core Tip: Myocardial infarction is a high-risk disease in the elderly, and reasonable cardiac rehabilitation nursing measures are extremely important. This paper aims to evaluate the effect of individualized cardiac rehabilitation on blood pressure variability (BPV) and baroreflex sensitivity (BRS) in elderly patients with myocardial infarction. Through randomized controlled trials and individualized cardiac rehabilitation, it was found that after individualized cardiac rehabilitation treatment, the BPV and BRS of elderly patients with myocardial infarction could be effectively improved, the cardiac function was significantly enhanced, and the prognosis was better. The results show that this nursing mode has important clinical nursing value.
- Citation: Liu HN, Gao B. Exploration of cardiac rehabilitation nursing for elderly patients with myocardial infarction based on individualized cardiac rehabilitation. World J Clin Cases 2024; 12(2): 256-266
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/256.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.256
Myocardial infarction refers to an acute condition in which the coronary arteries were blocked, the heart muscle ischemia had been caused necrosis, and the cardiac function was impaired. According to the 2021 Cardiovascular Health and Disease Report of China[1], the mortality rate of acute myocardial infarction in the urban areas of China from 2002 to 2018 increased from 16.46/100000 to 62.33/100000. In addition, the mortality rate in this realm of China has been increased year by year. It is important to explore the more effective intervention options for myocardial infarction. At present, the commonly used treatment options for patients with myocardial infarction include the drug treatment, surgical treatment and other programs. Drug therapy and surgery can exert good therapeutic effects, but there are obvious side effects of drug treatment[2], while the surgical treatment such as percutaneous coronary implantation (PCI) is expensive and unaffordable. In further situation, some patients will have the decreased cardiopulmonary function, anxiety and depression after this kind of surgery, which affects the patients’ life quality pretty much[3]. In recent years, the cardiac rehabilitation has gradually entered the field of clinicians’ research and the concerns of patients’ treatment.
The main contents of individualized cardiac rehabilitation include the comprehensive use of a variety of clinical strategies to help patients recover body functions under the premise of determining the patient’s cardiopulmonary function, including the assessment of health status, the guiding training of exercise intensity and exercise mode, the guidance of diet and lifestyle, as well as the improvement of patients’ compliance, making them to take drugs and to monitor their various health indicators in a regular manner. According to above methods, they can enhance the patient’s quality of life, strengthen the patient’s psychological state, make them back to the normal social life, and effectively prevent the recurrence of various cardiovascular events[4]. The core of cardiac rehabilitation is aerobic exercise, which is performed according to an individualized exercise program when implementing cardiac rehabilitation exercise pre
Blood pressure variability (BPV) is considered a new risk factor for cardiovascular disease. Previous studies have found that BPV will be a new independent risk factor for coronary atherosclerotic heart disease (coronary heart disease), myocardial infarction, stroke, heart failure, and all-cause mortality[7]. Systolic BPV (SBPV) has also been associated with the incidence of all-cause mortality, coronary heart disease, and stroke in numerous observational studies[8]; in addition, SBPV has shown that SBPV is an independent predictor factor of all-cause mortality, cardiovascular incidence, car
In this single-center and randomized controlled trial (RCT), we randomly selected 74 patients with myocardial infarction admitted to our hospital from January 2021 to January 2022. Inclusion criteria: (1) Met the relevant diagnostic criteria for myocardial infarction[14]; (2) The stable condition, clear consciousness, walking easily; and (3) Age ≥ 60 years. Exclusion criteria: (1) The acute left heart failure, acute exacerbation of chronic cardiac insufficiency, the killip class in 3-4 of cardiac function, congenital heart disease, hypertrophic cardiomyopathy, restrictive cardiomyopathy, vasculitis, dilated cardiomyopathy, acute myocarditis, aortic syndrome, acute pulmonary embolism, hypertension grade 3 very high-risk group blood pressure higher than 200/100 mmHg; (2) Other arrhythmias such as atrial fibrillation, atrial premature beat, atrioventricular block, ventricular arrhythmias; (3) The diseases of the musculoskeletal system such as joints and spine; and (4) Myocardial infarction with cerebral infarction, uremia, acute gastrointestinal bleeding, acute chronic obstructive pulmonary disease, pneumothorax, poor compliance or psychiatric disorders. This study was approved by the hospital’s ethics committee, and patient-level family members gave informed consent.
The control group was given the conventional medical treatment (aspirin enteric-coated tablets, clopidogrel tablets or ticagrelor tablets, atorvastatin calcium tablets or rosuvastatin calcium tablets, metoprolol sustained-release tablets, benazepril tablets, or perindopril tablets), and during the hospitalization observation period, the routine exercise guided by the routine nursing interventions, such as walking accompanied by others, etc. On the basis of the control group, the research group completed the 6-min walking test, and the exercise plate test within the first week of hospitalization, measured the maximum target heart rate and exercise tolerance, and formulated an individualized exercise prescription that accurately meets the actual situation of the patient according to the above data: (1) Exercise form: Warm up for 10 min to brisk walking (4-6 km/h) for 20-30 min, relax for 5-10 min; (2) Exercise intensity: Heart rate is kept within the target heart rate range; the self-feeling is subtly harder; and (3) Exercise frequency: At least once every other day. The patient’s exercise intensity is measured by the target heart rate law, that is, the heart rate during exercise is 60%-80% of the maximum target heart rate, and the heart rate control requirements of the oral β blockers can be lower than that of those without oral β blockers. What should be paid attention to when implementing the exercise of cardiac rehabilitation: Patients should carry the heart rate oximeters, wear sports bracelets or sports watches, and dynamically monitor their heart rate at any time; carrying a record of their name and address, family number, name of their illness, and first-aid medicines such as nitrates (such as nitroglycerin tablets). If chest tightness, chest pain and other symptoms occur during exercise, stop exercising immediately, and take nitroglycerin tablets under the tongue, and tell the family in time to avoid accidents.
BPV: Spacelabs90217 ambulatory blood pressure monitor was used to monitor the blood pressure level of the control group before and after treatment the next day and the AMI group. The daytime detection time is 08:00-22:00, the night detection time is 22:00-08:00, blood pressure is measured and recorded once every half hour, BPV is calculated as the standard deviation of systolic or diastolic blood pressure, and the diastolic BPV (24DBPSD) and 24 h SBPV (24SBPSD) of each group are counted.
BRS: The machine used to measure the BRS in this study is the continuous finger pulse pressure measuring instrument (Finometer, Finapres Medical systems, Netherlands). The patient did not take tea, coffee or other irritating food and beverages on the day of the examination, did not smoke 30 minutes before the examination, and emptied the bladder. The research subject laying quietly and flat, being measured the circumference of the second knuckle of the middle finger of the right hand with a special soft ruler, selecting a special pressure-sensing finger sleeve that matched it, wearing a cuff 1-2 cm on the elbow socket of the right arm to detect blood pressure, and connecting the finger sleeve and sleeving with the signal receiving end. Preheating and stepping up the machine and entering the patient’s personal information, including age, gender, height, weight, etc. Starting the measurement when all prepares are readied, firstly performing the physiological correction, and measuring the blood pressure of the right upper extremity twice, and correcting the finger pulse signal simulated blood pressure. After the pulse wave is stable and the physiological correction is completed (about 10 min), the detection will be officially started. Taking the supine position, instructing the patient to breathe calmly, avoiding severe coughing, moving or loud specking, and continuously collecting information for 5 min. The built-in analysis software (Beatscope) was used to derive continuous systolic blood pressure, diastolic blood pressure, heart rate, and calculating the BRS in the lying position, taking the above average.
Cardiopulmonary function indicators: After the start of intervention, T0, T1 and T2 were subjected to the cardiopulmonary exercise test in these two groups, and the changes of the peak heart rate and systolic blood pressure product (peak RPP), the 1 min heart rate recovery (HRR1), and the carbon dioxide ventilation equivalent slope (VE/VCO2 slope) were collected and compared. The process of cardiopulmonary exercise test: The exercise cardiopulmonary function test instrument produced by the Italian company COSMED is used to implement it with the help of power bicycles. Patients were smoke-free, liquor, tea and coffee 12 h before the test. Before testing, the gas flow, capacity and concentration are calibrated. The patient takes the riding position and adjusts the seat height. Connecting a 12-lead ECG, blood pressure monitor, and finger oximeter, wearing a mask, and checking that there are no air leaks before connecting to the gas analysis system. According to the individualized indicators of patients, a ramp-type power ramp test (Ramp) protocol of 10-20 W/min was selected. First resting for 3 min, then pedaling at 60 ± 5 r/min zero load for 3 min, continue to exercise at this speed, during which the treadmill power increases rampantly at a speed of 10-20 W/min, until any of the indications for termination of symptomatic restrictive exercise test are reached, then the exercise load is unloaded.
Adverse cardiovascular events: After the patients were discharged from the hospital after the intervention, these two groups of patients were followed up for six months, and the adverse cardiovascular events such as arrhythmia, angina pectoris, and myocardial infarction were statistically counted, and the incidence differences between these two groups were compared.
SPSS22.0 statistical software was used to analyze and process the collected data. The measurement data were confirmed to conform to the homogeneity of variance and approximately obey the normal distribution after the homogeneity test and normality test, expressed by the mean ± SD, t-test and repeated measurement design ANOVA, counting data expressed as percentages, χ2 test and rank sum test, and the difference was statistically significant in P < 0.05.
A total of 74 elderly patients with myocardial infarction were included in this study. Among them, the average age is 68.64 ± 7.36 years old, the average body mass index body mass index (BMI) is 24.52 ± 2.03 kg/m², and the average Gensini score is (55.95 ± 3.41) points. There are 60.81% men, 39.19% women, and 67.56% patients There was a history of smoking in the past, 74.32% of the patients underwent PCI, 45.94% of the patients were complicated with hypertension, 29.72% of the patients were complicated with diabetes, 78.37% of the patients had previously used β-blockers, and 33.78% of the patients had previously used diuretics. 70.27% of the patients used ACEI drugs in the past, and 6.75% of the patients used CCB drugs in the past. In addition, 74.32% of the patients were Killip classⅠ, and 25.68% of the patients were Killip class Ⅱ.
Compared with the control group, there were no significant differences in age, gender, BMI, smoking history, PCI, hypertension, diabetes, the Killip classification, the Gensini score, and medication history in the research group (P > 0.05) (Table 1).
| Item | Research group (n = 37) | Control group (n = 37) | t/χ2/Z | P value |
| Age (yr) | 68.36 ± 7.19 | 68.92 ± 7.62 | 0.325 | 0.746 |
| Gender | 0.056 | 0.811 | ||
| Man | 22 (59.46) | 23 (62.16) | ||
| Woman | 15 (40.54) | 14 (37.84) | ||
| BMI (kg/m²) | 24.69 ± 2.12 | 24.35 ± 1.94 | 0.719 | 0.474 |
| Smoking history | 26 (70.27) | 24 (29.73) | 0.246 | 0.619 |
| The PCI surgery | 27 (72.97) | 28 (75.67) | 0.070 | 0.790 |
| Complication | ||||
| Hypertension | 18 (48.65) | 16 (43.24) | 0.217 | 0.640 |
| Diabetes | 10 (27.02) | 12 (32.43) | 0.258 | 0.610 |
| The Killip classification | ||||
| The I class | 28 (75.67) | 27 (72.97) | 0.235 | 0.797 |
| The II class | 9 (24.32) | 10 (27.02) | ||
| The Gensini score | 55.75 ± 3.82 | 56.16 ± 2.99 | 0.514 | 0.608 |
| Medication history | ||||
| β-blockers | 28 (75.67) | 30 (81.08) | 0.318 | 0.572 |
| Diuretic | 12 (32.43) | 13 (35.13) | 0.060 | 0.805 |
| ACEI | 25 (67.56) | 27 (72.97) | 0.258 | 0.610 |
| CCB | 2 (5.40) | 3 (8.10) | 0.214 | 0.643 |
The differences in the inter-group comparison and the time comparison of 24DBPSD and 24SBPSD between these two groups were statistically significant (P < 0.05), while the differences in their interactive comparison of inter-groups*time between these two groups were not statistically significant (P > 0.05). Compared 24DBPSD and 24SBPSD at T0 in these two groups, there were no significant difference (P > 0.05). When T1 and T2, 24DBPSD and 24SBPSD were lower than those when T0 in the same group, the difference was statistically significant (P < 0.05). When 24DBPSD and 24SBPSD at T1 and T2 of the research group were lower than those of the control group, and the differences were statistically significant (P < 0.05) (Table 2, Figure 1).
| Item | Time | Research group (n = 37) | Control group (n = 37) | F/P value1 | F/P value2 | F/P value3 |
| 24DBPSD | T0 | 8.45 ± 0.62 | 8.57 ± 0.43 | 6.592/0.010 | 50.410/< 0.001 | 0.039/0.961 |
| T1 | 8.02 ± 0.23a,b | 8.14 ± 0.25b | ||||
| T2 | 7.83 ± 0.31a,b | 7.98 ± 0.27b | ||||
| 24SBPSD | T0 | 11.56 ± 1.05 | 11.74 ± 1.11 | 6.232/0.013 | 28.710/< 0.001 | 0.184/0.831 |
| T1 | 11.02 ± 0.79a,b | 11.35 ± 0.58b | ||||
| T2 | 10.48 ± 0.61a,b | 10.79 ± 0.57b |
The differences between the inter-group comparison of BRS and the time comparison of BRS in these two groups were statistically significant (P < 0.05), while the differences between the interactive comparison of inter-group and time in these groups was not statistically significant (P > 0.05). There was no significant difference in the comparison of BRS between these two groups at T0 (P > 0.05). The BRS at T1 and T2 in these two groups were higher than the BRS at T0 in the same group, and the difference was statistically significant (P < 0.05). BRS in T1 and T2 were higher than those in the control group, and the difference was statistically significant (P < 0.05) (Table 3, Figure 2).
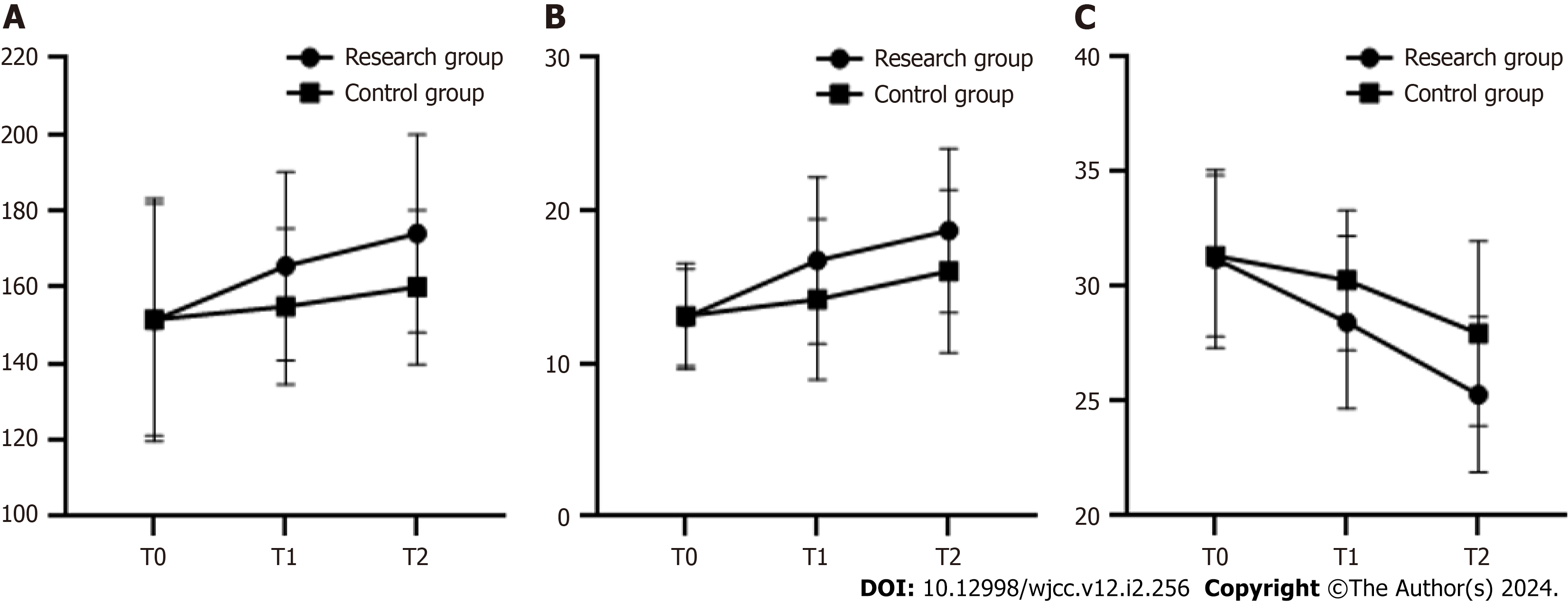
The differences in the inter-group comparison and the time comparison of peak RPP, HRR1, VE/VCO2 slope in these two groups were statistically significant (P < 0.05) but the interactive comparison of inter-group and time in these elements of these two groups were not statistically significant (P > 0.05). Compared with peak RPP, HRR1 and VE/VCO2 slope at T0, there was no significant difference between these two groups (P > 0.05). The difference was statistically significant when peak RPP and HRR1 were higher than those in the same group at T0, but VE/VCO2 slope were lower than those in the same group at T0, in both groups at T1 and T2 (P < 0.05). The peak RPP and HRR1 were higher in the study group than those in the control group at T1 and T2, and VE/VCO2 slope were lower than those in the control group, and the difference was statistically significant (P < 0.05) (Table 4, Figure 3).
| Item | Time | Research group (n = 37) | Control group (n = 37) | F/P value1 | F/P value2 | F/P value3 |
| Peak RPP (beat mmHg/100) | T0 | 151.36 ± 31.74 | 151.53 ± 30.39 | 5.482/0.020 | 6.732/0.001 | 1.512/0.223 |
| T1 | 165.53 ± 24.67a,b | 154.96 ± 20.43 | ||||
| T2 | 174.07 ± 25.92a,b | 160.02 ± 20.15b | ||||
| HRR1 (beat/min) | T0 | 13.02 ± 3.18 | 13.11 ± 3.46 | 7.079/0.008 | 15.040/< 0.001 | 1.964/0.142 |
| T1 | 16.74 ± 5.45a,b | 14.19 ± 5.25 | ||||
| T2 | 18.68 ± 5.37a,b | 16.03 ± 5.32 | ||||
| VE/VCO2 slope | T0 | 31.21 ± 3.88 | 31.34 ± 3.52 | 10.110/0.001 | 30.900/< 0.001 | 2.369/0.096 |
| T1 | 28.44 ± 3.76a,b | 30.27 ± 3.05 | ||||
| T2 | 25.29 ± 3.38a,b | 27.96 ± 4.02b |
The follow-up results showed that the incidence of adverse events such as angina, arrhythmia, and reinfarction in the study group was 5.40% (2/37), and the incidence of adverse event in the control group was 27.02% (10/37), and the incidence of adverse event in the study group was lower than that in the control group, and the difference was statistically significant (χ² = 6.365, P = 0.011) (Figure 4).
In this study, when the research group T1 and T2, 24DBPSD, 24SBPSD and VE/VCO2 slope were lower than those in the control group. BRS, peak RPP and HRR1 were higher than those in the control group, and the incidence of adverse event in the research group were lower than those in the control group, and the difference was statistically significant (P < 0.05). This shows that individualized cardiac rehabilitation can effectively improve BPV and BRS in elderly patients with myocardial infarction, enhance cardiac function and improve prognosis.
The post-myocardial infarction cardiac autonomic dysfunction is closely related to the occurrence of sudden cardiac death[15], manifested by increased sympathetic activity, decreased ventricular fibrillation value, decreased vagus nerve activity, and weakened protective effect on the heart, resulting in malignant ventricular arrhythmias, accounting for about 50% of patients who died early after myocardial infarction[16]. In addition, increased vagal tone in the heart can cause hyperpolarization of pacing cells and a slowdown in the rate of automatic depolarization, resulting in a decrease in heart rate. In experimental animals[17], stimulation of the vagus nerve elicits a short delay (approximately 150 ms) in cardiac chronotropic response. When sympathetic tension increases, the norepinephrine released by nerve endings acts on each ion channel, increasing the automatic depolarization rate of pacing cells during diastolic and accelerating the heart rate. The heart rate responds to changes in sympathetic tone with a long delay (about 1-2 s) and reaches a steady state about 30-60 s after the change. As a result, the heart responds much more rapidly to the vagus nerve, and the heart rate changing effect of the baroreflex is primarily mediated by the vagus nerve[18]. Studies in normal people have shown that the time difference from elevated arterial pressure to reflex bradycardia is approximately 475 ms, i.e., adjustment can be completed in one cardiac cycle[19]. In some pathological states, the mechanism of baroreflex is abnormal, impairing its tensile effect on cardiovascular regulation, resulting in increased sympathetic tone and decreased vagal tone.
BPV refers to the degree to which blood pressure fluctuates over a certain period of time. BPV has been strongly associated with adverse cardiovascular outcomes in the literature[20,21]. Potential mechanisms may include: First, increased BPV may be associated with stiffness and decreased compliance of large arteries; second, sympathetic activity is associated with BPV, and may be a determinant of BPV. The temporary instability in blood pressure may reflect the increased sympathetic activity with increased vascular risk; third, limited animal experimental data suggest that increased BPV leads to endothelial damage, activation of the renal angiotensin system, and accelerated apoptosis of cardiomyocytes, leading to end-organ damage.
BRS, on the other hand, reflects the balance of autonomic nerves. Decreased BRS indicates a loss of sympathetic vagus balance and decreased vagus modulation. Experiments by Cerati and Schwartz[22] demonstrated that BRS can quantitatively reflect the regulatory ability of the vagus nerve of the heart. They made an acute experimental myocardial ische
Cardiac rehabilitation exercise therapy is to improve lifestyle, by appropriate activities and exercise, especially moderate intensity aerobic exercise, to reduce, eliminate and improve the adverse effects of absolute bed rest, in order for patients to recover their physical health faster and better, while receiving the five major prescriptions to improve patients’ awareness of the disease, reasonable arrangement of nutritional combination, avoid staying up late overwork, improve psychological quality, in order to get a real sense of rehabilitation[25]. In this study, individualized cardiac rehabilitation can significantly improve BPV and BRS, and its pathophysiology may be based on: When patients have coronary artery occlusion or stenosis, myocardial cells will be ischemic and hypoxic, cardiomyocyte necrosis and apoptosis in a short period of time, vagus nerve and sympathetic nerve can’t get normal nutrient supply, regulatory function is correspondingly impaired, and the risk of malignant arrhythmias and sudden cardiac death will increase. Numerous studies have shown that cardiac rehabilitation exercises can improve cardiovascular endothelial function, increase coronary collateral circulation, increase coronary perfusion, and improve exercise tolerance in patients[26,27]. The ischemia and hypoxia of cardiomyocytes are significantly improved, which enhances the activity of cardiac autonomic regulation, helps to promote the recovery of cardiac function, improves myocardial contractility, and increases ventricular diastolic filling.
Grässler et al[28] found that exercise can increase heart rate variability in older adults. Other studies have found that heart rate variability indexes were significantly increased in the group of patients with coronary heart disease who had physical activity habits[29]. In a six-year RCT study of middle-aged adults, routine light to moderate-intensity exercise improved autonomic function[30]. Exercise can reflexively increase the activity of the cerebral cortex and hypothalamus, make the sympathetic nerve and vagus nerve in a higher state of equilibrium, strengthen the regulatory effect of autonomic nerves on cardiac function, improve the myocardial ischemic state of patients with coronary heart disease, increase oxygen supply, enhance myocardial contractility, and increase cardiac output[31]. The corresponding increase of stroke volume (SV) and output per minute (CO) can increase systemic blood flow, transport more oxygen and nutrients to various organs and tissues of the body, increase oxygen utilization, and improve cardiopulmonary fitness[32]. On the other hand, the increase of SV and CO enhances coronary perfusion and promotes the cardiac autonomic regulatory[33]. Cardiac rehabilitation exercises improve the patient’s cardiopulmonary function, and regulate the balance state of the sympathetic and vagus nerves by improving the patient’s exercise tolerance, thereby restoring normal heart rate oscillation function and reducing the incidence of malignant arrhythmias and sudden cardiac death[34].
There are some limitations in this study. The insufficient sample size of the participants included in this study and the failure to further clarify and exclude factors that may affect BPV and BRS, these may have biased the results. It is necessary to further expand the sample size, improve the research, and further analyze and discuss the research results.
In summary, individualized cardiac rehabilitation in patients with myocardial infarction can significantly improve BPV and BRS, improve cardiac function and prognostic outcomes. These results can be applied in practice to provide new therapeutic targets for clinical decision-making of myocardial infarction.
The rapid development of medicine, such as myocardial infarction, cerebral apoplexy, severe trauma and cancer difficult severe patients can save survival, but at the same time left down different degrees of dysfunction and disability, dysfunction and disability is surgery and drugs is difficult to work, which highlights the importance of rehabilitation and rehabilitation care.
Individualized rehabilitation care can effectively improve the depressed mood and quality of life of elderly myocardial infarction patients.
This study aims to assess the impact of personalized cardiac rehabilitation on blood pressure variability and baroreflex sensitivity in geriatric patients diagnosed with myocardial infarction.
Implement individualized cardiac rehabilitation based on routine pharmacotherapy and nursing-guided interventions.
Data on the implementation of individualized rehabilitation treatments are significantly better than usual care inter
Myocardial infarction is a kind of high-risk disease in the elderly, so cardiac rehabilitation is of great significance.
Patients who have experienced myocardial infarction may exhibit negative affective states following surgery, which can impede their progress in physical rehabilitation. Therefore, conducting research on cardiac rehabilitation holds significant importance.
I would like to express my gratitude to all those helped me during the writing of this thesis. I acknowledge the help of my colleagues, they have offered me suggestion in academic studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiu HW, Taiwan S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH
| 1. | Writing committee of the report on cardiovascular health and diseases in China. Report on Cardiovascular Health and Diseases in China 2021: An Updated Summary. Biomed Environ Sci. 2022;35:573-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 71] [Reference Citation Analysis (2)] |
| 2. | Zhao J, Zeng Y, Shen X. Efficacy and safety of early initiation of Sacubitril/Valsartan in patients after acute myocardial infarction: A meta-analysis. Clin Cardiol. 2021;44:1354-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Zhang JZ, Wu HL, Chang PF, Huang KL. [Therapeutic effect of auriculotherapy with miniature bian needle on anxiety in the patients after percutaneous coronary intervention]. Zhen Ci Yan Jiu. 2021;46:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Zanini M, Nery RM, de Lima JB, Buhler RP, da Silveira AD, Stein R. Effects of Different Rehabilitation Protocols in Inpatient Cardiac Rehabilitation After Coronary Artery Bypass Graft Surgery: A RANDOMIZED CLINICAL TRIAL. J Cardiopulm Rehabil Prev. 2019;39:E19-E25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, Sanderson BK, Whooley MA. Home-Based Cardiac Rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69-e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (1)] |
| 6. | Rahmani R, Niyazi S, Sobh-Rakhshankhah A, Guazzi M, Mazaheri R, Hashemi N, Khoddami-Vishteh HR. Effects of a Cardiac Rehabilitation Program Versus Usual Care on Cardiopulmonary Function in Patients With Cardiac Syndrome X. J Cardiopulm Rehabil Prev. 2020;40:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Izar MC, Fonseca FAH. Blood Pressure Variability and Cardiovascular Risk in ELSA-Brasil: A Potential Surrogate Marker for Predicting Mortality and Cardiovascular Outcomes? Arq Bras Cardiol. 2022;119:512-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Bakkar NZ, El-Yazbi AF, Zouein FA, Fares SA. Beat-to-beat blood pressure variability: an early predictor of disease and cardiovascular risk. J Hypertens. 2021;39:830-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 582] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 10. | Soh MS, Park JS, Seo KW, Yang HM, Lim HS, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Visit-to-visit systolic blood pressure variability in patients with ST-elevation myocardial infarction predicts long-term cardiovascular outcomes. J Hum Hypertens. 2019;33:259-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Moštak I, Višnjić N, Junaković A, Krbot Skorić M, Habek M. Comparison of baroreflex sensitivity indices with standard tests of autonomic nervous system function. J Neurol Sci. 2021;426:117473. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Tomoto T, Repshas J, Zhang R, Tarumi T. Midlife aerobic exercise and dynamic cerebral autoregulation: associations with baroreflex sensitivity and central arterial stiffness. J Appl Physiol (1985). 2021;131:1599-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Hissen SL, Macefield VG, Brown R, Taylor CE. Sympathetic baroreflex sensitivity is inversely related to vascular transduction in men but not women. Am J Physiol Heart Circ Physiol. 2019;317:H1203-H1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 2491] [Article Influence: 355.9] [Reference Citation Analysis (1)] |
| 15. | Manolis AA, Manolis TA, Apostolopoulos EJ, Apostolaki NE, Melita H, Manolis AS. The role of the autonomic nervous system in cardiac arrhythmias: The neuro-cardiac axis, more foe than friend? Trends Cardiovasc Med. 2021;31:290-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Yang HJ, Kong B, Shuai W, Zhang JJ, Huang H. Knockout of MD1 contributes to sympathetic hyperactivity and exacerbates ventricular arrhythmias following heart failure with preserved ejection fraction via NLRP3 inflammasome activation. Exp Physiol. 2020;105:966-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Haberbusch M, Kronsteiner B, Kramer AM, Kiss A, Podesser BK, Moscato F. Closed-loop vagus nerve stimulation for heart rate control evaluated in the Langendorff-perfused rabbit heart. Sci Rep. 2022;12:18794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Capilupi MJ, Kerath SM, Becker LB. Vagus Nerve Stimulation and the Cardiovascular System. Cold Spring Harb Perspect Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 19. | Parati G, Di Rienzo M, Mancia G. How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J Hypertens. 2000;18:7-19. [PubMed] |
| 20. | Kaze AD, Santhanam P, Erqou S, Yuyun M, Bertoni AG, Ahima RS, Echouffo-Tcheugui JB. Long-Term Variability of Blood Pressure, Cardiovascular Outcomes, and Mortality: The Look AHEAD Study. Am J Hypertens. 2021;34:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Mallamaci F, Tripepi G, D'Arrigo G, Borrelli S, Garofalo C, Stanzione G, Provenzano M, De Nicola L, Conte G, Minutolo R, Zoccali C. Blood Pressure Variability, Mortality, and Cardiovascular Outcomes in CKD Patients. Clin J Am Soc Nephrol. 2019;14:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Cerati D, Schwartz PJ. Single cardiac vagal fiber activity, acute myocardial ischemia, and risk for sudden death. Circ Res. 1991;69:1389-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 132] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Seravalle G, Quarti-Trevano F, Dell'Oro R, Gronda E, Spaziani D, Facchetti R, Cuspidi C, Mancia G, Grassi G. Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. J Hypertens. 2019;37:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Sano N, Way D, McGrath BP. Renal norepinephrine spillover and baroreflex responses in evolving heart failure. Am J Physiol. 1990;258:F1516-F1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Dibben GO, Faulkner J, Oldridge N, Rees K, Thompson DR, Zwisler AD, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease: a meta-analysis. Eur Heart J. 2023;44:452-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 168] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 26. | do Nascimento DM, Machado KC, Bock PM, Saffi MAL, Goldraich LA, Silveira AD, Clausell N, Schaan BD. Cardiopulmonary exercise capacity and quality of life of patients with heart failure undergoing a functional training program: study protocol for a randomized clinical trial. BMC Cardiovasc Disord. 2020;20:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Borges JP, Mendes FSNS, Rangel MVDS, Lopes GO, da Silva GMS, da Silva PS, Mazzoli-Rocha F, Saraiva RM, de Sousa AS, Tibirica E, Mediano MFF. Exercise training improves microvascular function in patients with Chagas heart disease: Data from the PEACH study. Microvasc Res. 2021;134:104106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Grässler B, Thielmann B, Böckelmann I, Hökelmann A. Effects of different exercise interventions on heart rate variability and cardiovascular health factors in older adults: a systematic review. Eur Rev Aging Phys Act. 2021;18:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Jug B, Vasić D, Novaković M, Avbelj V, Rupert L, Kšela J. The Effect of Aquatic Exercise Training on Heart Rate Variability in Patients with Coronary Artery Disease. J Cardiovasc Dev Dis. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Tuomainen P, Peuhkurinen K, Kettunen R, Rauramaa R. Regular physical exercise, heart rate variability and turbulence in a 6-year randomized controlled trial in middle-aged men: the DNASCO study. Life Sci. 2005;77:2723-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Macagnan FE, Feoli AMP, Russomano T. Acute Physical Effort Increases Sympathovagal Balance Responses to Autonomic Stimulation in Metabolic Syndrome. Metab Syndr Relat Disord. 2019;17:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Li D, Chen P, Zhu J. The Effects of Interval Training and Continuous Training on Cardiopulmonary Fitness and Exercise Tolerance of Patients with Heart Failure-A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J. 2015;36:1974-182b. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 193] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 34. | Boyes NG, Marciniuk DD, Haddad H, Tomczak CR. Autonomic cardiovascular reflex control of hemodynamics during exercise in heart failure with reduced ejection fraction and the effects of exercise training. Rev Cardiovasc Med. 2022;23:72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |