Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.249
Peer-review started: September 19, 2023
First decision: October 24, 2023
Revised: November 7, 2023
Accepted: December 22, 2023
Article in press: December 22, 2023
Published online: January 16, 2024
Processing time: 113 Days and 13.2 Hours
Post-stroke epilepsy is a common and easily overlooked complication of acute cerebrovascular disease. Long-term seizures can seriously affect the prognosis and quality of life of patients. Electroencephalogram (EEG) is the simplest way to diagnose epilepsy, and plays an important role in predicting seizures and guiding medication.
To explore the EEG characteristics of patients with post-stroke epilepsy and improve the detection rate of inter-seizure epileptiform discharges.
From January 2017 to June 2020, 10 patients with post-stroke epilepsy in our hospital were included. The clinical, imaging, and EEG characteristics were collected. The stroke location, seizure type, and ictal and interictal EEG manifestations of the patients with post-stroke epilepsy were then retrospectively analyzed.
In all 10 patients, epileptiform waves occurred in the side opposite to the stroke lesion during the interictal stage; these manifested as sharp wave, sharp-wave complex, or spike discharges in the anterior head lead of the side opposite to the lesion.
In EEG, epileptiform waves can occur in the side opposite to the stroke lesion in patients with post-stroke epilepsy.
Core Tip: Post-stroke epilepsy refers to epileptic seizures occurring after stroke in patients without a history of epilepsy or any brain or systemic disease causes. Post-stroke epilepsy can occur any time after stroke. The most common type of post-stroke epilepsy is focal or tonic-clonic seizures, which then progress to bilateral clonic seizures. The present study retrospectively analyzed the electroencephalogram characteristics of 10 patients with post-stroke epilepsy; these mainly manifested as epilepsy-like waves on the side opposite to the stroke lesion during the intervals between seizures.
- Citation: Wen LM, Li R, Wang YL, Kong QX, Xia M. Electroencephalogram findings in 10 patients with post-stroke epilepsy: A retrospective study. World J Clin Cases 2024; 12(2): 249-255
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/249.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.249
Post-stroke epilepsy (PSE) refers to seizures that occur within a certain time after stroke in patients without a prior history of epilepsy or brain and systemic diseases. The epileptic discharges detected by electroencephalogram (EEG) are generally consistent with the lesion site of the stroke[1].
PSE can be divided into two categories based on the time between the stroke and the first seizure. Seizures that occur within 1 week after stroke are called “early-onset” seizures, also known as “acute symptomatic epilepsy.” Epileptic seizures that occur 1 wk or more after stroke are known as “late-onset” seizures, and are most common 6-12 mo after stroke. A later onset is associated with an increased risk of recurrent stroke, also known as “stroke-related epilepsy”[2]. The most common seizure types in PSE are focal seizures or tonic-clonic seizures that progress to bilateral focal seizures. Although generalized convulsive status epilepticus is very rare in PSE, nonconvulsive status epilepticus occurs in 4%-19% of patients with acute stroke[3,4]. Focal status epilepticus occurs occasionally, and epileptic seizures may be the first or only manifestation of acute stroke; they thus require prompt recognition and active management[4].
PSE is a common complication after acute cerebrovascular events, and is a common etiology in older patients with epilepsy. It can prolong the hospitalization time of patients with cerebrovascular diseases, increase disability and mortality rates, and affect the long-term prognosis and quality of life of patients[1,5]. EEG is the most objective auxiliary examination method for the diagnosis of PSE, and plays a very important role in epileptic seizure prediction, disease monitoring, drug selection, and prognosis evaluation[6]. In the present study, the special EEG findings of 10 PSE patients were retrospectively reviewed and are briefly reported.
From January 2017 to June 2020, data were collected from 10 patients with PSE in our hospital. Through retrospective analysis, their EEG abnormalities were noted as sharp or spike waves on the side opposite to the stroke lesion, which occurred between seizures. Of the 10 patients, there were seven males and three females aged 58 to 71 years. Five of the patients had cerebral hemorrhage and five had cerebral infarction. The study was conducted following the principles of the Helsinki Declaration and was approved by the Affiliated Hospital of Jining Medical University. Any images or data included in this article are anonymized, and written informed consent was not required.
The clinical data of the 10 patients — including age, sex, type and location of stroke, complications, time between stroke and first seizure, type of seizure, interictal EEG, ictal EEG, drug treatment, and prognosis—were analyzed retros
After admission, the patients were examined using 16-hour long-range video EEG. For the EEG (Nicolet V32, Natus Medical, Middleton, WI, United States), the scalp electrodes were placed according to the international 10-20 system estimation method. Bilateral earlobes or average leads were used as reference points, and bipolar leads were recorded. The patients were recorded during the open-close eyes test, hyperventilation, and photic stimulation activation.
Of the 10 patients with PSE (Table 1), two had early-onset epilepsy after cerebral infarction, with times between stroke and first seizure of 3 h and 2 d, respectively. The other eight patients had late-onset epilepsy, with times between stroke and first seizure ranging from 5 mo to 16 years. The seizures mostly manifested as focal seizures that evolved into bilateral tonic-clonic seizures, although one patient had non-convulsive seizures. The stroke site was located in the cerebral cortex in five cases, in the basal ganglia region in four cases, and in the corona radiata and centrum semiovale in one case.
| Case | Sex/age (yr) | Type of stroke | Location of stroke | Type of seizure | Time between the first seizure and stroke | Background EEG | Interictal EEG |
| Case 1 | Male/62 | CH | Right basal ganglia | Focal to bilateral tonic-clonic (motor) | 1 yr | 4-7 Hz slow waves in the right anterior head | High amplitude sharp waves in the left temporal lobe |
| Case 2 | Male/58 | CH | Right frontotemporal lobe | Generalized clonic (motor) | 7 yr | 9-10 Hz α rhythm, low amplitude in the right temporal lobe | Medium-low amplitude sharp waves and sharp-wave complex in lead F7 and T3, especially F7 |
| Case 3 | Male/71 | CH | Right basal ganglia | Focal to bilateral tonic-clonic (motor) | 16 yr | Slow waves in all the right leads | Epileptic waves in the left anterior head |
| Case 4 | Female/64 | CH | Right basal ganglia | Focal onset (nonmotor) | 2 d | Slow waves in the right temporal lobe | Paroxysmal sharp waves in the left temporal lobe |
| Case 5 | Female/64 | CH | Right frontoparietal lobe | Focal to bilateral tonic-clonic (motor) | 5 mo | 9-10 Hz α rhythm | Bilateral sharp waves and sharp-wave complex, especially left |
| Case 6 | Male/54 | CI | Right basal ganglia and temporoparietal lobe | Focal to bilateral tonic-clonic (motor) | 2 yr | 10-11 Hz α rhythm | Medium-high amplitude sharp waves in the left temporal lobe |
| Case 7 | Male/58 | CI | Left corona radiata and centrum semiovale | Focal to bilateral tonic-clonic (motor) | 5 yr | Low voltage in all the left leads | Low amplitude sharp-wave complex in lead Fp2, F4, F8 |
| Case 8 | Male/59 | CI | Left cerebral hemisphere | Focal onset (nonmotor) | 8 mo | Slow waves in lead O1, low voltage in all the left leads | Sharp wave and sharp-wave complex in lead Fp2, F4, C4, Cz |
| Case 9 | Female/58 | CI | Right frontal lobe and corpus callosum | Focal to bilateral tonic-clonic (motor) | 3 h | Slow waves in the right anterior head | Asynchronous sharp waves in the bilateral anterior head |
| Case 10 | Male/61 | CI | Right frontal temporal parietal lobe | Focal to bilateral tonic-clonic (motor) | 8 mo | Slow waves in lead O2 | Medium-high amplitude sharp waves in the left anterior head |
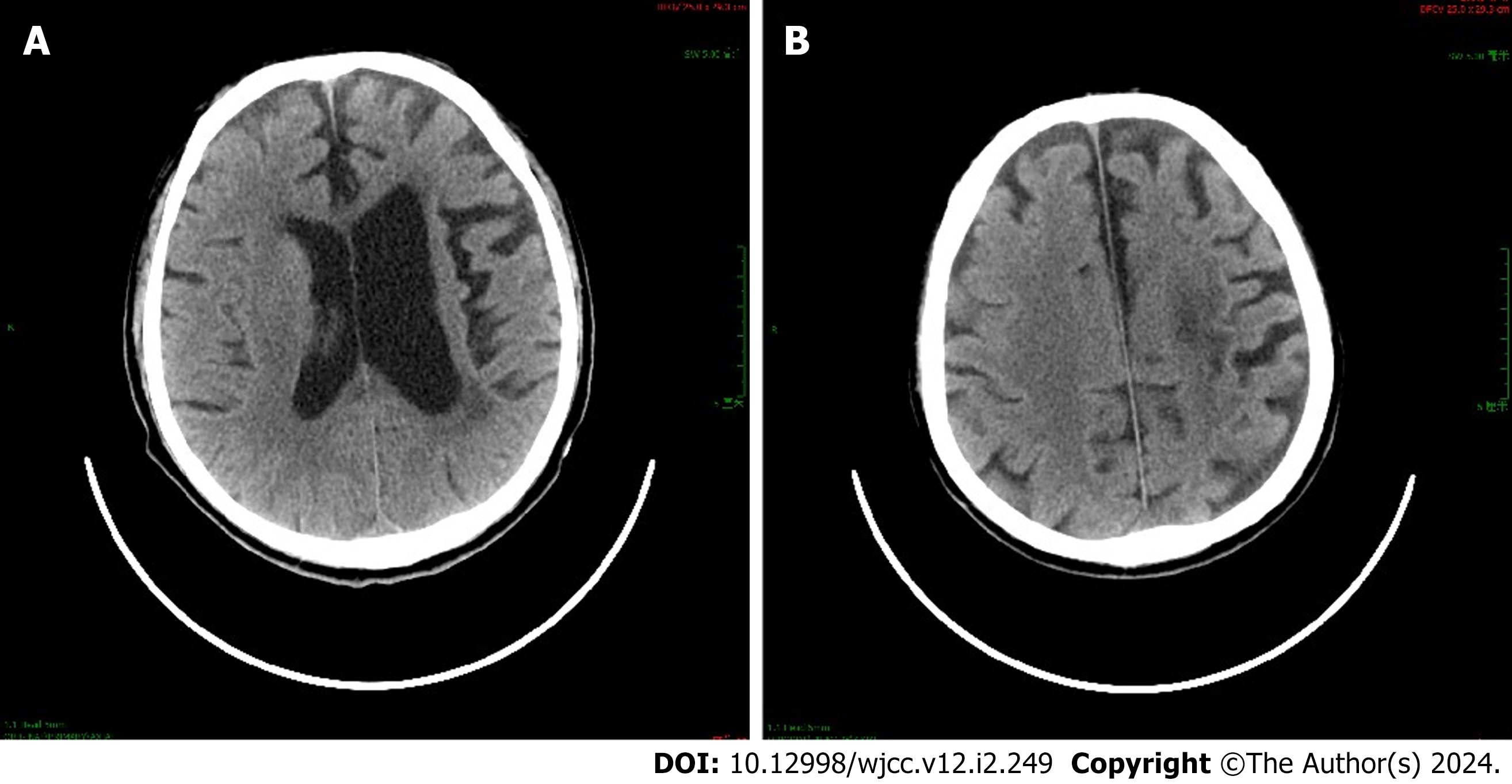
Case 2 was a 58-year-old man with cerebral hemorrhage in the right frontotemporal lobe. The time between stroke and first seizure was 7 years, and he had generalized clonic (motor) seizures. At the time of seizures, he showed convulsions of the extremities with loss of consciousness, upturned eyes, foaming at the mouth, tongue biting, and urinary incontinence; these lasted for 10 minutes. Case 7 was a 58-year-old man with cerebral infarction in the left corona radiata and centrum semiovale (Figure 1). The time between stroke and first seizure was 5 years, and he had focal to bilateral tonic-clonic (motor) seizures. Seizures were characterized by a rightward deviation of the head and eyes as well as facial twitching on the right side. Case 8 was a 59-year-old man with cerebral infarction in the left cerebral hemisphere. The time between stroke and first seizure was 8 mo, and the type of seizure was focal onset (nonmotor). Seizures manifested as a loss of consciousness, upward gaze, and lack of motion.
Of the 10 patients with PSE (Table 1), six exhibited slow waves on the lesion side, two showed low voltage on the lesion side, and two had normal EEG backgrounds on the lesion side. During the interictal stage, epileptiform waves were observed in the side opposite to the stroke lesion in all 10 patients; these manifested as sharp wave, sharp-wave complex, or spike discharges in the anterior head lead on the side opposite to the lesion (frontal and/or temporal lobe).
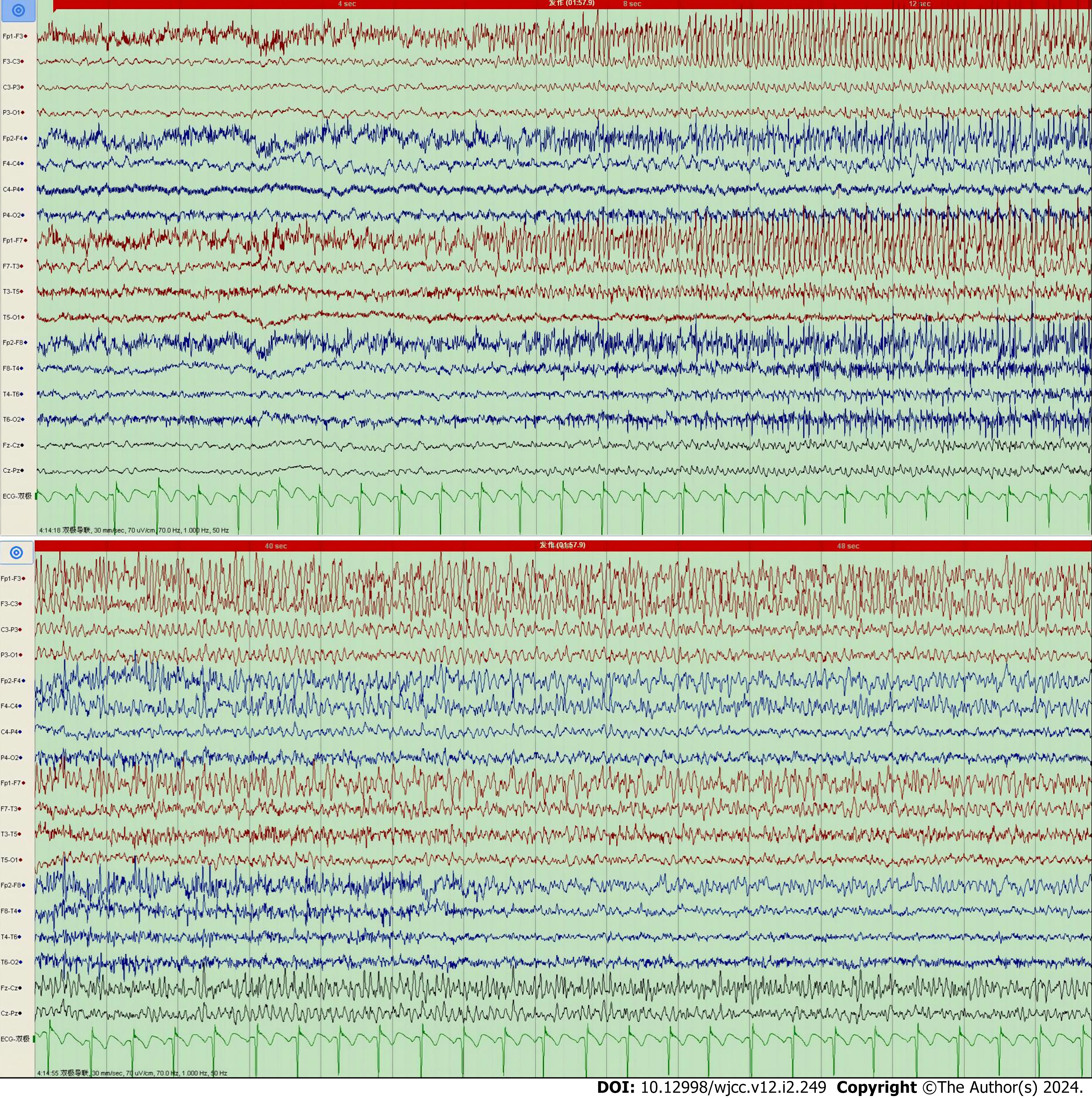
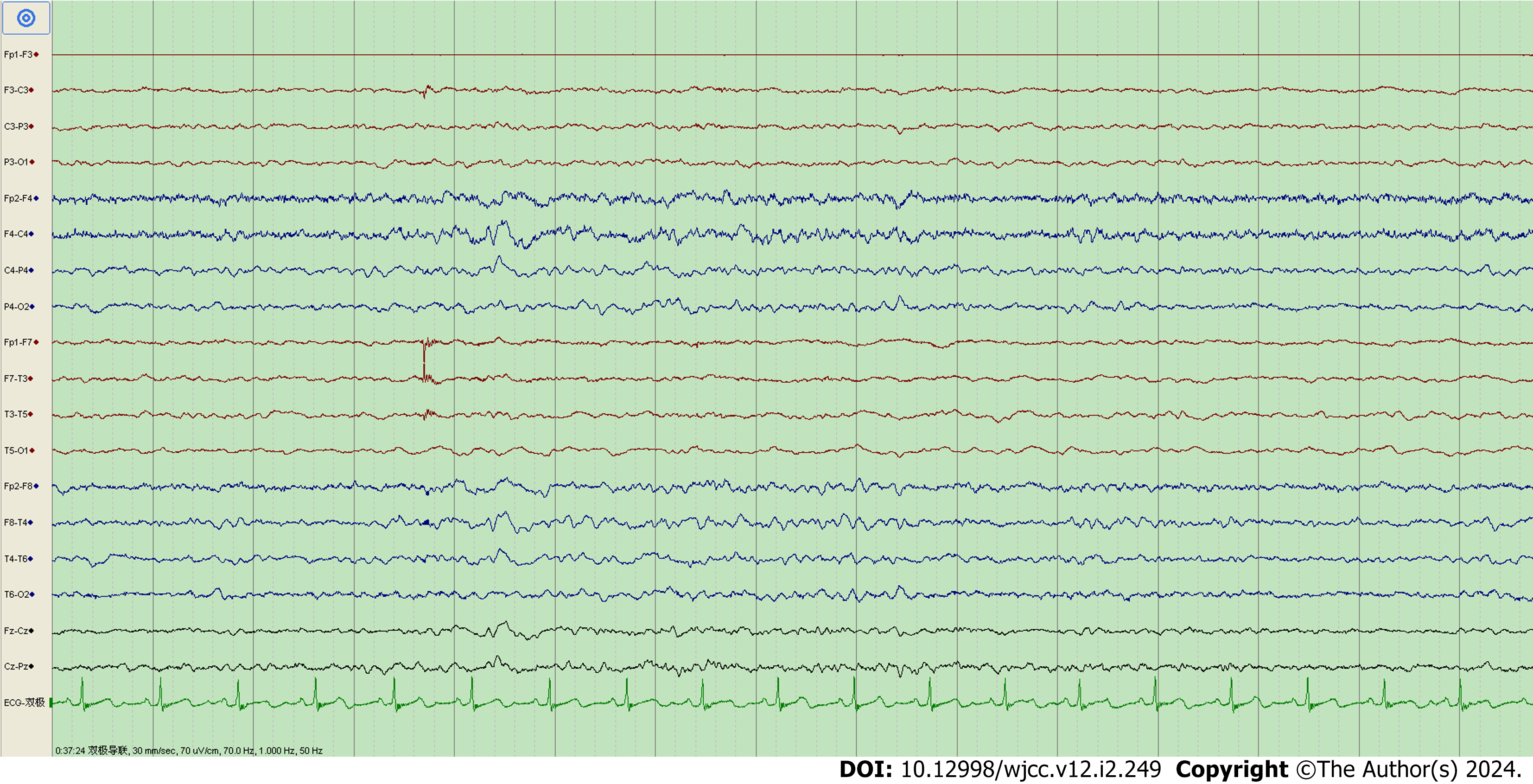
Ictal EEG was captured in Case 7 only. The EEG of Case 7 during seizures showed low-amplitude fast waves originating from the left frontal lobe (lead F3) and left anterior temporal lobe (lead F7), conducting to the frontal zero (lead Fz), central zero (lead Cz), and left center (lead C3) areas; each lead had gradually increasing amplitude and decreasing frequency (Figure 2). This patient’s background EEG showed low voltage in all left leads, and his interictal EEG showed low-amplitude sharp-wave complexes in the right frontal pole (lead Fp2), right frontal lobe (lead F4), and right anterior temporal lobe (lead F8) (Figure 3).
The background EEG of Case 2 exhibited 9-10 Hz α rhythm and low amplitude in the right temporal lobe. His interictal EEG showed intermittent and asynchronous discharges of medium- to low-amplitude sharp waves and sharp-wave complexes in the left middle temporal lobe (lead T3), and especially in the left anterior temporal lobe (lead F7). The background EEG of Case 8 exhibited slow waves in the left occipital lobe and low voltage in all left leads. His interictal EEG showed a few intermittent and synchronous discharges of sharp wave and sharp-wave complexes in the right frontal pole (lead Fp2), right frontal lobe (lead F4), right center (lead C4), and central zero (lead Cz).
Brain tissue damage caused by stroke is the main cause of PSE. Acute stroke results in local brain tissue hypoxia, abnormal brain metabolism, whole-brain low/high perfusion, glutamate excitotoxicity, ion channel dysfunction, and blood–brain barrier damage; these changes can lead to early-onset PSE. By contrast, glial scarring, chronic inflammation, angiogenesis, neurodegeneration, neurogenesis, selective neuronal loss, and synaptic plasticity secondary to stroke are closely related to late-onset PSE[3,7]. The occurrence of PSE can also have a negative impact on stroke; PSE can lead to decreased intracranial blood flow and increased intracranial pressure, aggravate secondary damage of neurological function, and worsen patient prognosis. Simultaneously, the risk of recurrent stroke increases[8,9].
The most common EEG findings in acute stroke are focal slow waves and diffuse slow waves on the infarct or hemorrhage side[10]. The presence of focal spikes, sharp waves, or unilateral periodic epileptiform discharges suggests an increased risk of PSE[11]. For patients with symptomatic epilepsy who have structural lesions in the brain, bioelectrical activity decreases as a result of damage at the stroke site and in surrounding neurons, and scalp EEG asymmetries—such as focal slow waves or low voltage—can occur. These background abnormalities are generally consistent with the structural lesions[2,12]. However, in some cases, such as those of cerebral trauma or cerebral perforation deformity, the focal or unilateral epileptiform discharges are inconsistent with the structural lesions, and the discharge sites are located distant from the structural lesions, or even in the contralateral hemisphere[13].
In the 10 PSE patients in the present study, symptom onset was consistent with the structural abnormal lesions that were observed on imaging; however, the interictal epileptic waves were located contralaterally to the lesions. The underlying mechanisms of this phenomenon may be as follows: (1) Local or unilateral pathological changes and abnormal sites of background EEG mean that EEG activity is almost lost; there is therefore no structural and functional basis for producing epileptiform discharges, and the discharges thus come from the relatively normal lobes or hemispheres. For example, it has been reported that when most of one hemisphere is damaged and necrotic, EEG shows generalized low voltage in this hemisphere, and discharges often come from the relatively normal hemisphere (i.e., on the same side of the hemiplegia)[14]; and (2) A local structural or functional brain injury affects distant sites via specific known or unknown intermediate links, thus producing epileptoid discharges[13].
PSE can lead to an important increase in the disability and mortality rates of stroke patients. However, compared with other types of symptomatic epilepsy, the refractory rate of PSE drugs is relatively low, and overall prognosis is better[5]. Together, the current findings indicate that the use of long-range video EEG monitoring should be refined in patients with severe cerebrovascular disease, to identify atypical seizures and unexplained disturbances of consciousness as soon as possible and to improve the detection rate of interictal epileptiform discharges[6]. For patients with PSE presenting with a first seizure (especially a focal nonmotor seizure), if scalp EEG discharges are on the side contralateral to the lesion during seizure intervals, the following steps should be taken: (1) Carefully examine the patient’s medical history; (2) analyze whether the clinical manifestations of seizure onset correspond to the softening lesions of stroke; and (3) capture the ictal EEG (if possible). A diagnosis of epilepsy should be considered for such focal seizures, and the possible etiology should first be presumed to be stroke.
In EEG, epileptiform waves can occur in the side opposite to the stroke lesion in patients with post-stroke epilepsy.
In electroencephalogram (EEG), epileptiform waves can occur in the side opposite to the stroke lesion in patients with post-stroke epilepsy.
EEG is the most objective auxiliary examination method for the diagnosis of post-stroke epilepsy (PSE). This imaging modality plays a very important role in epileptic seizure prediction, disease monitoring, drug selection, and prognosis evaluation.
This study aims to explore the EEG characteristics of patients with PSE and improve the detection rate of inter-seizure epileptiform discharges.
The clinical data, imaging characteristics, seizure intervals, and EEG characteristics of 10 patients with PSE in our hospital (from January 2017 to June 2020) were analyzed retrospectively.
During the interictal stage, epileptiform waves occurred in the side opposite to the stroke lesion in all 10 patients. These manifested as sharp wave, sharp-wave complex, or spike discharges in the anterior head lead of the side opposite to the lesion.
In patients with PSE, epileptiform waves can occur in the side opposite to the stroke lesion in EEG.
The current findings indicate that long-range video EEG monitoring should be refined in patients with PSE, to improve the detection rate of interictal epileptiform discharges.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sotelo J, Mexico S-Editor: Gong ZM L-Editor: A P-Editor: Cai YX
| 1. | Labovitz DL, Hauser WA, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology. 2001;57:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Tanaka T, Ihara M. Post-stroke epilepsy. Neurochem Int. 2017;107:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Myint PK, Staufenberg EF, Sabanathan K. Post-stroke seizure and post-stroke epilepsy. Postgrad Med J. 2006;82:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Bentes C, Franco AC, Peralta AR, Viana P, Martins H, Morgado C, Casimiro C, Fonseca C, Geraldes R, Canhão P, Pinho E Melo T, Paiva T, Ferro JM. Epilepsia partialis continua after an anterior circulation ischaemic stroke. Eur J Neurol. 2017;24:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Arntz RM, Maaijwee NA, Rutten-Jacobs LC, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, de Leeuw FE. Epilepsy after TIA or stroke in young patients impairs long-term functional outcome: the FUTURE Study. Neurology. 2013;81:1907-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Feyissa AM, Hasan TF, Meschia JF. Stroke-related epilepsy. Eur J Neurol. 2019;26:18-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Reddy DS, Bhimani A, Kuruba R, Park MJ, Sohrabji F. Prospects of modeling poststroke epileptogenesis. J Neurosci Res. 2017;95:1000-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Stefanidou M, Das RR, Beiser AS, Sundar B, Kelly-Hayes M, Kase CS, Devinsky O, Seshadri S, Friedman D. Incidence of seizures following initial ischemic stroke in a community-based cohort: The Framingham Heart Study. Seizure. 2017;47:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Huang CW, Saposnik G, Fang J, Steven DA, Burneo JG. Influence of seizures on stroke outcomes: a large multicenter study. Neurology. 2014;82:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Bentes C, Martins H, Peralta AR, Morgado C, Casimiro C, Franco AC, Fonseca AC, Geraldes R, Canhão P, Pinho E Melo T, Paiva T, Ferro JM. Early EEG predicts poststroke epilepsy. Epilepsia Open. 2018;3:203-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Bentes C, Martins H, Peralta AR, Casimiro C, Morgado C, Franco AC, Fonseca AC, Geraldes R, Canhão P, Pinho E Melo T, Paiva T, Ferro JM. Post-stroke seizures are clinically underestimated. J Neurol. 2017;264:1978-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Sueiras M, Thonon V, Santamarina E, Sánchez-Guerrero Á, Riveiro M, Poca MA, Quintana M, Gándara D, Sahuquillo J. Is Spreading Depolarization a Risk Factor for Late Epilepsy? A Prospective Study in Patients with Traumatic Brain Injury and Malignant Ischemic Stroke Undergoing Decompressive Craniectomy. Neurocrit Care. 2021;34:876-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Liu XY, Wu X. Clinical EEG. Beijing: People's Health Publishing House, 2017: 235-236. |
| 14. | Garzon E, Gupta A, Bingaman W, Sakamoto AC, Lüders H. Paradoxical ictal EEG lateralization in children with unilateral encephaloclastic lesions. Epileptic Disord. 2009;11:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |