Published online Jan 16, 2024. doi: 10.12998/wjcc.v12.i2.240
Peer-review started: August 30, 2023
First decision: December 7, 2023
Revised: December 18, 2023
Accepted: December 26, 2023
Article in press: December 26, 2023
Published online: January 16, 2024
Processing time: 133 Days and 16.1 Hours
Umbilical artery thrombosis (UAT) is extremely uncommon and leads to adverse perinatal outcomes. Hypercoagulation of blood in pregnant women is suspected to be an important risk for UAT. Ultrasound is an effective way to detect throm
To investigate ultrasonic blood signal after UAT in the umbilical artery, and eva
We described a case of a newly formed UAT with markedly altered ultrasonic indices of umbilical artery blood flow, and retrospectively studied it with 18 UAT patients confirmed by histopathology from October 2019 and March 2023 in Xia
A patient with normal findings in second and third-trimester routine ultrasound scan developed UAT with severe changes in ultrasonic indices of umbilical artery blood flow (within 2.5th of reference ranges) in a short period of time. Statistical analysis of umbilical artery blood flow ultrasound indices for 19 patients with UAT showed that the decrease in S/D, RI, and PI and increase of PSV during the disease process was greater than that of non-UAT. All 18 patients delivered in our hospital showed characteristic manifestations of UAT on histological examination after delivery, most of which (16/18) showed umbilical cord abnormalities, with 15 umbilical cord torsion and 1 pseudoknot. Coagulation parameters were not significantly changed in UAT patients compared with normal pregnancy women.
Significant changes in ultrasound indicators after UAT were demonstrated. PSV can play important roles in the diagnosis of UAT. Hypercoagulability alone is not sufficient for the occurrence of UAT.
Core Tip: A case of umbilical artery thrombosis (UAT) occurring within a short period of time was reported, resulting in significant changes in the ultrasound flow indices of the umbilical artery. Then, a retrospective study of 19 patients revealed a high prevalence of umbilical cord abnormalities among those with UAT, while the coagulation status did not significantly differ from that of normal pregnant women. Notably, the proportion of decrease in systolic-diastolic duration ratio, resistance index and pulsatility index, and increase in peak systolic velocity were considerably higher in UAT cases compared to normal pregnant women, which might help to detect UAT in advance using ultrasound.
- Citation: Hong SJ, Hong LW, He XQ, Zhong XH. Ultrasound blood flow characteristics changes in fetal umbilical artery thrombosis: A retrospective analysis. World J Clin Cases 2024; 12(2): 240-248
- URL: https://www.wjgnet.com/2307-8960/full/v12/i2/240.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i2.240
Umbilical artery thrombosis (UAT) is a rare pregnancy and delivery problem, but it can threaten the life of a fetus. UAT is a risk factor for intrauterine asphyxia and fetal growth restriction during pregnancy or even stillbirth[1]. Early detection of UAT allows for rapid treatment to guarantee the optimum fetal development. The pathogenesis of UAT has not yet been fully understood.
Previous studies have shown that blood hypercoagulation, abnormalities of the umbilical cord may lead to stagnant blood flow and the formation of blood clots[2-4]. The blood of pregnant women was reported to be hypercoagulable and that the risk of thrombosis during pregnancy increases over 4-fold compared to non-pregnant cohorts, suggesting that hypercoagulable mechanical injury may be an important trigger for UAT[5,6].
Ultrasound is one of the best techniques to evaluate the fetus vascular function in both laboratory investigations and clinical contexts due to its relative low cost, simplicity, non-invasiveness, and the capacity to spot minor abnormalities[7]. By detecting various frequencies of sound waves reflected off red blood cells, Doppler ultrasound is used to image blood flow[8]. Although some studies have shown the clinical value of cord blood flow changes in the assessment of fetal anomalies or distress, the association of ultrasound flow indices of the umbilical cord vessels with cord thrombosis has rarely been reported.
Diagnose data of 19 UAT cases were obtained for this study from the accumulative database of 51969 patients who gave birth in Xiamen Women and Children's Hospital from October 2019 and March 2023. Patients were screened by serial routine ultrasound scans during the second and third trimesters, and the diagnosis was further confirmed by postnatally pathological examination except the first case who insisted on being discharged from hospital. Initial clinical information of patients in UAT and non-UAT groups was collected for baseline analysis, including age, body mass index at booking and ethnicity. Transabdominally ultrasonic examination was performed using 4-6 MHz transducers (Philips iU22 or GE Voluson E8). The diagnosis of UAT was confirmed by two experienced sinologists independently. Doppler measurements were obtained from both umbilical arteries following the International Society of Ultrasound in Obstetrics and Gyne
Statistical analyses were performed using IBM SPSS version 24.0 software (IBM Corp., Armonk, NY, United States). We applied descriptive analyses to detect sample differences. To ensure that both samples actually differed in two groups compared, we calculated matched samples t-test or Mann-Whitney U test (Wilcoxon rank sum test).
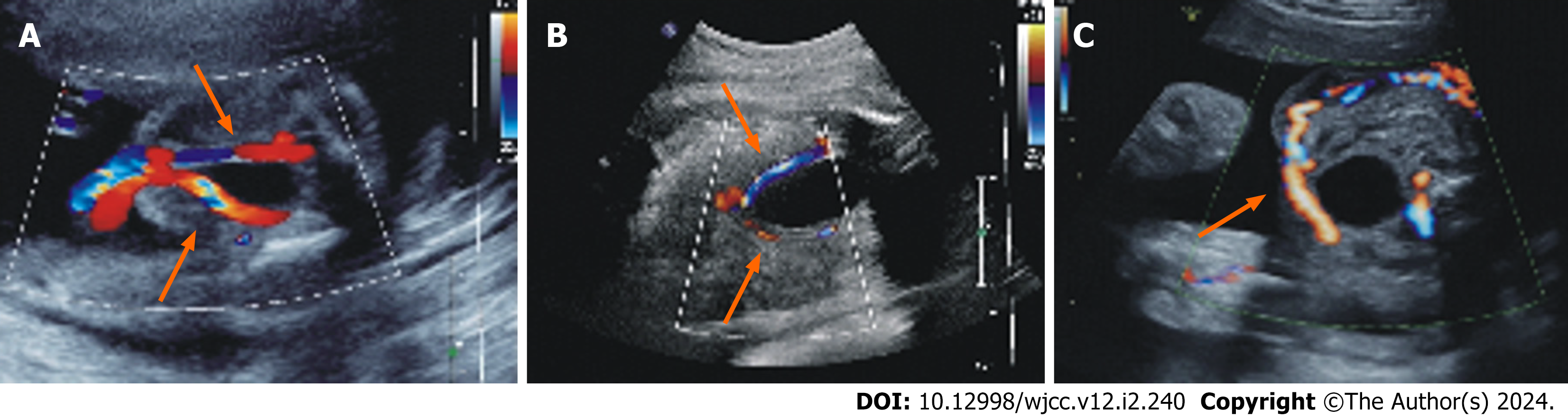
A 33-year-old, gravida 2 para 0, pregnant woman had an unremarkable gestational course before 30 wk and 4 d. An ultrasound scan for fetal malformations at 24+1 wk of the pregnancy showed normal findings. Two umbilical arteries were detected during an ultrasound examination (Figure 1A). The corresponding systolic-diastolic duration ratio (S/D), re
| Diagnosis gestational weeks | S/D | RI | PI | PSV (cm/s) |
| 24+1 | 3.70 (50th-75th) | 0.73 (50th-75th) | 1.20 (50th-75th) | 37.9 |
| 30+4 | 2.30 (10th-25th) | 0.57 (10th-25th) | 0.93 (25th-50th) | 38.7 |
| 30+6 | 1.82 (2.5th) | 0.45 (2.5th) | 0.62 (2.5th) | 59.1 |
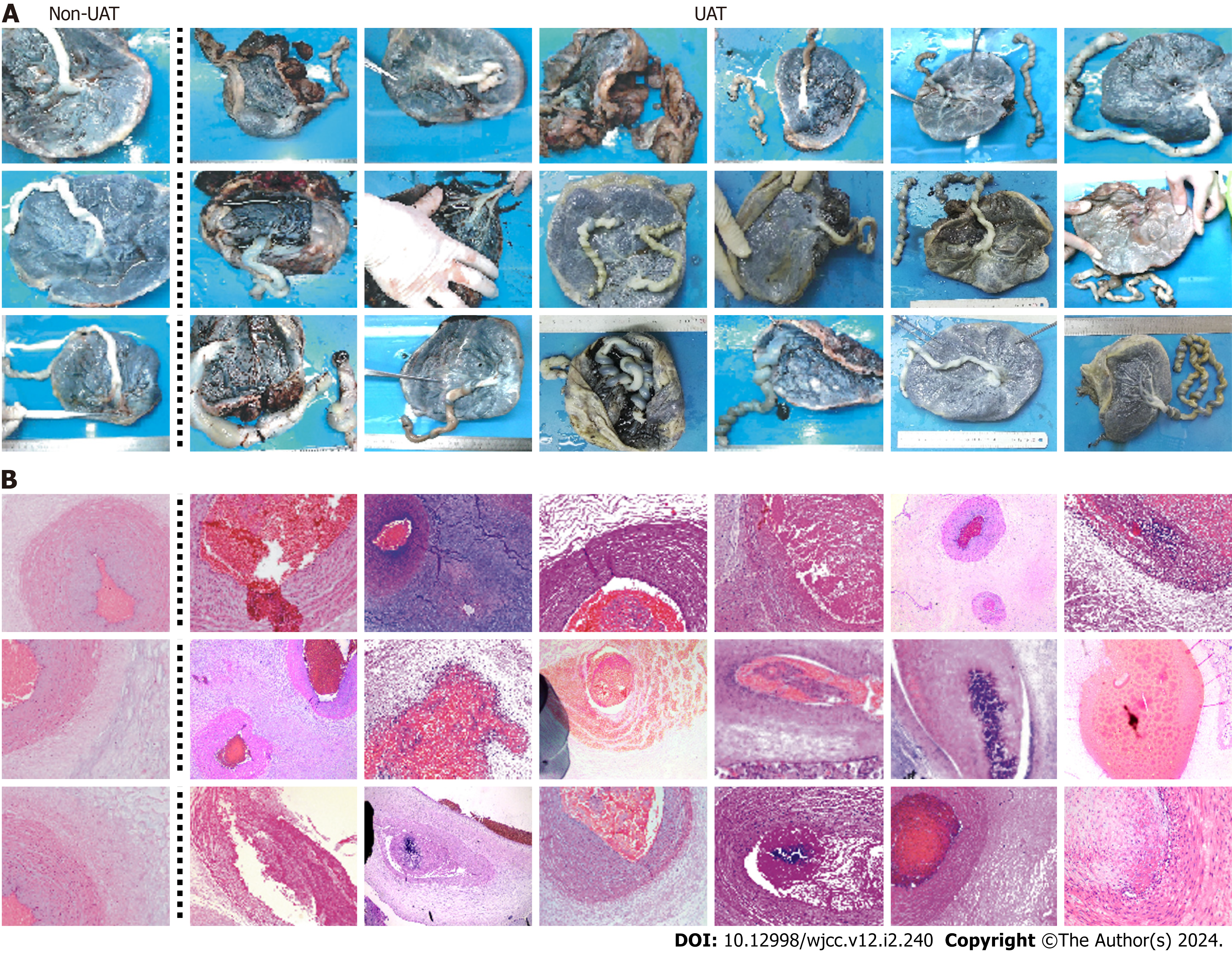
To further investigate the changes of ultrasonic blood signal after UAT, we retrospectively studied 19 patients with UAT from 51,969 pregnant women in last 3.5 years, and 19 patients without UAT were set as control group. Two groups of patients did not show statistically differences at the baseline (Supplementary Table 1). The incidence of cord artery thrombosis is about 1 in 2600 deliveries. In all cases, the routine second-trimester ultrasound scans exhibited normal findings with one umbilical vein and two umbilical arteries observed, and UAT occurred in gestational age of the third trimester. Maternal clinical data and neonatal information were listed in Table 2. 16 cases showed umbilical cord abnormalities, with 15 umbilical cord torsion and 1 pseudoknot, which represents a significantly higher proportion and severity of umbilical cord abnormalities compared to those in normal pregnancies (Supplementary Table 2). Finally, except for one patient who required automatic discharge, 18 patients were delivered by cesarean section, and all the maternal outcomes were good. Eight patients were full-term (8/18), 10 patients (10/18) were preterm.9 fetal (9/18) were low birth weight, 2 fetal (2/18) was very low birth weight. All 18 patients showed characteristic manifestations of UAT on histological examination after delivery compared to normal umbilical artery (Figure 2).
| No. | Age (yr) | Gravidity (times) | Parity (times) | Diagnosis gestational weeks | Delivery gestational weeks | Apgar score | Umbilical cord abnormalities | Birth weight (g) |
| 1 | 32 | 2 | 0 | 30+6 | - | - | - | - |
| 2 | 28 | 3 | 2 | 30+6 | 31+2 | 6-8-8 | Torsion for 48 cycles | 1380 |
| 3 | 28 | 1 | 1 | 36+3 | 37+1 | 10-10-10 | Torsion for 44 cycles | 2330 |
| 4 | 27 | 1 | 1 | 33+6 | 33+6 | 9-9-9 | Torsion for 30 cycles | 2315 |
| 5 | 28 | 2 | 1 | 31+5 | 32 | 8-9-9 | Torsion for 45 cycles | 1300 |
| 6 | 35 | 2 | 2 | 34+4 | 34+4 | 9-9-9 | Torsion for 45 cycles | 2442 |
| 7 | 24 | 1 | 1 | 35+2 | 35+3 | 10-10-10 | Torsion for 40 cycles | 2220 |
| 8 | 25 | 1 | 1 | 32 | 36+3 | 10-10-10 | Torsion for 38 cycles | 2475 |
| 9 | 31 | 2 | 2 | 37+2 | 37+2 | 1-7-7 | Torsion for 41 cycles | 3480 |
| 10 | 31 | 1 | 1 | 29+3 | 32+3 | 8-9-9 | Torsion for 38 cycles | 1690 |
| 11 | 31 | 2 | 1 | 36+6 | 37+1 | 9-10-10 | Torsion for 35 cycles | 2915 |
| 12 | 30 | 2 | 1 | 36 | 36 | 10-10-10 | Torsion for 30 cycles | 3023 |
| 13 | 27 | 1 | 1 | 39+1 | 39+1 | 9-9-10 | Pseudoknot | 3000 |
| 14 | 35 | 4 | 2 | 37+4 | 37+4 | 10-10-10 | - | 2477 |
| 15 | 35 | 3 | 2 | 39+5 | 39+5 | 10-10-10 | - | 3468 |
| 16 | 25 | 1 | 1 | 34+3 | 34+3 | 9-9-9 | Torsion for 45 cycles | 1830 |
| 17 | 35 | 3 | 2 | 31 | 31 | 9-9-9 | Torsion for 41 cycles | 1715 |
| 18 | 32 | 1 | 1 | 28+4 | 37 | 10-10-10 | Torsion for 55 cycles | 2590 |
| 19 | 36 | 3 | 2 | 37+2 | 37+3 | 8-10-10 | Narrow, torsion for 30 cycles with tight spirals | 3055 |
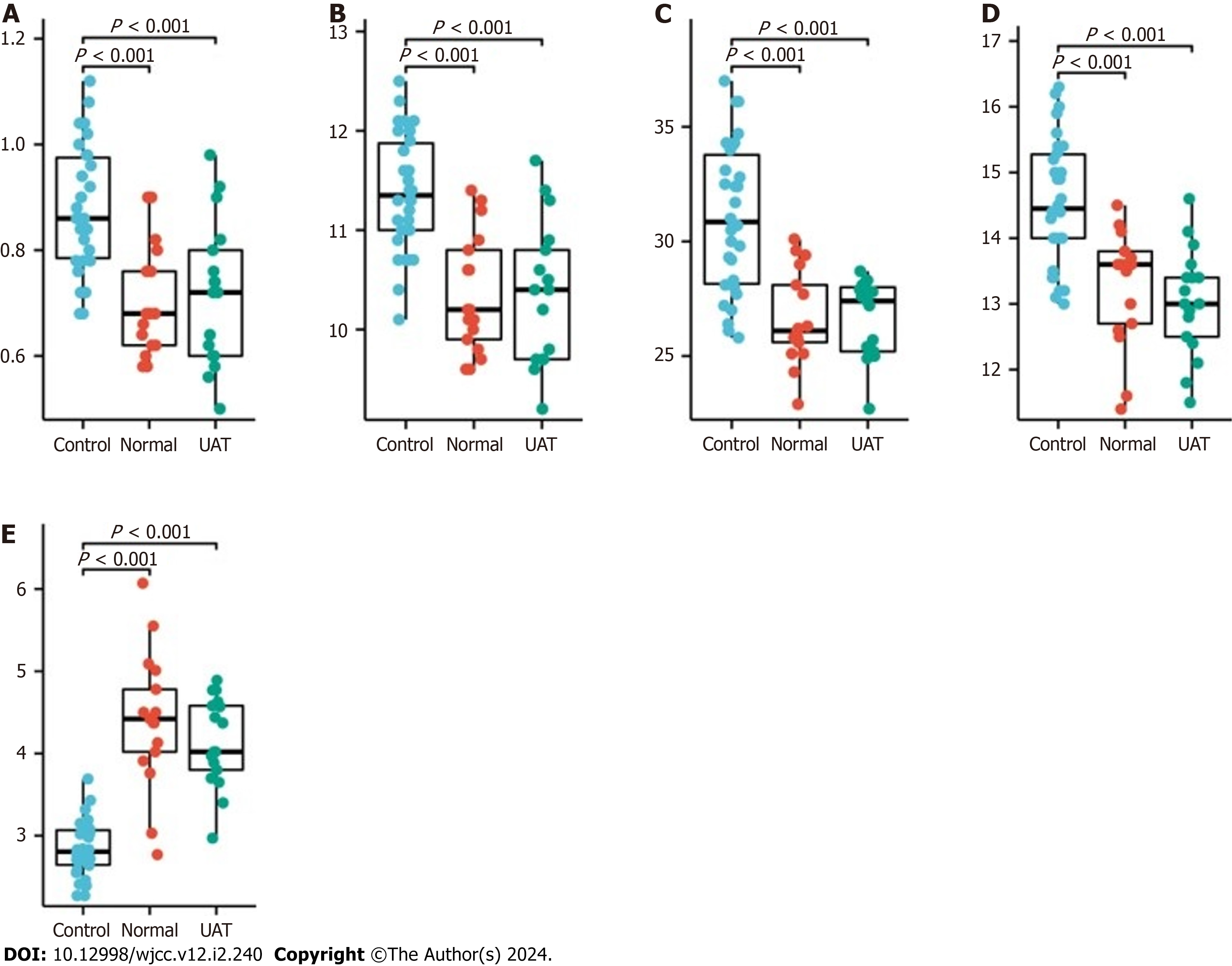
Previous studies have reported that hypercoagulability is one of the main risks leading to thrombosis in pregnancy[10]. So we collected coagulation data from the patients with UAT (17/19, 2 patients with UAT did not undergo coagu
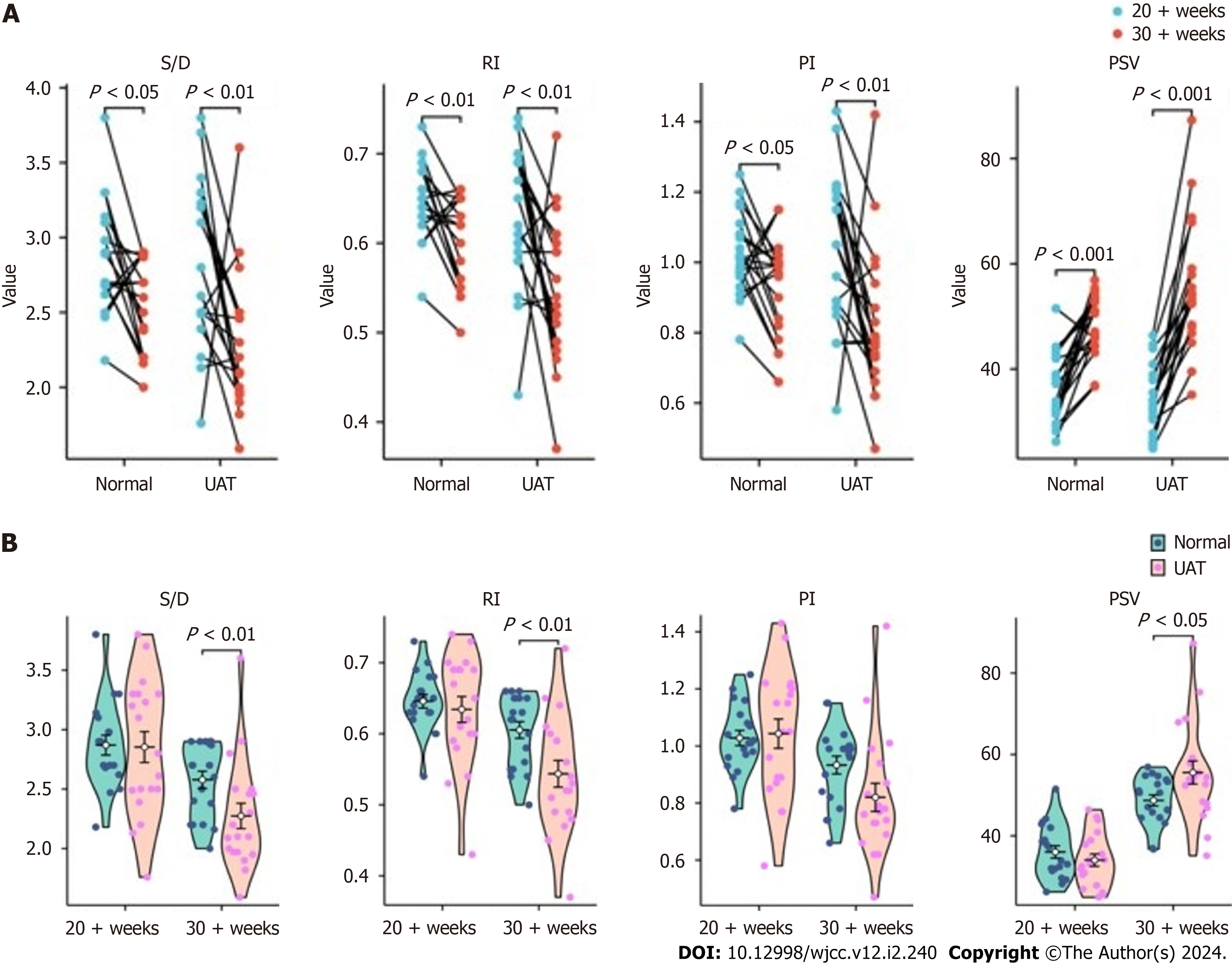
Finally, the ultrasound indices of umbilical artery flow were analyzed (Supplementary Table 3). We found that the umbilical artery flow ultrasound indices S/D, RI and PI decreased significantly from second to third trimester, while PSV increased significantly in both normal pregnant women and UAT group (Figure 4A). However, the proportion of de
Infants rarely suffer from UAT, which is a difficult-to-predict disorder[11]. Although previous research exhibits that certain factors like umbilical cord structural dysplasia, maternal coagulopathy, vascular endothelial damage, elevated blood glucose, and intrauterine transfusion increase the chances of UAT[12,13], it still happens without warning. More
In the routine practice, we encountered a patient who seemed to be normal during second-trimester. The umbilical artery ultrasound flow indicators ranged from 50th to 75th. Later, at 30+4 wk of late pregnancy, the routine ultrasound images showed no significant abnormalities in the flow of either umbilical artery, but the ultrasound flow indicators changed (within the 10th-25th reference range). It probably meant that the UAT was progressing but not causing physiological changes. Nevertheless, just two days later, the woman was back with abdominal tightness, and ultrasound findings showed that only one of the two umbilical artery flows that should have been surrounding the bladder was left, indicating that the patient suffered from an UAT. Ultrasonic results revealed significantly abnormal S/D, RI and PI of the umbilical artery flow (lower than the 2.5th reference range) and a 1.53-fold increase in PSV of the remaining umbilical artery after UAT, indicating a significantly increased flow rate of the remaining umbilical artery. This might be due to the dilation of remaining artery in compensation to maintain the blood flow from the fetus to the placenta as previously reported[14]. This case suggests that umbilical artery flow ultrasound indices change gradually during the course of thrombosis, and alter dramatically when thrombosis eventually leads to physiological structural changes, which may be used as a predictor of the occurrence of UAT. This hypothesis needs to be validated by more cases that real-time ultrasound tracking was performed to observe the UAT forming.
For second best, we retrospectively analyzed 19 cases of UAT diagnosed in our department from October 2019 to March 2023, and further obtained some useful clues to understand UAT. All 19 patients with UAT we reported occurred during late pregnancy, and most patients associated with cord abnormalities, likely because the umbilical cord is more prone to robbery, entanglement, and torsion in later pregnancy.
It was reported that the risk of thrombosis during pregnancy is over 4 times higher than that in the non-pregnant women, indicating that hypercoagulability may indeed be a potential cause of UAT. Compared to normal women, both non-UAT and UAT pregnant women showed a phenomenon of hypercoagulability, while there was no significant difference between these two groups of pregnant women, suggesting that hypercoagulability alone is not sufficient for the occurrence of UAT, and other key factors need further investigation.
Our findings showed that in both UAT and non-UAT groups, the umbilical artery flow ultrasound indices S/D, RI and PI decreased significantly from mid to late pregnancy, while PSV increased significantly, which indicating that the demand for blood flow between the fetus and the mother is elevated as the fetus develops. The proportion of decrease in S/D and RI as well as the increase in PSV in the UAT group were greater than in the non-UAT group, revealing an increase in blood flow in the remaining vessel, which supports our hypothesis about the compensatory effect of blood flow in the described case. PSV can also be used as an observer of umbilical artery anomalies, something that is usually overlooked by previous studies, and reference ranges for PSV at different gestational times remains to be developed.
However, it is important to acknowledge the limitations of our study, such as the relatively limited number of cases due to the rarity of UAT, which may not fully represent all the changes in the disease process. Furthermore, as a retro
For the first time, here we demonstrate statistically significant changes in ultrasound indices of umbilical artery flow after the occurrence of UAT, which provides a basis for clinical follow-up of people at high risk for UAT. Our results show that PSV, which was previously overlooked, can be used as a diagnostic indicator for UAT, in combination with RI, PI and S/D. We also show that blood hypercoagulation during pregnancy is not sufficient for the cause of UAT.
Umbilical artery thrombosis (UAT) is a rare condition that poses a serious threat to the lives of both the mother and the fetus. Its occurrence has been reported to be potentially associated with hypercoagulability in pregnant women. While ultrasound is a routine method for assessing umbilical artery blood flow, there is currently limited research on the blood flow changes before and after the occurrence of UAT.
Studies of changes in ultrasound flow indices before and after the onset of UAT can help in early detection and intervention to reduce the risk to the pregnant woman and the fetus.
The results of this study will help to understand the changes in blood flow before and after the onset of UAT and will hopefully lead to the use of ultrasound flow indices as an effective means of tracking the condition of people at high risk for UAT.
In this study, blood flow ultrasound indices and coagulation parameters of patients before and after the occurrence of UAT were collected and analyzed statistically.
Patients with UAT did not display a significantly abnormal blood coagulation status compared with normal pregnant controls. In patients with UAT, the changes in ultrasound blood flow indices were significantly greater than normal pregnant women.
Hypercoagulability alone is not sufficient for the occurrence of UAT. Significant changes in ultrasound indicators after UAT were demonstrated. Peak systolic velocity can play important roles in the diagnosis of UAT.
Prospective studies with large sample sizes can help to further explore the physiologic changes in the process of umbilical artery embolization by setting up well-defined control groups.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alsaidan A, Saudi Arabia S-Editor: Li L L-Editor: A P-Editor: Xu ZH
| 1. | Wei J, Li Q, Zhai H. Umbilical artery thrombosis diagnosed at different gestational ages and fetal outcomes: a case series. BMC Pregnancy Childbirth. 2021;21:788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Shah SJ, Dasika J, McEnerney DC. Hypercoagulation and Thrombotic Disorders. In: Scher CS, Kaye AD, Liu H, Perelman S, Leavitt S, editors. Essentials of Blood Product Management in Anesthesia Practice. Cham: Springer International Publishing, 2021: 51-56. [DOI] [Full Text] |
| 3. | Redline RW. Clinical and pathological umbilical cord abnormalities in fetal thrombotic vasculopathy. Hum Pathol. 2004;35:1494-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Pinar H, Carpenter M. Placenta and umbilical cord abnormalities seen with stillbirth. Clin Obstet Gynecol. 2010;53:656-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Konkle BA. Diagnosis and management of thrombosis in pregnancy. Birth Defects Res C Embryo Today. 2015;105:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tian Y, Fu Z, Lan H, Ma X, Wu S, Zhou X, Shen W, Lou Y. Chinese traditional herbs enhanced the clinical efficacy of low-molecular-weight heparin in the treatment of recurrent spontaneous abortion complicated with thrombophilia. Heliyon. 2023;9:e13120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Shung KK. Diagnostic ultrasound: Imaging and blood flow measurements. 2nd ed. Boca Raton (FL): CRC press, 2015. [DOI] [Full Text] |
| 8. | Reilly RM. Ultrasound Imaging Technology. In: Reilly RM. Medical Imaging for Health Professionals: Technologies and Clinical Applications. New York: John Wiley & Sons, Inc., 2019: 107-113. [DOI] [Full Text] |
| 9. | Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol. 2005;192:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 10. | Hui C, Lili M, Libin C, Rui Z, Fang G, Ling G, Jianping Z. Changes in coagulation and hemodynamics during pregnancy: a prospective longitudinal study of 58 cases. Arch Gynecol Obstet. 2012;285:1231-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Wu X, Wei C, Chen R, Yang L, Huang W, Huang L, Yan X, Deng X, Gou Z. Fetal umbilical artery thrombosis: prenatal diagnosis, treatment and follow-up. Orphanet J Rare Dis. 2022;17:414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Zhu Y, Beejadhursing R, Liu Y. 10 cases of umbilical cord thrombosis in the third trimester. Arch Gynecol Obstet. 2021;304:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 13. | Oliveira GH, Dias Cde M, Vaz-Oliani DC, Oliani AH. Intrauterine thrombosis of umbilical artery - case report. Sao Paulo Med J. 2016;134:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |