Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.3995
Revised: April 26, 2024
Accepted: May 22, 2024
Published online: July 6, 2024
Processing time: 114 Days and 1.9 Hours
Owing to the advancement in bacterial identification techniques, the detection rate of non-tuberculous mycobacterium (NTM) has been on the rise. Different from Mycobacterium tuberculosis, the clinical symptoms of NTM are not easily detected, and the clinical efficacy and prognosis are somewhat heterogeneous. To report a case of Mycobacterium gordoniasis of cervical lymph node diagnosed in Anhui Chest Hospital in July 2022.
Upon examination, the patient who weighed 67.5 kg, was human immunodeficiency virus negative, healthy, without hypertension, diabetes, heart disease and other basic diseases microscopic analysis revealed granulomatous inflammation with coagulation necrosis in the lymphocyte, and tuberculosis was not ruled out. Plain computed tomography scans of the neck and chest indicated the presence of a single grayish-yellow and grayish-brown tissue, the dimensions of which was top of form 10.5 cm × 3.0 cm × 1.5 cm. After pathological consultation in our hospital, the diagnosis was confirmed as NTM infection.
This case report and the clinical epidemiological research on improving NTM have important guiding significance for improving decision-making in clinical treatments.
Core Tip: This case highlights the growing challenge of diagnosing and managing non-tuberculous mycobacterium (NTM) infections, with an emphasis on Mycobacterium gordoniasis in the cervical lymph node. This underscores the significance of employing sophisticated bacterial identification methods to differentiate NTM from tuberculosis, considering their nuanced clinical presentations and implications for treatment approaches. The report serves as a pivotal reminder of the diverse clinical outcomes linked to NTM infections, advocating for increased vigilance and personalized therapeutic interventions in analogous scenarios.
- Citation: Peng L, Ma R, Li Y, Cheng J. Mycobacterium gordoniasis of the cervical lymph nodes: A case report. World J Clin Cases 2024; 12(19): 3995-4002
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/3995.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.3995
Mycobacterium gordoniasis belongs to the category of non-tuberculous mycobacterium (NTM), which has certain regional and ethnic characteristics[1,2]. It is commonly found in the lungs, but rarely in the lymph nodes. The clinical manifestations are extremely atypical and can be misdiagnosed as tumor or lymph node tuberculosis[3]. This paper reports a case of Mycobacterium gordoniasis of cervical lymph node diagnosed in Anhui Chest Hospital in July 2022.
The main complaint was "no obvious cause found a lump in the right neck for 1 month, gradually increased to about the size of an egg, accompanied by pain, and no fever".
Upon examination, the patient was human immunodeficiency virus (HIV) negative, healthy, without hypertension, diabetes, heart disease and other basic diseases, and weighed 67.5 kg.
The patient was a 61-year-old male farmer with a smoking history of 20 cigarettes per day for 30 years. The patient underwent functional neck lymph node dissection under general anesthesia outside our hospital on June 21, 2022.
The patient denied any family history related to other diseases related to this type of disease.
Nodules and cords of the upper lobe of the right lung increased in density, nodules of the left lung, multiple mediastinal lymph nodes of varying sizes, and bilateral pleural hypertrophy. Under general anesthesia, "functional neck lymph node dissection (right side)", postoperative anti-infection and other symptomatic treatment.
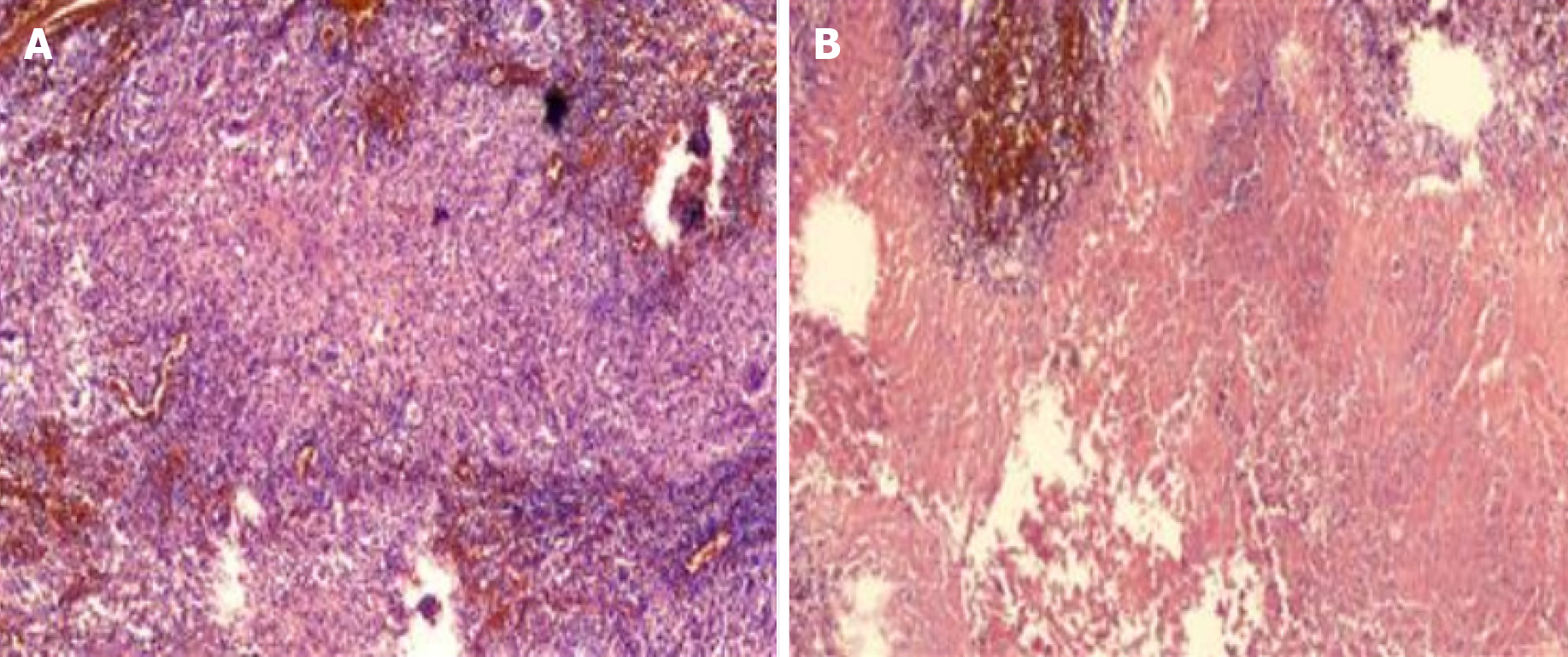
Microscopic examination showed that the lymphocyte showed granulomatous inflammation with coagulation necrosis, and tuberculosis could not be excluded. Further examination should be combined with clinical and related etiology. Immunohistochemical results: Sex determining region Y-box 10 (SOX 10) negative, S-100 (scattered +), CD 68 positive, Ki-67 (scattered +), CK negative, antacid negative (Figure 1).
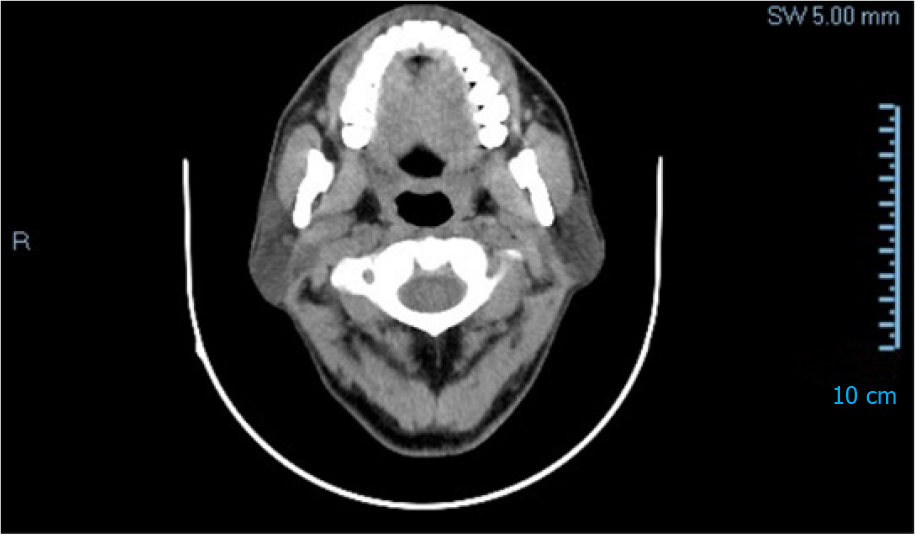
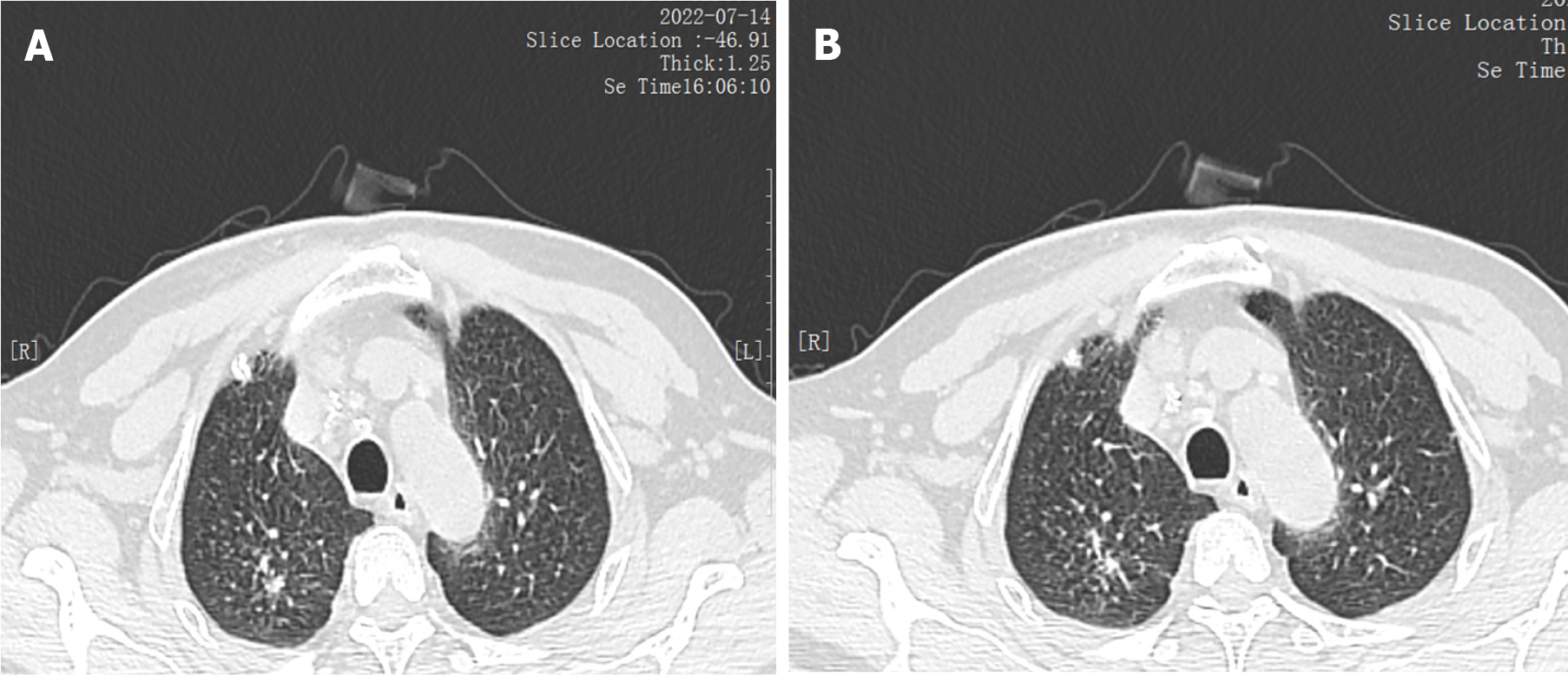
There was no obvious cough, phlegm, chest tightness, chest pain and discomfort. Neck and chest computed tomography (CT) plain scan + enhanced scan indicated that the right cervical sheath area was occupied and neurogenic tumors were possible (Figure 2). Pathology showed that there was one grayish-yellow and grayish-brown tissue, the size of which was 10.5 cm × 3.0 cm × 1.5 cm. When the largest surface was cut open, a grayish-gray mass with a size of 2.0 cm × 1.5 cm × 1.5 cm was found. The section was cystic and solid, and salivary gland tissue was seen next to the mass, 6.0 cm × 3.0 cm × 1.0 cm in size.
After pathological consultation in our hospital, the diagnosis was confirmed as NTM infection. (Suspected to be a neurogenic tumor before surgery, postoperative pathology suggests that tuberculosis cannot be ruled out. For tuberculosis and NTM infections, surgery should be performed with caution before treatment, as the incision may be prolonged and unable to heal.)
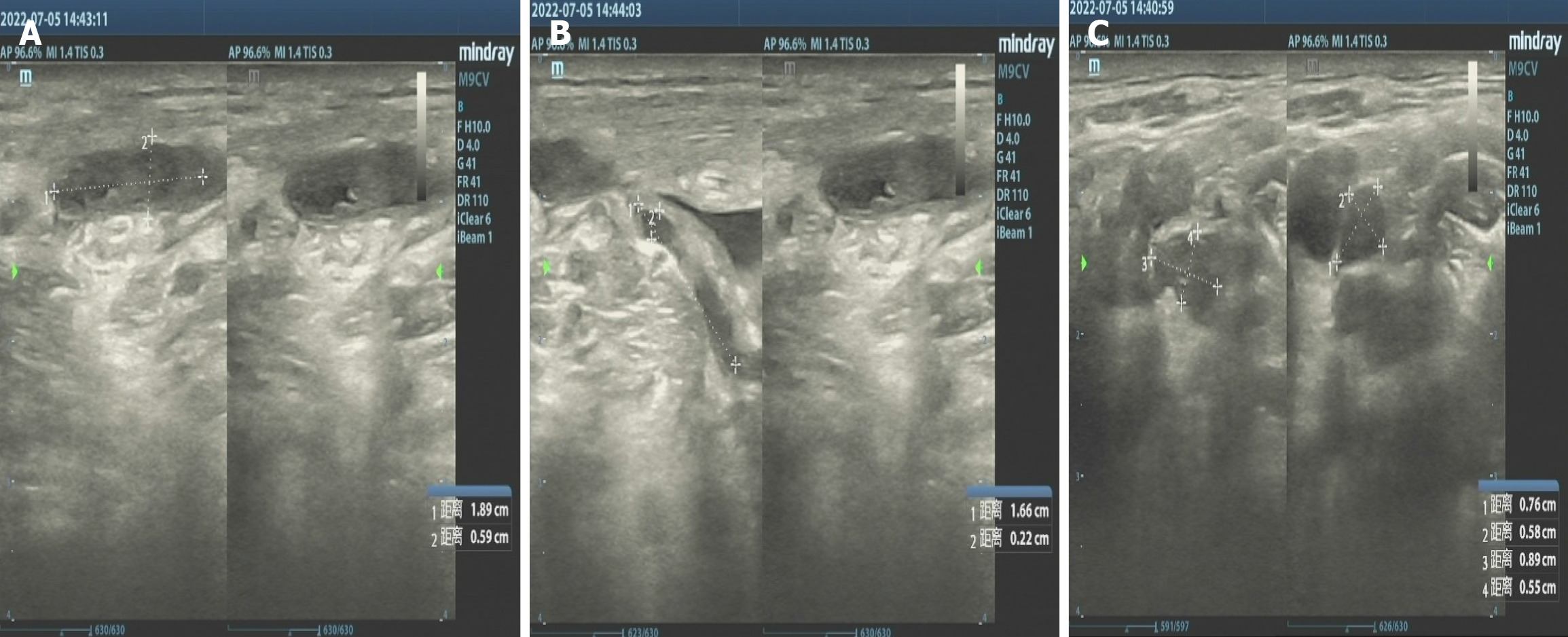
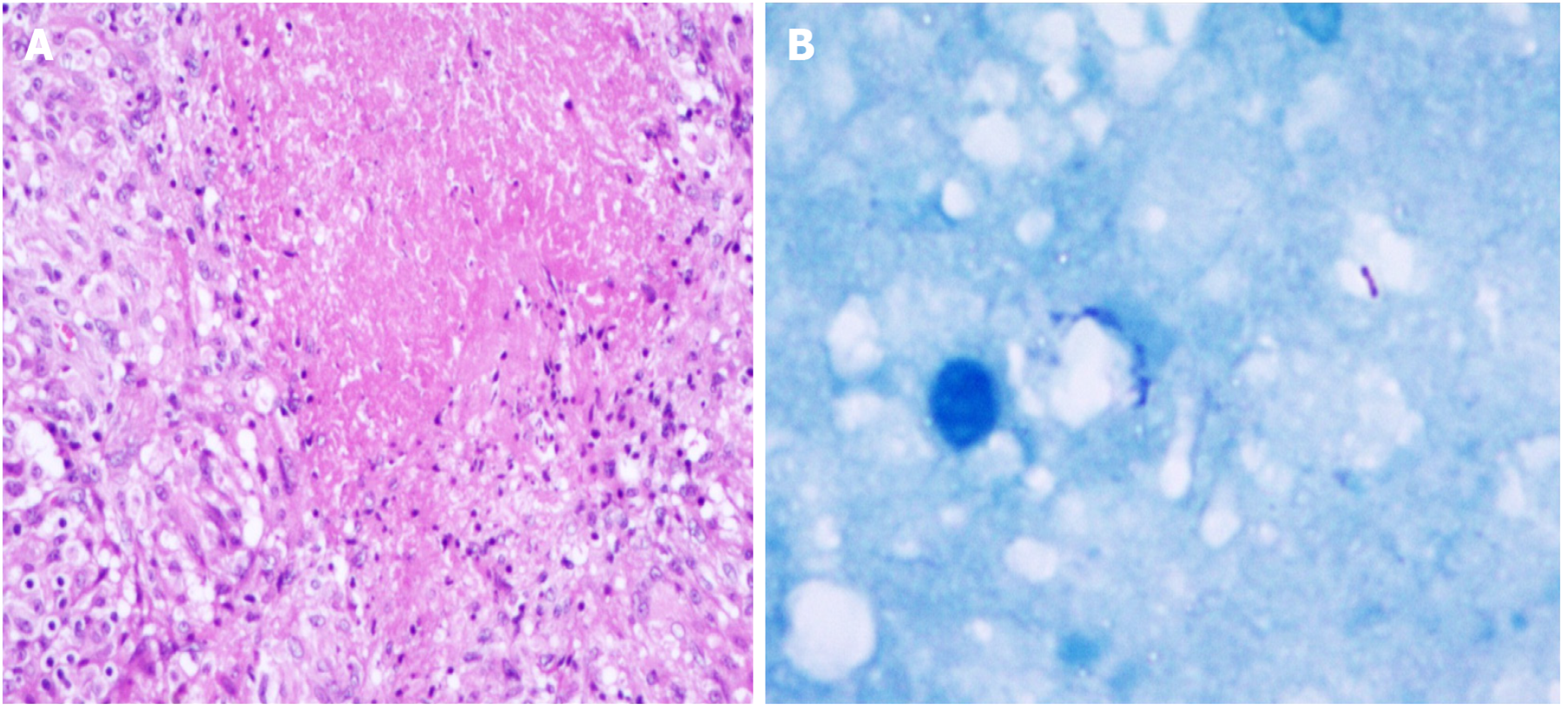
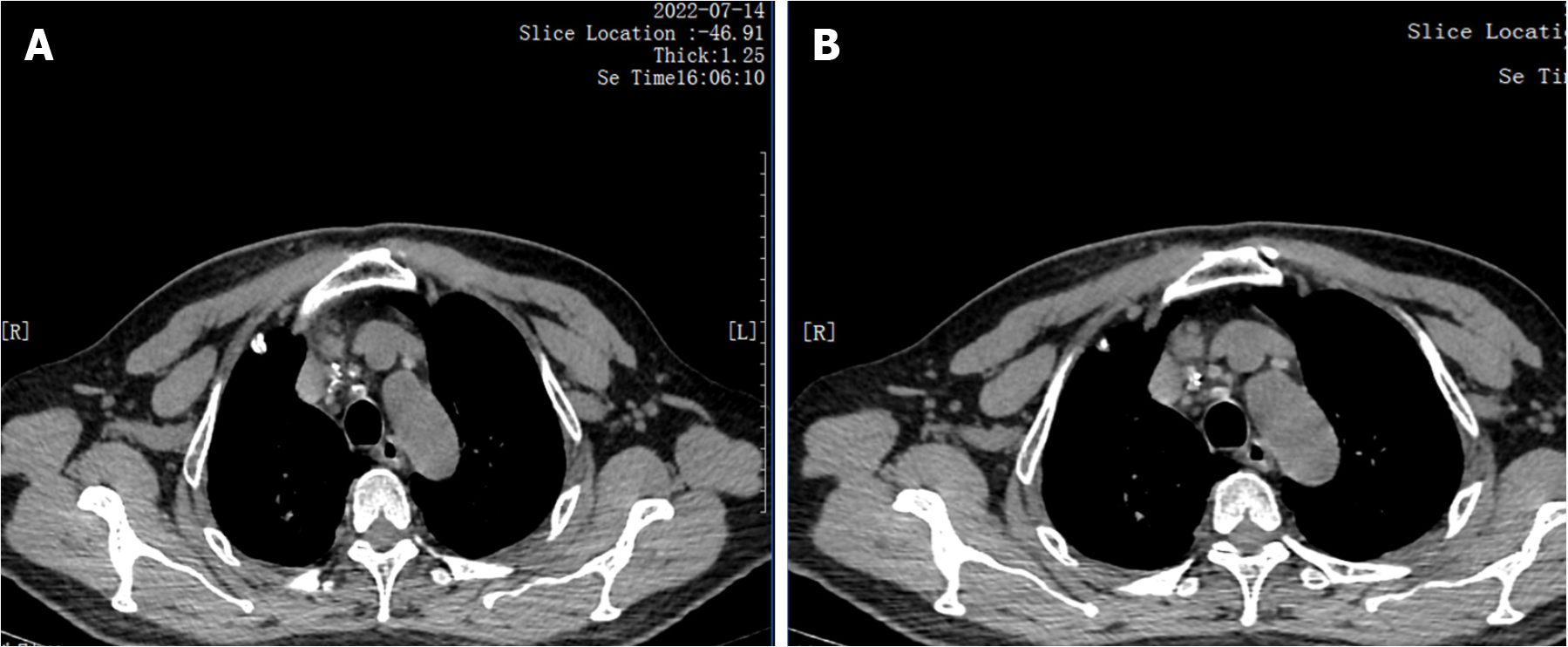
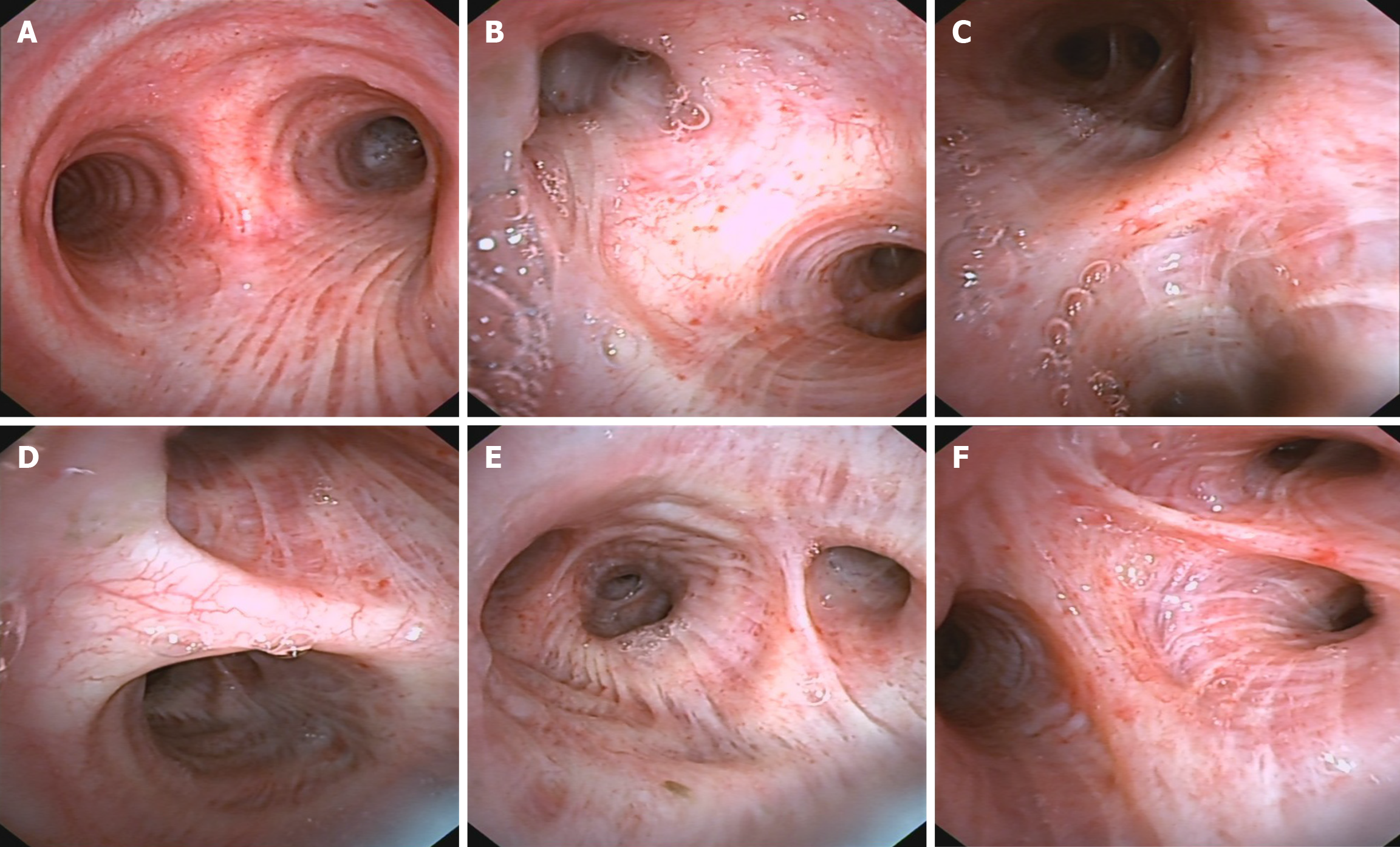
After being transferred to our hospital, the tuberculin test [purfied protein derivatives (PPD)], blood tuberculosis antibody and blood T-SPOT were all negative. Sputum tests for acid-fast bacilli were negative for many times. B-ultrasonography of superficial lymph nodes in the neck, groin and armpit showed low echo in the right neck with a little liquefaction. Please refer to clinical history, multiple lymph nodes in the left neck with partial enlargement. (Figure 3) Pathological consultation: Necrotizing granulomatous inflammation of (right neck mass), negative silver hexamine staining, negative acid-fast staining, and negative Mycobacterium tuberculosis polymerase chain reaction (PCR) results. The results of Mycobacterium tuberculosis identification were both positive for Mycobacterium gordoniasis, indicating NTM infection (Figure 4). Further improvement of chest CT showed that there were multiple miliary, nodular and cable strip shadows with increased density in both lungs, the boundary was unclear, the density was uneven, and calcification shadows were seen in part; there were enlarged and calcified lymph nodes in the mediastinum. Calcification shadow was observed in coronary artery. The pleura thickened on the right side and there was no significant effusion on both sides of the chest. Impressions: (1) Possible NTM lesions in both lungs; and (2) right pleurisy (Figures 5 and 6). Bronchoscopy showed that the trachea and the mucosa of the left and right bronchus were mildly hyperemia, edema, hyperplasia and hypertrophy. A few mucous secretions in the lumen were removed by suction, and the lumen was smooth without any new organisms. According to CT, bronchoalveolar lavage fluid (BALF) was collected in the upper lobar bronchus, and the brush examination for acid-fast bacilli was negative twice, while the DNA, RNA and Gxpert of Mycobacterium tuberculosis in BALF were negative. The culture of Mycobacterium tuberculosis BALF was negative for 42 d (Figure 7).
The patient weighs 67.5 kg and upon examination, HIV is negative. Treat with isoniazid 0.3qd, rifampicin 0.6 qd, ethambutol 1.0 qd, pyrazinamide 1.5 qd, moxifloxacin 0.4 qd, and clarithromycin 0.5 bid, respectively. Simultaneously monitor the patient's blood routine and liver and kidney function electrolytes. During the Q-T interval of electrocardiogram monitoring, acid bacteria smear and sputum culture of mycobacteria should be conducted at least once a month. Review B-type cervical or chest CT every 2-3 months. A follow-up examination two months later showed that the neck incision healed well without obvious redness or swelling. Neck ultrasound shows multiple lymph nodes on both sides of the neck. After analysis and comparison, the left side of the patient's neck is about 9 mm × 4 mm smaller than the front, and the right side is about 5 mm × 4 mm smaller than the front. Compared with the examination results on July 14, 2023, the absorption of both types of lung lesions in the patient has improved. The sputum culture results showed that the sputum smear of acid-fast bacteria was negative, and the sputum culture of mycobacteria was negative. The patient's blood cell count, liver and kidney function electrolytes, and electrocardiogram are normal. Afterwards, stop taking pyrazinamide. Continue to use isoniazid, rifampicin, ethambutol, moxifloxacin, and clarithromycin. The results of regular follow-up showed that the patient had no significant enlarged lymph nodes in the neck after surgery. No obvious abnormalities were found in both lungs, with calcification of mediastinal lymph nodes and bilateral pleural thickening. There is no obvious effusion in both chest cavities. The sputum acid-fast bacterial smear and Mycobacterium tuberculosis sputum culture were both negative. At this point, stop taking medication. The total treatment period is 14 months.
The follow-up results three months after discontinuation of medication showed that the patient had old surgical scars on the neck and no enlarged lymph nodes on both sides of the neck. No obvious respiratory symptoms such as cough, sputum, wheezing, fever, etc., auscultation of both lungs, clear breathing sounds, and no dry or wet rales. The neck chest CT is similar to 3 months ago. The sputum acid fast bacilli smear is negative.
NTM, defined as any strain of Mycobacterium tuberculosis other than Mycobacterium tuberculosis complex, is a distinct group of pathogens that contribute to a substantial but often under-appreciated global burden of disease. NTM causes disease similar to Mycobacterium tuberculosis, but usually does not respond to classical tuberculosis drug regimens[4-7]. Yeh and Tsai[8] reported a case of NTM infection after liposuction and fat transplantation. In the absence of bacterial growth in conventional cultures, NTM must be considered when facing a refractory infection after surgery or invasive surgery. Acid-fast staining and Mycobacterium tuberculosis culture are strongly recommended. The incidence of NTM in China has gradually exceeded that of tuberculosis, and the incidence of NTM in Asian population has increased to about 39.6 cases/100000 people per year, and is still rising rapidly at a rate of 19 cases/100000 people per year[9]. There are many kinds of NTM, and at least 50 kinds of NTM have been found. It can widely infect human lungs, skin, soft tissue, lymph nodes, blood and other parts. The clinical symptoms depend on the infection site. The symptoms of pulmonary infection are similar to those of Mycobacterium tuberculosis, and the clinical diagnosis is difficult. Naturally resistant to most anti-tuberculosis drugs, the anti-tuberculosis efficacy is often poor for patients who cannot correctly identify Mycobacterium tuberculosis or NTM in the early stage, and it is easy to lead to the emergence of drug-resistant bacteria[10-12].
NTM infections are growing rapidly, and it is difficult to achieve early and correct diagnosis with existing technologies. Even the NTM diagnosis rate of the strong top three hospitals is lower than 40%[13,14]. Clinical laboratory methods for the differential diagnosis of mycobacteria mainly include acid-fast staining, colony culture, drug test and PCR detection, but the disadvantages are also obvious, such as low positive rate, long cycle, low accuracy, many experimental consumables, high failure rate, and little drug resistance information, which are the main adverse factors for delaying disease diagnosis and clinical treatment. NTM infection is more difficult to treat than tuberculosis due to its complicated drug regimen, long course of treatment, poor efficacy and easy recurrence[15-17].
As shown in this study, the main symptoms of the patient were only the dominant cervical mass, which gradually increased, without the typical symptoms of early tuberculosis such as fever, cough, etc., which was not easy to detect in the early stage. It is easily misdiagnosed as neurogenic tumor or lymph node tuberculosis. Chest CT showed signs of atypical pulmonary and mediastinal tuberculosis, but it could not be used as a basis for differential diagnosis of NTM. If the tuberculosis occurs in the superficial position (such as skin, lymph nodes, etc.), further biopsy and pathological examination can be done. Pathological findings showed that lymphocyte showed granulomatous inflammation with coagulative necrosis, which could be used as the diagnostic basis for tuberculosis, but still could not be used for NTM differential diagnosis and strain identification. Immunohistochemical SOX 10 is usually used as a sensitivity marker for cutaneous malignancies, S-100 protein is usually used as a marker for neurogenic tumors, and CK protein is keratin. CD68 mostly indicates malignant tumors of macrophage origin, Ki-67 is a nuclear antigen associated with proliferating cells, and its elevated value usually indicates the possibility of malignant cells. Acid-fast staining is an important diagnostic basis for Mycobacterium tuberculosis.
PPD, blood TB antibody, blood T-SPOT and sputum examination for acid-fast bacilli are commonly used in clinical diagnosis of Mycobacterium tuberculosis. Nucleic acid time-of-flight mass spectrometry ("nucleic acid mass spectrometry" for short) enables the identification and strain identification of Mycobacterium tuberculosis and NTM, and provides sensitive antibiotics[18,19]. The main principle is multiplex PCR+ mass spectrometry detection of PCR products, with the high sensitivity of PCR technology, high throughput of chip technology, high precision of mass spectrometry technology and intelligent analysis of biological information, high repeatability, can detect pathogens and molecular typing, drug metabolism gene mutation, DNA methylation analysis and tumor mutation. The distribution of NTM strains in China may be regional, and the common strains mainly include intracellular type, abscess type, maasai type, bovine type, kansas type, secondary type and non-chromogenic type[20,21]. The drug resistance rate is about 92% for isoniazid, 95% for streptomycin, 70% for prothioisonicamide, 80% for rifampicin, 72% for capreomycin, 98% for cycloserine, 45% for ethambutol, 70% for amikacin, and 85% for ofloxacin[22].
At present, there is no clear treatment method for Gordon's disease, whether it is an intrapulmonary or extrapulmonary infection. Therefore, in terms of treatment methods, it is recommended to refer to the Diagnosis and Treatment Guidelines for NTM (2020 edition)[23]. Adequate, long-term, powerful anti-tuberculosis regimen is also suitable for NTM treatment. Sun et al[24] discussed the in vitro activity of fedasmin against NTM. Hong et al[25] pointed out that physiologically based pharmacokinetic modeling was used to guide the treatment of drug interactions between NTM and antibiotics. Explore the relationship between drug-drug interactions (DDI) and cystic fibrosis transmembrane conductance regulator (CFTR), The intrinsic relationship of CFTR regulation therapy is an important way to improve the clinical efficacy of Mycobacterium tuberculosis and NTM. Esacaffor, Tezacaffor, and ivacaftor (ETI) were evaluated for DDI risk in combination with ETI and NTM therapies, including rifambutin, clofazimine, and clarithromycin, primarily through cytochrome P450 3A-mediated metabolic elimination, in order to develop more efficient treatment options. When administered in combination with rifambutin, clofazimine, or clarithromycin, the predicted area under the dose-time curve ratios of ivacaftor were 0.31, 2.98, and 9.64, respectively, indicating moderate and strong interactions.
Careful selection of the right patient to treat for benefit is the first step in NTM clinical decision making. Although most patients diagnosed with NTM have no obvious pulmonary symptoms in the early stage, the lung disease will progress if timely drug intervention is not taken. NTM patients with positive acidobacter stain on sputum smear and pulmonary cavity should be treated with combined antituberculosis drugs immediately. The second step is to assess clinical benefits and risks. Since most NTM patients have broad-spectrum resistance, sputum culture Yin should not be the only treatment target for elderly patients with underlying diseases, and the main basis should be to reduce disease damage. The third step is strict adherence to the treatment regimen recommended by the guidelines. For example, macrolide antibiotics combined with aminoglycoside antibiotics can improve the cure rate of Mycobacterium tuberculosis avium complex group, and azithromycin is better than clarithromycin. The initial treatment of abscess disease in Marseille and Bolle should consist of injections with no less than 3 drugs, azithromycin instead of clarithromycin and imipenem instead of cefoxitin. The fourth step is to objectively understand the importance of drug sensitivity tests. The minimum inhibitory concentration of anti-infective drugs on NTM is not necessarily related to the efficacy. For example, Mycobacterium tuberculosis kansans should be tested for rifampicin susceptibility. Drug susceptibility test is limited to strains with high pathogenic potential, and liquid medium is recommended. The critical point of resistance of macrolide antibiotics to fast growing Mycobacterium tuberculosis is 8 mg/L, and drug susceptibility test should be actively conducted for NTM with possible drug resistance.
Through this case report and literature analysis of Mycobacterium gordoniasis in cervical lymph nodes, clinical data with certain reference value can be provided for the clinical diagnosis and treatment of this type of disease. However, due to limitations in the number of samples and literature, more clinical cases and literature data are needed to support it.
| 1. | Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev. 2014;27:727-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 2. | Ghielmetti G, Hilbe M, Friedel U, Menegatti C, Bacciarini L, Stephan R, Bloemberg G. Mycobacterial infections in wild boars (Sus scrofa) from Southern Switzerland: Diagnostic improvements, epidemiological situation and zoonotic potential. Transbound Emerg Dis. 2021;68:573-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Remot A, Carreras F, Coupé A, Doz-Deblauwe É, Boschiroli ML, Browne JA, Marquant Q, Descamps D, Archer F, Aseffa A, Germon P, Gordon SV, Winter N. Mycobacterial Infection of Precision-Cut Lung Slices Reveals Type 1 Interferon Pathway Is Locally Induced by Mycobacterium bovis but Not M. tuberculosis in a Cattle Breed. Front Vet Sci. 2021;8:696525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 4. | Li JX, Li G, Chen J, Jia W. [Clinical characteristics of nontuberculous mycobacterium lung disease]. Ningxia Yixue Zazhi. 2019;45:322-325. |
| 5. | Kumar K, Loebinger MR. Nontuberculous Mycobacterial Pulmonary Disease: Clinical Epidemiologic Features, Risk Factors, and Diagnosis: The Nontuberculous Mycobacterial Series. Chest. 2022;161:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Koh WJ. Nontuberculous Mycobacteria-Overview. Microbiol Spectr. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Jamal F, Hammer MM. Nontuberculous Mycobacterial Infections. Radiol Clin North Am. 2022;60:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 8. | Yeh JP, Tsai YJ. Nontuberculosis Mycobacteria Infection After Liposuction and Fat Grafting for Augmentation Mammoplasty: A Case Report and Literature Review. Ann Plast Surg. 2023;90:S55-S59. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Chen XK, Qiu DH, Shen WW, Zhu YL. [Distribution characteristics and epidemic status of non-tuberculous mycobacterium in Taizhou]. Zhongguo Weisheng Jianyan Zazhi. 2019;33:536-538. |
| 10. | Duan HF. [Timely grasp the research progress and effectively improve the treatment level of nontuberculous mycobacteriosis]. Zhongguo Fanglao Zazhi. 2019;45:329-332. |
| 11. | Mei L, Liu SS, Lin WH, Zhang YB. [Distribution, clinical characteristics and drug resistance of non-tuberculous Mycobacterium pneumonitis strains in a special hospital in Anhui province]. Zhongguo Kangshengsu Zazhi. 2019;8:1-7. |
| 12. | Khawbung JL, Nath D, Chakraborty S. Drug resistant Tuberculosis: A review. Comp Immunol Microbiol Infect Dis. 2021;74:101574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | Huang LP, He HQ, Zou W, Hu XL, Li HR, Chen YS, Xie BS, Xu NL. [Clinical characteristics and strain identification of nontuberculous mycobacterium pulmonary disease]. Zhonghua Yiyuan Ganranxue Zazhi. 2019;33:641-646. |
| 14. | Gopalaswamy R, Shanmugam S, Mondal R, Subbian S. Of tuberculosis and non-tuberculous mycobacterial infections - a comparative analysis of epidemiology, diagnosis and treatment. J Biomed Sci. 2020;27:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 15. | Zhou M, Zhou Y, Huang H, Peng J. [Clinical characteristics of fast growing and slow growing non-tuberculous mycobacterium lung disease]. Linchuang Neike Zazhi. 2002;39:755-757. |
| 16. | Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR Jr. Management of drug-resistant tuberculosis. Lancet. 2019;394:953-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 17. | Kroó M, Cernoch Z, Rezác O. [Tactics in the treatment of brain abscesses in the CT era-- experience, problems, perspectives]. Cesk Neurol Neurochir. 1987;50:125-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 18. | Liang JQ, Wu XQ, An HR. [Clinical application of nucleic acid matrix assisted laser desorption ionization time-of-flight mass spectrometry in the diagnosis of tuberculosis and nontuberculous mycobacteriosis]. Zhongguo Fanglao Zazhi. 2019;45:543-558. |
| 19. | Procop GW. Laboratory Diagnosis and Susceptibility Testing for Mycobacterium tuberculosis. Microbiol Spectr. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Liu HL, Li YK, Dong WM, Zheng D, Yu SJ, Song YZ. [Distribution of non-tuberculous mycobacterium strains in alveolar lavage fluid of patients with suspected pulmonary tuberculosis and anti-tuberculosis drug resistance]. Zhonghua Yiyuan Ganranxue Zazhi. 2019;33:1926-1930. |
| 21. | Fröberg G, Maurer FP, Chryssanthou E, Fernström L, Benmansour H, Boarbi S, Mengshoel AT, Keller PM, Viveiros M, Machado D, Fitzgibbon MM, Mok S, Werngren J, Cirillo DM, Alcaide F, Hyyryläinen HL, Aubry A, Andres S, Nadarajan D, Svensson E, Turnidge J, Giske CG, Kahlmeter G, Cambau E, van Ingen J, Schön T; EUCAST AMST and ESCMYC study groups. Towards clinical breakpoints for non-tuberculous mycobacteria - Determination of epidemiological cut off values for the Mycobacterium avium complex and Mycobacterium abscessus using broth microdilution. Clin Microbiol Infect. 2023;29:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 22. | Velanovich V, McHugh TP, Smith DJ Jr, Geldner P, Robson MC, Boertman J, Heggers JP. Digital replantation and revascularization. Factors affecting viability, prognosis, and pattern of injury. Am Surg. 1988;54:598-601. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Liu SS, Tang SJ. [Interpretation of the Diagnosis and Treatment Guidelines for Non-Mycobacterial Tuberculosis (2020 Edition)]. Jiehe Yu Feibu Jibing Zazhi. 2021;2:108-115. |
| 24. | Sun Q, Liao X, Wang C, Jiang G, Yang J, Zhao J, Huang H, Wang G, Li H. In vitro activity of fidaxomicin against nontuberculosis mycobacteria. J Med Microbiol. 2022;71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Hong E, Almond LM, Chung PS, Rao AP, Beringer PM. Physiologically Based Pharmacokinetic Modeling To Guide Management of Drug Interactions between Elexacaftor-Tezacaftor-Ivacaftor and Antibiotics for the Treatment of Nontuberculous Mycobacteria. Antimicrob Agents Chemother. 2022;66:e0110422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |