Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.3978
Revised: April 23, 2024
Accepted: May 10, 2024
Published online: July 6, 2024
Processing time: 122 Days and 8.9 Hours
Congenital sideroblastic anemia (CSA) is a rare and heterogeneous group of genetic disorders. Conventional treatment include pyridoxine (vitamin B6) and allogeneic hematopoietic stem cell transplantation (allo-HSCT), and can alleviate anemia in the majority of cases. Nevertheless, some CSA cases remain unres
We share our experience in luspatercept in a 4-year-old male patient with CSA. Luspatercept was administered subcutaneously at doses of 1.0 mg/kg/dose to 1.25 mg/kg/dose every 3 wk, three consecutive doses, evaluating the hematological response. Luspatercept leading to a significant improvement in the patient's anemia. The median hemoglobin during the overall treatment with three doses of luspatercept was 90 (75-101) g/L, the median absolute reticulocyte count was 0.0593 (0.0277-0.1030) × 1012/L, the median serum ferritin was 304.3 (234.4-399) ng/mL, and the median lifespan of mature red blood cells was 80 (57-92) days. Notably, no adverse reactions, such as headaches, dizziness, vomiting, joint pain, or back pain, were observed during the treatment period.
We believe that luspatercept might emerge as a viable therapeutic option for the maintenance treatment of CSA or as a bridging treatment option before hemato
Core Tip: Congenital sideroblastic anemia (CSA) is a rare and heterogeneous group of genetic disorders. Conventional treatment include pyridoxine (vitamin B6) and allogeneic hematopoietic stem cell transplantation (allo-HSCT), and can alleviate anemia in the majority of cases. Nevertheless, some CSA cases remain unresponsive to pyridoxine or are unable to undergo allo-HSCT. Novel management approaches is necessary to be developed. We present a case of luspatercept administered to enhance hemoglobin Levels.
- Citation: Li Y, Ye L, Zhou K, Fan HH, Li JP, Xiong YZ, Yang Y, Peng GX, Yang WR, Zhao X, Jing LP, Zhang L, Zhang FK. Luspatercept enhances hemoglobin levels in a Chinese boy with congenital sideroblastic anemia: A case report. World J Clin Cases 2024; 12(19): 3978-3984
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/3978.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.3978
Congenital sideroblastic anemia (CSA) is a rare and heterogeneous group of genetic disorders. It is characterized by ineffective erythropoiesis and the presence of bone marrow ring sideroblasts, which reflect excess mitochondrial iron deposition in erythroid precursors[1,2]. Mutations in genes related to heme biosynthesis, iron-sulfur cluster biogenesis, or mitochondrial protein synthesis underlie the fundamental pathogenesis of this condition. In the condition, production of the heme of hemoglobin is reduced, and maturation failure and apoptosis of erythroid precursors appeared in the bone marrow, known as ineffective erythropoiesis. X-linked sideroblastic anemia (XLSA) associated with mutations in the ALAS2 gene is the most common subtype, while others, such as autosomal recessive CSA linked to mutations in the SLC25A38 gene, are exceedingly rare. Traditional treatment options for CSA encompass primary disease management and symptomatic treatment. Primary disease management primarily involves interventions such as allogeneic hematopoietic stem cell transplantation and pharmacotherapy. However, allogeneic hematopoietic stem cell transplantation is limited by disease severity, patient tolerability, and donor availability, with limited clinical data available. Pharmacotherapy includes pyridoxine (vitamin B6), which can improve ineffective erythropoiesis and alleviate anemia in the majority of XLSA cases. Nevertheless, some XLSA and other CSA types remains unresponsive to pyri
Luspatercept[3], is a recombinant fusion protein composed of a modified extracellular domain of human activin receptor IIB (ActR-IIB) linked to the crystallizable fragment region of human immunoglobulin G1. Luspatercept is a novel clinical drug with its primary active component being a ligand trap protein for ActR-II, targeting ineffective erythropoiesis. This medication works by binding to transforming growth factor β superfamily ligands, thereby blocking their interaction with ActR-II and subsequently inhibiting downstream SMAD2/3 signaling pathways, enhanced latestage erythroid maturation in the bone marrow, leading to an improvement in ineffective erythropoiesis. Luspatercept has demonstrated clear efficacy and good safety profiles in anemia-related conditions characterized by ineffective erythropoiesis, such as thalassemia. Due to the shared pathophysiological characteristics of ineffective erythropoiesis in CSA and similar clinical features to anemias like thalassemia, it is hypothesized that luspatercept may also be applicable in the treatment of CSA.
Currently, there are no clinical reports of luspatercept monotherapy being used to treat CSA in domestic settings[4]. In this report, we described the application of luspatercept in the treatment of one CSA patient who had no response to pyridoxine, resulting in an improvement in anemia.
The patient, a 4-year-old male, initially presented to our hospital on April 14, 2023, with the chief complaint of "discovered anemia for 2.5 years".
At the age of 1.5 years, he exhibited pallor, and a blood routine examination revealed "microcytic anemia". Oral iron supplementation was initiated, but the anemia showed no improvement. In the 6 months leading up to his admission, his hemoglobin (HGB) levels consistently ranged between 70 g/L and 75 g/L. One month before his admission, he had a follow-up examination at a local hospital, which yielded the following results: HGB 75 g/L, MCV (mean corpuscular volume) 68.9 fL, MCHC (mean corpuscular hemoglobin concentration) 293 g/L, ARC (absolute reticulocyte count) 0.05 × 1012/L, RET (reticulocyte percentage) 1.47%, WBC (white blood cell count) 4.58 × 109/L, PLT (platelet count) 548 × 109/L. Serum iron levels were 52.14 µmol/L, total iron-binding capacity (TIBC) was 52.15 µmol/L, transferrin saturation (TSAT) was 99.98%, and serum ferritin (SF) was 1595 ng/mL. Bone marrow iron staining indicated the presence of 50% ringed sideroblasts. A provisional diagnosis of "sideroblastic anemia" was made, and two months of treatment commenced with vitamin B6 at 100 mg per day, along with folic acid and chelation therapy. Unfortunately, despite these interventions, the anemia did not show any improvement.
The patient denied past illness was identified.
The patient denied any family history of anemia and malignant tumours.
On physical examination, the anemia appearance was seen.
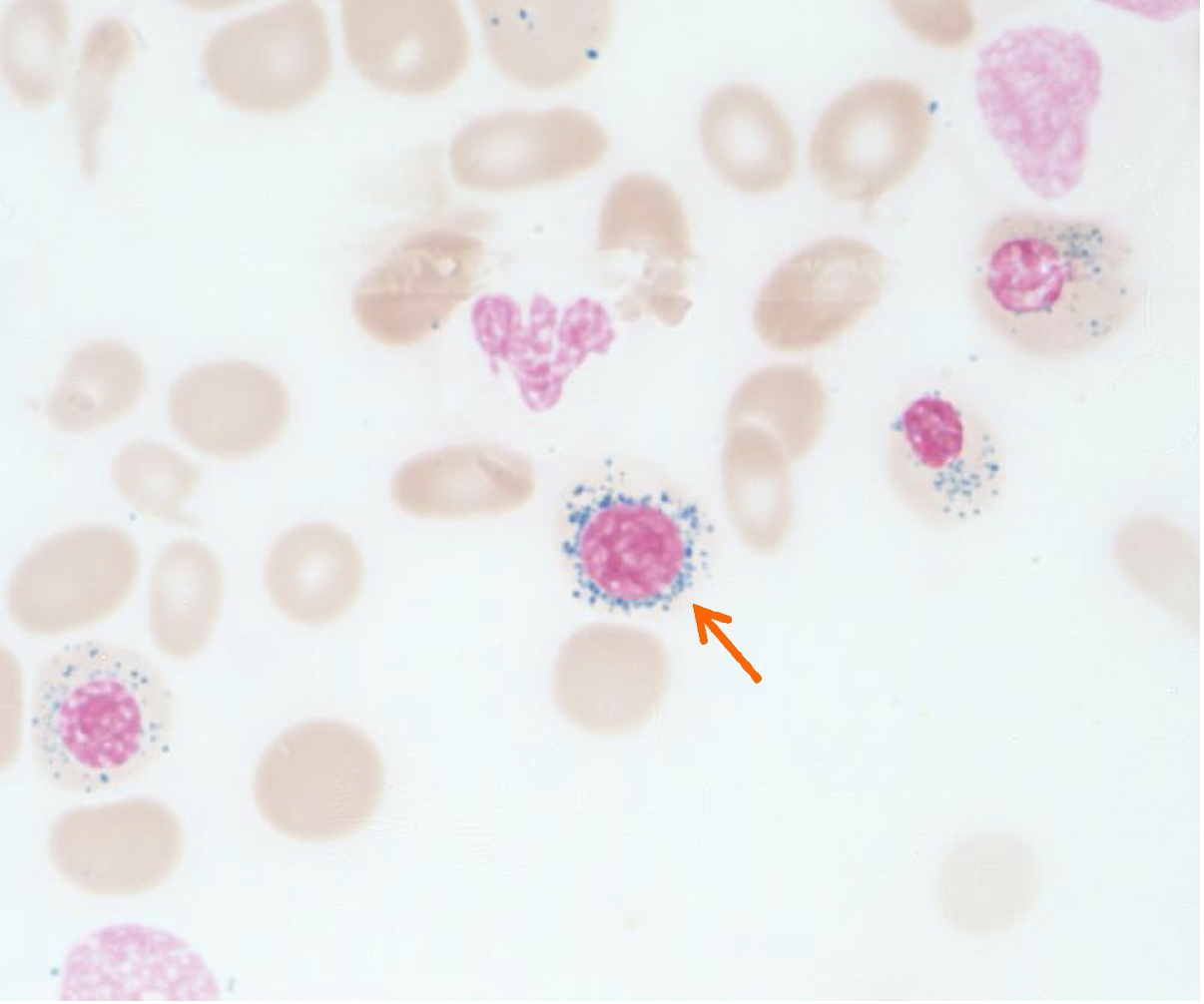
Upon admission, a blood routine examination showed: HGB 74 g/L, MCV 68 fL, MCHC 289 g/L, ARC 0.05 × 1012/L, RET 1.46%, WBC 5.56 × 109/L, and PLT 439 × 109/L. Mature red blood cells were predominantly small, with some cells exhibiting enlarged central pallor. Serum iron levels were 48.2 µmol/L, TIBC was 53.48 µmol/L, TSAT was 90%, and SF was 234.4 ng/mL (Table 1). The CO breath test indicated a red cell lifespan of 57 d. HGB electrophoresis did not reveal any abnormal bands. Liver function, renal function, cardiac function, HBA2, HBF, and other parameters were all within the normal range. The bone marrow examination showed active hematopoiesis, with the erythroid lineage comprising 35.5% of the total, primarily consisting of intermediate and late erythroblasts (10.5% and 24%, respectively) with noticeable areas of cytoplasmic pallor. Additionally, 12 megakaryocytes were observed throughout the smear, accompanied by platelet aggregates. Bone marrow iron staining indicated extracellular iron deposits (++), 97% of sideroblasts, and 18% of ringed sideroblasts within the erythroblasts (Figure 1). Genetic analysis for congenital red blood cell disorders did not reveal any mutations in known CSA-related pathogenic genes, such as ALAS2 and SLC25A38, nor were mutations related to conditions like thalassemia identified.
| Subject | Value |
| Gender | Male |
| Age (yr) | 4 |
| Complete blood count | |
| WBC (× 109/L) | 4.58 |
| RBC (× 1012/L) | 3.66 |
| HGB (g/L) | 74 |
| PLT (× 109/L) | 548 |
| MCV (fL) | 68.9 |
| MCH (pg) | 20.2 |
| MCHC (g/L) | 293 |
| ANC (× 109/L) | 1.33 |
| RET (%) | 1.47 |
| ARC (× 1012/L) | 0.05 |
| ALT (U/L) | 31.6 |
| AST (U/L) | 40.7 |
| DBIL (µmol/L) | 4.1 |
| TBIL (µmol/L) | 14.9 |
| LDH (U/L) | 215 |
| SF (ng/mL) | 228.9 |
| Iron (µmol/L) | 44.95 |
| UIBC (µmol/L) | 5.06 |
| TIBC (µmol/L) | 50.01 |
| ISAT | 0.9 |
| EPO (mIU/mL) | 16.31 |
| Mature red blood cell lifespan (d) | 57 |
| IgG (g/L) | 4.95 |
| IgA (g/L) | 0.66 |
| IgM (g/L) | 1.09 |
| Erythroid lineage cells (%) | 35.5 |
| Megakaryocytes | 12 |
| Extracellular iron deposits | 2+ |
| Sideroblasts (%) | 97 |
| Ringed sideroblasts within the erythroblasts (%) | 18 |
| Bone marrow PAS positivity (%) | 24 |
| Bone marrow PAS positivity index | 46 |
| NGS gene | pathogenic mutations in responsible genes not detected |
Ultrasonography did not reveal hepatosplenomegaly.
Combined with the patient’s medical history, the final diagnosis was congenital sideroblastic anemia.
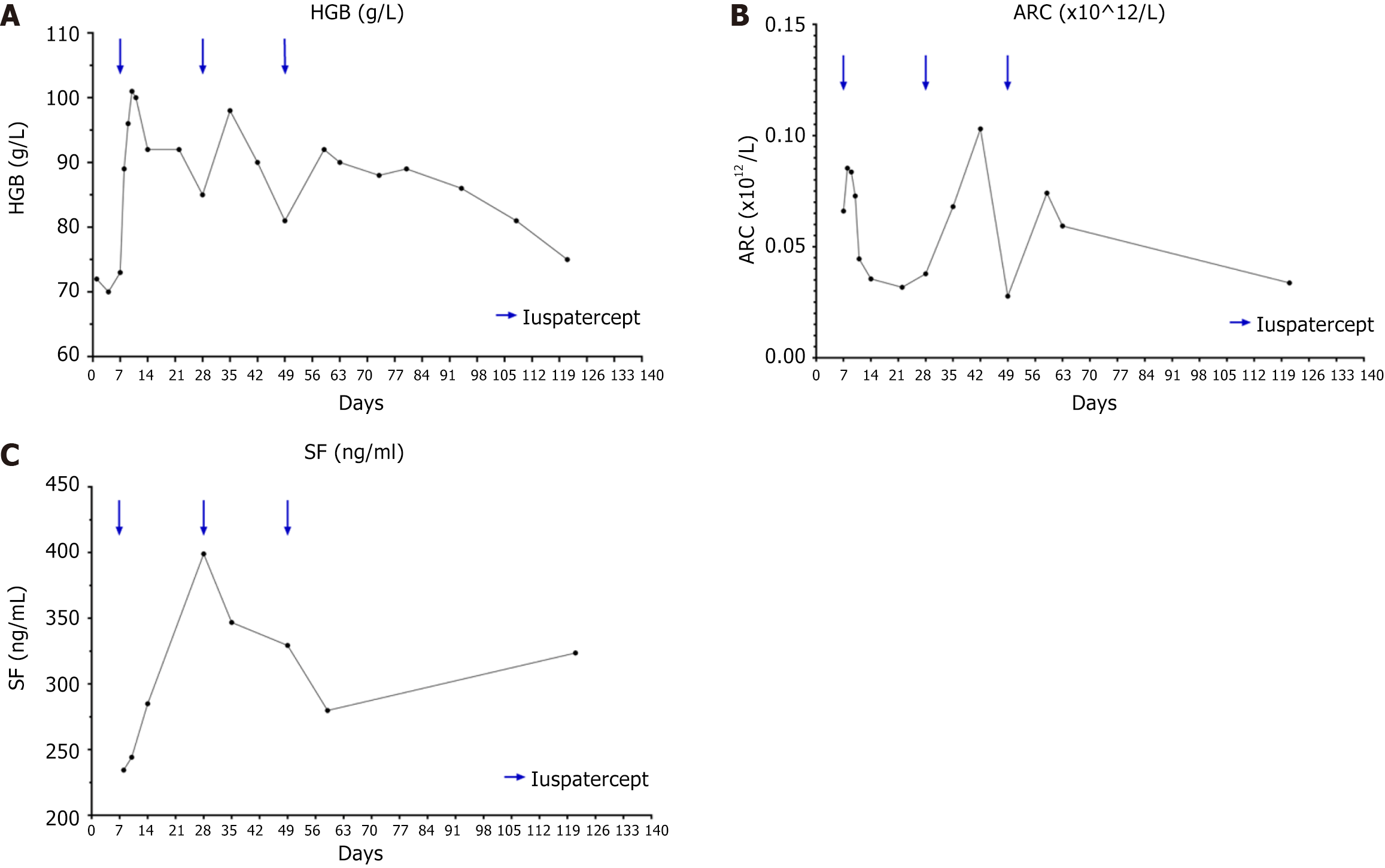
The treatment with luspatercept was initiated (Figure 1). Luspatercept was administered on days 7, 28, and 49 after admission at doses of 1.0 mg/kg, 1.25 mg/kg, and 1.25 mg/kg, respectively.
The treatment leading to a significant improvement in the patient's anemia (Figure 2A). Following the first dose, HGB levels began to rise on day 8, peaked on day 9, and started to decline on day 12, returning to pre-treatment levels by day 27, with an effective duration of 21 d. The change in HGB levels following the second dose mirrored the first. After the third dose, the timing of HGB increase, peak, and decrease matched the first dose, but it took until day 108 after the third dose for HGB to return to pre-treatment levels, with an effective duration of approximately 2 months. The median HGB during the overall treatment with three doses of luspatercept was 90 (75-101) g/L, the median ARC was 0.0593 (0.0277-0.1030) × 1012/L (Figure 2B), the median SF was 304.3 (234.4-399) ng/mL (Figure 2C), and the median lifespan of mature red blood cells was 80 (57-92) d. Notably, no adverse reactions, such as headaches, dizziness, vomiting, joint pain, or back pain, were observed during the treatment period. A fourth dose of luspatercept was planned.
In this study, luspatercept demonstrated rapid and effective improvement in CSA, alleviating anemia, eliminating the need for blood transfusions, and prolonging the lifespan of mature red blood cells, achieving the desired therapeutic outcomes. After the administration of luspatercept, the median HGB level reached 90 (range: 75-101) g/L. Compared to the highest HGB level of 75 g/L observed in the 6 months prior to starting luspatercept treatment, the median improvement in anemia was 15 g/L (20%), with a maximum improvement of 27 g/L (36%). When considering the research endpoint criteria for luspatercept treatment in myelodysplastic syndrome (MDS) established by Fenaux et al[5] and the efficacy criteria for luspatercept treatment in MDS with ringed sideroblasts (MDS-RS) as outlined by Zeidan et al[6], the patient felt within the category of a favorable treatment response. Similar to our study, Van Dijck et al[7] have used luspatercept to treat adult XLSA and achieved an increase in HGB to 90 g/L, with a median improvement in anemia of approximately 30 g/L (30%). This improvement is slightly higher than the one observed in our study, possibly due to the ongoing transfusion support during their treatment. In this case, the patient exhibited a remarkably swift improvement in anemia following luspatercept treatment, with the maximum response reached within 1 wk. Similar to Van Dijck et al[7], Piga et al[8], and Cappellini et al[9], we also observed a rapid response to luspatercept, with a median time to initial response of 8 d.
However, in contrast to previous studies[6-8], the duration of treatment efficacy in this patient after luspatercept treatment was relatively short. Clinical trials conducted by Piga et al[8] have suggested that the high-dose luspatercept group achieves a more favorable response rate and response quality. Piga et al[8] showed that eighteen non–transfusion-dependent thalassemia patients (58%) receiving higher dose levels of luspatercept (0.6-1.25 mg/kg) achieved mean hemoglobin increase ≥ 1.5 g/dL over ≥14 days vs baseline. In contrast to Piga et al[8], Cappellini et al[9], and Van Dijck et al[7], our study employed luspatercept less frequently. Furthermore, we observed that as the luspatercept dose and frequency increased, the rate of HGB decline slowed, and there was a trend toward a longer duration of effect. Therefore, the relatively infrequent use of luspatercept in our study might have contributed to the shorter duration of anemia improvement.
In this study, following the administration of the initial dose of luspatercept, SF levels continued to increase compared to pre-treatment levels. However, with subsequent doses (the second and third doses), SF levels remained relatively stable, with no further significant increase. This finding suggested that the initial rise in SF might be attributed to transfusions before the first dose. In addition, the subsequent stability in SF levels indicated that luspatercept slowed down the progression of iron overload, effectively reducing the iron burden. The maximum decrease observed in SF was approximately 120 ng/mL, accompanied by a maximum decrease in TSAT of around 72%. While the assessment parameters of iron metabolism employed in this study might differ from those in other research, these findings were in line with the conclusions drawn by Piga et al[8] and Cappellini et al[9]. In Cappellini et al's phase III clinical trial evaluating luspatercept treatment for transfusion-dependent beta-thalassemia[9], a significantly higher proportion of patients in the luspatercept group achieves a 33% reduction in transfusion burden during any 12-wk period compared to the placebo group (70.5% vs 29.5%; odds ratio, 5.69; 95%CI: 3.46 to 9.35). Additionally, at week 48, there is a significant decrease in SF levels in the luspatercept group compared to the placebo group (−348 μg/L, 95% confidence interval, -517 to -179). These results support the notion that luspatercept's mechanism of action likely involves reducing iron burden, possibly by improving ineffective erythropoiesis. In our present study, we utilized the CO breath test[10] to assess the lifespan of mature red blood cells in peripheral blood. This method indirectly estimates the lifespan of mature red blood cells by measuring the concentration of CO produced during hemolysis. It can serve as an indicator of the severity of hemolytic anemia and provide a reference point for assessing the extent of ineffective erythropoiesis. Through dynamic monitoring, our study identified an improvement in the red blood cell lifespan that correlated with the clinical course, thereby partially validating the hypothesis of ameliorating ineffective erythropoiesis through luspatercept treatment.
This study has certain limitations. First, the sample size was small, and to arrive at more objective conclusions, larger-scale clinical trials are necessary. Second, in this study, no adverse events related to the use of luspatercept occurred. However, it is worth noting that luspatercept is currently indicated for adult patients. To our knowledge, there have been no reports of luspatercept being used to treat pediatric patients. An ongoing clinical study (NCT04143724) is investigating the use of luspatercept in treating pediatric patients with thalassemia, and we anticipate that it will provide reliable and favorable safety data.
In summary, this study utilized luspatercept to treat a single pediatric patient with CSA, resulting in improvements in anemia and iron overload. We also analyzed potential mechanisms of luspatercept's efficacy through measurements of mature red blood cell lifespan. We believe that luspatercept might emerge as a viable therapeutic option for the maintenance treatment of CSA or as a bridging treatment option before hematopoietic stem cell transplantation. In the future, we intend to conduct additional clinical trials to further explore its potential.
| 1. | Cazzola M. Ineffective erythropoiesis and its treatment. Blood. 2022;139:2460-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Fujiwara T, Harigae H. Molecular pathophysiology and genetic mutations in congenital sideroblastic anemia. Free Radic Biol Med. 2019;133:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Hatzimichael E, Timotheatou D, Koumpis E, Benetatos L, Makis A. Luspatercept: A New Tool for the Treatment of Anemia Related to β-Thalassemia, Myelodysplastic Syndromes and Primary Myelofibrosis. Diseases. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Shao Y, He L, Ding S, Fu R. Luspatercept for the treatment of congenital sideroblastic anemia: Two case reports. Curr Res Transl Med. 2024;72:103438. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Fenaux P, Platzbecker U, Mufti GJ, Garcia-Manero G, Buckstein R, Santini V, Díez-Campelo M, Finelli C, Cazzola M, Ilhan O, Sekeres MA, Falantes JF, Arrizabalaga B, Salvi F, Giai V, Vyas P, Bowen D, Selleslag D, DeZern AE, Jurcic JG, Germing U, Götze KS, Quesnel B, Beyne-Rauzy O, Cluzeau T, Voso MT, Mazure D, Vellenga E, Greenberg PL, Hellström-Lindberg E, Zeidan AM, Adès L, Verma A, Savona MR, Laadem A, Benzohra A, Zhang J, Rampersad A, Dunshee DR, Linde PG, Sherman ML, Komrokji RS, List AF. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N Engl J Med. 2020;382:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 369] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 6. | Zeidan AM, Platzbecker U, Garcia-Manero G, Sekeres MA, Fenaux P, DeZern AE, Greenberg PL, Savona MR, Jurcic JG, Verma AK, Mufti GJ, Buckstein R, Santini V, Shetty JK, Ito R, Zhang J, Zhang G, Ha X, Backstrom JT, Komrokji RS. Longer-term benefit of luspatercept in transfusion-dependent lower-risk myelodysplastic syndromes with ring sideroblasts. Blood. 2022;140:2170-2174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Van Dijck R, Goncalves Silva AM, Rijneveld AW. Luspatercept as Potential Treatment for Congenital Sideroblastic Anemia. N Engl J Med. 2023;388:1435-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Piga A, Perrotta S, Gamberini MR, Voskaridou E, Melpignano A, Filosa A, Caruso V, Pietrangelo A, Longo F, Tartaglione I, Borgna-Pignatti C, Zhang X, Laadem A, Sherman ML, Attie KM. Luspatercept improves hemoglobin levels and blood transfusion requirements in a study of patients with β-thalassemia. Blood. 2019;133:1279-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Cappellini MD, Viprakasit V, Taher AT, Georgiev P, Kuo KHM, Coates T, Voskaridou E, Liew HK, Pazgal-Kobrowski I, Forni GL, Perrotta S, Khelif A, Lal A, Kattamis A, Vlachaki E, Origa R, Aydinok Y, Bejaoui M, Ho PJ, Chew LP, Bee PC, Lim SM, Lu MY, Tantiworawit A, Ganeva P, Gercheva L, Shah F, Neufeld EJ, Thompson A, Laadem A, Shetty JK, Zou J, Zhang J, Miteva D, Zinger T, Linde PG, Sherman ML, Hermine O, Porter J, Piga A; BELIEVE Investigators. A Phase 3 Trial of Luspatercept in Patients with Transfusion-Dependent β-Thalassemia. N Engl J Med. 2020;382:1219-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 10. | Ye L, Ji Y, Zhou C, Luo J, Zhang L, Jing L, Zhao X, Guo J, Gao Q, Peng G, Li Y, Li J, Fan H, Yang W, Yang Y, Ma Y, Zhang F. Comparison of Levitt's CO breath test and the (15) N-glycine labeling technique for measuring the lifespan of human red blood cells. Am J Hematol. 2021;96:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |