Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.3942
Revised: April 24, 2024
Accepted: May 11, 2024
Published online: July 6, 2024
Processing time: 128 Days and 3.9 Hours
This study presents the clinical and genetic mutation characteristics of an unusual case of adult-onset diabetes mellitus occurring in adolescence, featuring a unique mutation in the peroxisome proliferator-activated receptor gamma (PPARG) gene. Data Access Statement: Research data supporting this publication are available from the NN repository at www.NNN.org/download/.
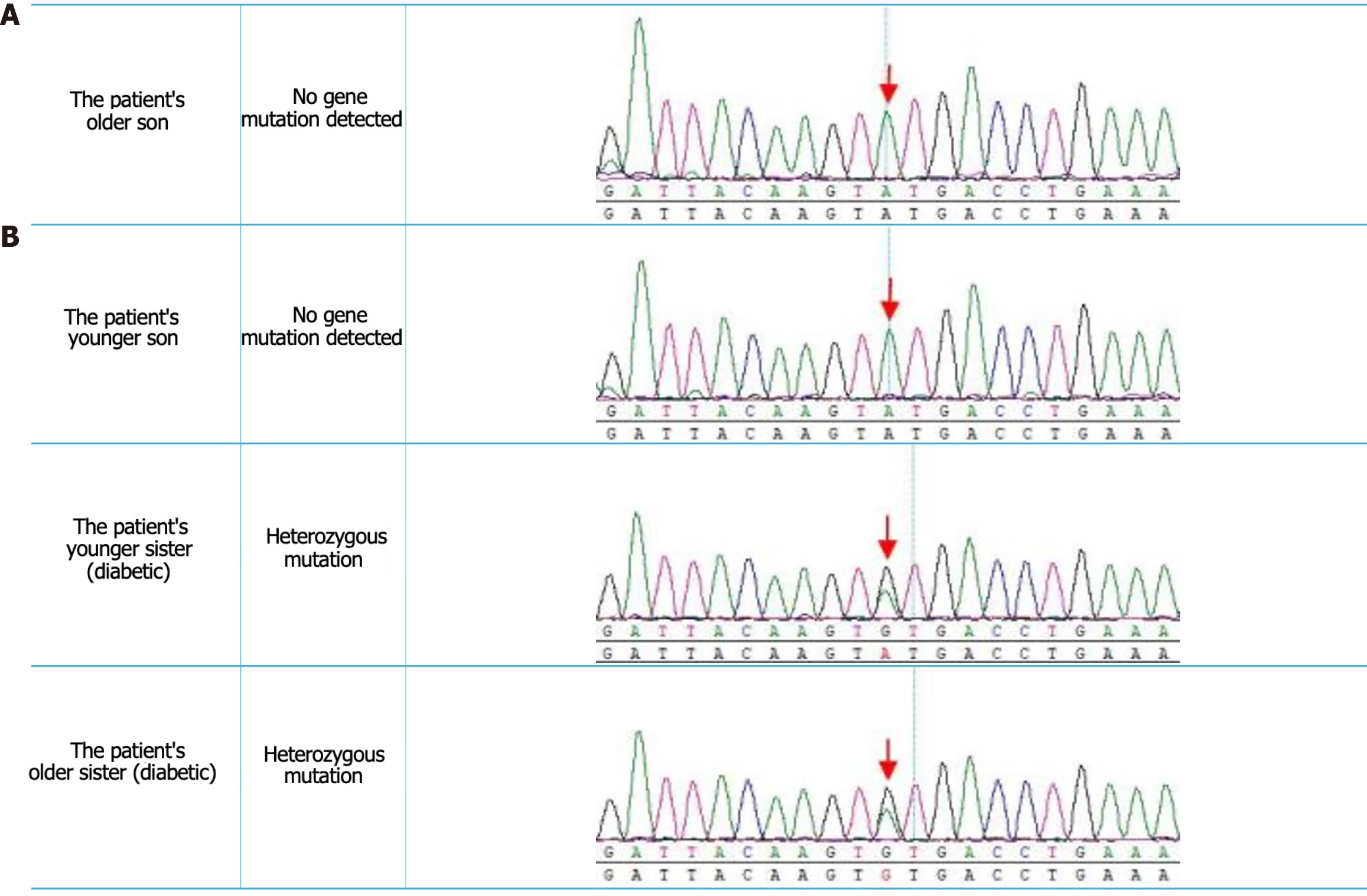
The methodology employed entailed meticulous collection of comprehensive clinical data from the probands and their respective family members. Additionally, high-throughput sequencing was conducted to analyze the PPARG genes of the patient, her siblings, and their offspring. The results of this investigation revealed that the patient initially exhibited elevated blood glucose levels during pregnancy, accompanied by insulin resistance and hypertriglyceridemia. Furthermore, these strains displayed increased susceptibility to diabetic kidney disease without any discernible aggregation patterns. The results from the gene detection process demonstrated a heterozygous mutation of guanine (G) at position 284 in the coding region of exon 2 of PPARG, which replaced the base adenine (A) (exon2c.284A>Gp.Tyr95Cys). This missense mutation resulted in the substitution of tyrosine with cysteine at the 95th position of the translated protein. Notably, both of her siblings harbored a nucleotide heterozygous variation at the same site, and both were diagnosed with diabetes.
The PPARG gene mutation, particularly the p.Tyr95Cys mutation, may represent a newly identified subtype of maturity-onset diabetes of the young. This subtype is characterized by insulin resistance and lipid metabolism disorders.
Core Tip: The results from the gene detection process demonstrated a heterozygous mutation of guanine (G) at position 284 in the coding region of exon 2 of peroxisome proliferator-activated receptor gamma (PPARG), which replaced the base adenine (A) (exon2c.284A>Gp.Tyr95Cys). This missense mutation resulted in the substitution of tyrosine with cysteine at the 95th position of the translated protein. Notably, both of the patient’s siblings harbored a nucleotide heterozygous variation at the same site, and both were diagnosed with diabetes, suggesting that the PPARG gene mutation, particularly the p.Tyr95Cys mutation, may represent a newly identified subtype of maturity-onset diabetes of the young. This subtype is characterized by insulin resistance and lipid metabolism disorders.
- Citation: Li WX, Xu LL, Liu CF, Dong BZ, Wang YY. Analysis of an adult diabetes mellitus caused by a rare mutation of the gene: A case report. World J Clin Cases 2024; 12(19): 3942-3949
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/3942.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.3942
Maturity-onset diabetes of the young (MODY) is a distinct form of diabetes mellitus that typically develops during adolescence. This condition is characterized by subtle onset, gradual progression, and a diverse range of clinical presentations[1]. This often leads to misdiagnosis as either type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM) because of the absence of a typical family history and investigative challenges. The lack of familial clustering in individuals with MODY contributes to frequent misdiagnoses. MODY encompasses a group of monogenic diabetes disorders that are inherited in an autosomal dominant manner. The diagnostic criteria for MODY include a family history spanning at least three generations, onset before the age of 25, absence of autoantibodies associated with T1DM, and absence of ketosis-prone episodes requiring insulin therapy. Currently, there are 14 known subtypes of MODY, with GCK, HNF1a, HNF4a, and HNF1B mutations being the most commonly observed in clinical practice. On the other hand, MODY caused by peroxisome proliferator-activated receptor gamma (PPARG) gene mutations is relatively rare. We present a case involving a heterozygous mutation of the PPARG gene that contributed to the clinical diagnosis and treatment of MODY. Our aim was to provide valuable insights and references for medical professionals managing this unique form of diabetes mellitus.
In 2023, our hospital admitted a 38-year-old female patient with persistent hyperglycemia lasting 10 years.
A decade ago, during pregnancy, she had elevated blood glucose levels, ranging from 144.0 to 180 mcg/dL (8-10 × 106 nmol/L), exceeding the reference range of 70.2-108 mcg/dL (3.9-6.0 × 106 nmol/L).
Insulin administration during pregnancy was poorly adhered to, and therapy ceased after gestation. Throughout this period, sporadic blood glucose monitoring occurred, with no reported symptoms such as dry mouth, excessive thirst, frequent urination, weight changes, numbness, visual impairments, or blurred vision. Recent physical exams revealed fasting blood glucose fluctuations of 144.0 to 320.4 mcg/dL (8-17.8 × 106 nmol/L), which are outside the reference range. Subsequently, the patient sought further evaluation and treatment at our hospital, with medical records indicating no unhealthy habits or a significant personal history.
In the process of conducting a thorough pedigree investigation, the patient’s father underwent a comprehensive physical examination, which revealed that the parents had entered into marriage without any consanguinity. Additionally, the patient's siblings, particularly her older and younger sisters, were diagnosed with diabetes. However, neither the patient's spouse nor her two sons exhibited any indicators of diabetes on evaluation.
The patient was a middle-aged woman who was thoroughly examined. Her height was 158 cm, and she weighed 51 kg, resulting in a body mass index (BMI) of 20.4 kg/m2. The patient's growth and development appeared normal, and her mental state was clear. However, the patient exhibited a slow response and unclear speech. Additionally, the patient had sparse hair. During the examination, the patient's light reflex had normal response. The tongue protruded without deviation or tremors. Comprehensive examination of the heart, lungs, and abdomen revealed no abnormalities. The patient's sensory perception in the limbs was within the normal range, and there were no signs of muscle weakness, with strength graded as 5. However, there was a slight increase in muscle tension in both lower limbs. The knee and Achilles tendon reflexes were normal. Notably, pathological signs were detected in both lower limbs, while signs of eye closure were absent. The clinical diagnosis confirmed that the patient had experienced the onset of adult diabetes mellitus with diabetic ketosis during her adolescence.
The patient's medical condition remained consistently stable during routine follow-up appointments, and no significant alterations were observed. Furthermore, her fasting blood glucose levels fluctuated within the range of > 180.0 mcg/dL (> 10 × 106 nmol/L) (reference range: 0.00-30.60 mcg/dL, 0.00-1.7 × 106 nmol/L). Ancillary testing revealed the following results: Triglyceride levels of 103.5 mcg/dL (5.75 × 106 nmol/L) (reference range: 0.00-30.60 mcg/dL, 0.00-1.70 × 106 nmol/L), cholesterol levels of 117.82 mcg/dL (6.54 × 106 nmol/L) (reference range: 52.20-102.24 mcg/dL, 2.90-5.68 × 106 nmol/L), low-density lipoprotein levels of 73.26 mcg/dL (4.07 × 106 nmol/L) (reference range: 0.00-60.66 mcg/dL, 0.00-3.37 × 106 nmol/L), and high-density lipoprotein levels of 18.72 mcg/dL (1.04 × 106 nmol/L) (reference range: 19.62-41.04 mcg/dL, 1.09-2.28 × 106 nmol/L). Furthermore, urine analysis revealed the presence of ketone bodies (3+) and glucose (4+). Fasting C-peptide levels were measured at 2.21 ng/mL (reference range: 0.8-4.2 ng/mL), and fasting insulin levels were 6.19 µIL/mL (reference range: 2.6-24.9 µIL/mL).
No pertinent imaging examinations were conducted.
Auxiliary inspection: Insufficient management of blood glucose levels prompted the patient to make several visits to the outpatient clinic. Routine urine analysis on August 2023 and islet function tests revealed the presence of ketone bodies (3+) and glucose (4+) in the urine, indicating elevated levels. Moreover, her fasting C-peptide level was 2.21 ng/mL, and her fasting insulin concentration was 6.19 uIL/mL. Considering the aforementioned results, it can be deduced that the patient's fundamental islet function was within the normal range. However, the primary factor contributing to high blood glucose levels was insulin resistance, suggesting the presence of a metabolic disorder. Regrettably, the patient did not undergo further examinations, such as the oral glucose tolerance test, assessment of islet-related antibodies, abdominal ultrasound, or quantitative measurement of body fat. Owing to persistent inadequate control of blood glucose levels and evident dyslipidemia, her condition deviated from conventional diabetes. After consulting pertinent literature for a comprehensive clarification of diabetes classification, it was observed that the clinical presentation of the patient closely resembled diabetes linked to a PPARG gene mutation. To enhance diagnostic precision and tailor treatment strategies accordingly, we elucidated the prevailing circumstances with the patient and proceeded with genetic sequencing following their informed consent.
Gene detection: Based on the results obtained from the gene sequencing analysis, a genetic abnormality was detected in the coding region of PPARG, specifically at position 284 of exon 2. This particular mutation (exon2c.284A>Gp.Tyr95Cys) was found to be heterozygous, resulting in the substitution of tyrosine with cysteine at the 95th position of the protein translated from this gene. Furthermore, it was observed that the patient's sister also possessed a nucleotide heterozygous variation at the same site on one of her sex chromosomes. Interestingly, her other sex chromosome exhibited a normal genotype without any genetic alterations, and extensive investigations encompassing both domestic and international research studies, as well as thorough exploration of various internet databases such as www.x-aid.nl, revealed no previous reports or discussions regarding the occurrence of the c.284A>Gp.Tyr95Cys mutation within PPARG. Thus, it can be concluded that this mutation represents a unique variant that has not been previously documented.
Methods and steps: All participants consented to genetic testing in strict accordance with the principles of informed consent. Prior to conducting the tests, a thorough explanation was provided to each individual concerning the aims, methodologies, potential discoveries, and the current scope of genetic testing. Rigorous confidentiality measures were enforced, and utmost consideration was given to their rights to privacy. Extraction of Genomic DNA from Peripheral Blood: A total of 2 mL of peripheral venous blood was obtained from the patient, along with her sisters, sisters-in-law, sons, and nieces. Ethylenediamine tetraacetic acid anticoagulant tubes were used for collection. Genomic DNA was then isolated using a QIAamp DNA extraction kit and the cross-column method. This ensured the accurate and precise extraction of DNA in a medical setting.
PCR amplification of the PPARG gene: Extracted DNA was fragmented using DNA enzymes and purified using the magnetic bead method. Specific primers were designed and synthesized using Primer Premier software (version 5.0) to target the exonic coding region of the gene. The subsequent step involved PCR amplification and ligation of the adapter sequences. The PCR conditions included an initial denaturation at 95 °C for 6 min, followed by 30 cycles of denaturation at 94 °C for 45 s, annealing at 55-58 °C for 45 s, and extension at 72 °C for 60 s. The final cycle was concluded with extension at 72 °C for 5 min.
Purification and sequencing: After conducting rigorous double purification of PCR products using the TruSight One Sequencing Panel, the subsequent sequencing process was carried out using the advanced Illumina sequencing platform.
Data analysis: Utilizing the BWA algorithm, a comparison between the entirety of the data and the reference sequence (PPARG exon 2) was performed, adhering to the default settings of the instrument. Furthermore, annotation of the data was conducted following the methodology described in the relevant literature[2]. Subsequent to a meticulous screening process, the functionalities, variations, and genetic patterns of each gene were exhaustively examined through the amalgamation of clinical data and prediction outputs from various bioinformatics software tools, including PolyPhen2, LRT, and Mutation Taster. This all-encompassing analysis has facilitated the identification of prospective candidate mutations, necessitating further investigation.
The validation process for candidate mutation sites involved Sanger sequencing in the field of medicine. PCR primers were used to amplify the regions of interest, after which thorough analysis and verification of the resulting sequences were conducted. This rigorous procedure ensured the accuracy and reliability of confirming the presence of mutations.
Considering factors such as the patient's height, weight, BMI, medical history, and personal background, the final clinical diagnosis was determined as “MODY, a form of adult diabetes mellitus that affects adolescents.”
An effective treatment protocol for this medical condition involves the administration of subcutaneous saiyinling injections in combination with acarbose. Additionally, Gehuazhi oral medication was prescribed to regulate blood sugar levels. To address abnormal lipid levels in the bloodstream, fenofibrate combined with pusen capsules may be administered concurrently with other suitable therapies. Throughout regular outpatient re-evaluation, the patient's condition remained stable, with no noticeable alterations. Moreover, fasting blood glucose levels fluctuated within the range of 180.0 mcg/dL (reference range: 0.00-30.60 mcg/dL, 0.00-1.70106 nmol/L).
In the process of investigating family history, the father of the proband underwent a comprehensive physical examination, revealing that the parents had no blood relationship. Additionally, the patient's siblings, specifically her older and younger sisters, were confirmed to have diabetes. However, neither the patient's spouse nor her two sons displayed any diabetes symptoms. Moreover, gene testing results (Figure 1; Table 1) were obtained from the patient and her relatives, demonstrating a meticulous and precise approach in the field of medical genetics.
PPARG, a ligand-dependent transcription factor, significantly impacts adipocyte differentiation, adipogenesis, glucose homeostasis, and inflammation within the body. Importantly, certain rare mutations in PPARG have frequently been associated with familial partial lipodystrophy, insulin resistance, and diabetes. The PPARG gene consists of two distinct subtypes, PPARG1 and PPARG2, with PPARG2 playing a more prominent role in regulating glucose and lipid metabolism. Within PPARG, there are four functional domains: The activation domain (AF1), DNA-binding domain, hinge domain, and ligand-binding domain (LBD). Previous studies have shown that individuals carrying rare variants of PPARG2 often present with significant obesity and/or hyperglycemia[3]. Patients with mutations in genes other than AF1 commonly exhibit reduced adipose tissue and insulin resistance. Notably, several studies have suggested a potential association between P.Tyr95Cys mutations and the onset of hyperglycemia[4]. Further research demonstrated that diabetes was present in all siblings of the probands who shared the same mutations. Additionally, patients with P.Tyr95Cys mutations showed decreased insulin resistance, lower triglyceride levels, and more pronounced fat accumulation than patients with other PPARG mutation variants. It has been reported that certain rare or low-frequency variants specific to particular populations (such as p.Gly319Ser in HNF1a in Oji-Cree, p.Glu508Lys in HNF1a in Latinos, and p.Arg1420His in ABCC8 in southwestern American Indians)[5-7] have been found to be associated with an increased risk of diabetes and exhibit various effects. Moreover, no individuals carrying p.Tyr95Cys variants have been identified in online databases, such as the Exac and 1000 Genomes databases. Therefore, p.Tyr95Cys may represent a unique genetic mutation specifically found in Chinese individuals that is linked to the development of diabetes.
PPARG2 regulates glycolipid metabolism through its AF1 domain. Previous studies have shown that AF1 translation affects the binding affinity of LBD regions[8] to ligands through phosphorylation[9,10], acetylation[11], O-GlcNAcylation[12], and SUMOylation[13,14]. Consequently, these modifications contribute to the development of glycolipid metabolic disorders in individuals with mutations. In line with earlier research, different types of mutations have been observed to lead to diverse clinical manifestations. For instance, individuals carrying P.Pro113Gln mutations may experience a range of symptoms, including obesity, T2DM, and elevated fasting insulin levels[15,16]. Conversely, non-AF1 mutations are commonly associated with localized adipose atrophy. These variations in phenotypic expression among patients may be partially explained by the interactions between PPARG and cofactors or environmental factors.
The initial manifestation of this case involved elevated blood glucose levels during pregnancy with an inadequate hypoglycemic response to insulin therapy. Subsequent evaluations revealed a slight increase in triglyceride levels and no evident abnormalities in adipose tissue distribution on physical examination. There was no discernible localized fat atrophy or accumulation. This MODY study presents a distinct clinical screening approach and diagnostic pathway, encompassing assessment of the patient's medical history, personal background, height, weight, and relevant laboratory tests. Ultimately, the definitive clinical diagnosis was MODY, ruling out the possibility of type 1, type 2, or other specific forms of diabetes.
To enhance the classification of MODY in patients, gene sequencing was conducted with explicit consent from both the patients and their families. The results revealed the presence of a heterozygous mutation (exon2c.284A>Gp.Tyr95Cys) within the coding region of exon 2 of PPARG. This specific mutation resulted in a missense alteration within this genetic framework, leading to the substitution of tyrosine with cysteine at the 95th position of the translated product. According to relevant research and descriptions of patient cases, it has been hypothesized that the PPARG gene mutation causes alterations in the AF1 domain, resulting in abnormal protein modification during translation. Various studies have suggested that the p.Tyr95Cys variant not only affects phosphorylation but also hinders adipocyte differentiation by affecting the secretion and expression of fibroblast growth Factor 21 (FGF21)[4]. Notably, FGF21 is predominantly found in the liver rather than in adipose tissue and primarily influences glucose uptake. This may explain the significant hyperglycemia observed in the reported patients despite the absence of pronounced hypertriglyceridemia and adipose tissue atrophy. Additionally, this mutation can suppress the expression of several adipose tissue-secreted factors, including adiponectin, resistin, leptin, and tumor necrosis factor-α, consequently inhibiting 3T3-L1 preadipocyte differentiation. Furthermore, it affects the activation of macrophages and hepatic stellate cells, which subsequently induces systemic inflammation and disrupts lipid metabolism. As a result, this exerts a considerable impact on systemic insulin sensitivity. However, the specific signaling pathway involved in the regulation of this gene, the aberrant alterations in its modification process, and the underlying molecular mechanisms leading to the development of diabetes require substantially more clinical evidence and further research. Moreover, Bonofiglio et al[15] have conducted research that significantly contributes to our understanding of the regulatory mechanisms involved in the expression of phosphatase and tensin homologous gene (PTEN) deletion on chromosome 10. These researchers suggested that PPARG plays a role in this regulation by binding to the peroxisome proliferator response element located upstream of the PTEN promoter. Previous investigations have established the involvement of PTEN in various processes, such as the transformation of renal tubular epithelial cells into mesenchymal cells, renal tubulointerstitial fibrosis[16], and the rearrangement of the podocyte cytoskeleton[17]. These findings underscore the importance of PTEN in renal health and highlight its potential implications for the development of diabetic kidney disease (DKD). Furthermore, a recent study[4] revealed significant associations between PPARG mutations and the risk of DKD in patients with diabetes. Specifically, individuals with these mutations were found to have a 12.5 times greater likelihood of developing DKD than other diabetic patients. These findings emphasize the crucial role of PPARG in the progression of DKD and underscore the need for further research to understand its mechanisms and potential therapeutic interventions.
The current management approach for PPARG mutation-induced MODY primarily focuses on conventional treatment strategies. These strategies include the following: Implementing lifestyle modifications: This involves adopting a low-carbohydrate and low-calorie diet, reducing the intake of sugary beverages and food, promoting healthy lifestyle habits, and creating personalized exercise plans. It is important to note that diligence and academic rigor are necessary when treating PPARG mutant MODY. The use of various types of hypoglycemic drugs specific to different MODY subtypes is recommended for patients with diabetes caused by PPARG mutations, and thiazolidinediones are recommended. Research has shown that patients with partial inactivation of PPARG respond well to thiazolidinediones, which effectively improve blood glucose levels, lipid metabolism, and inflammatory indicators. Additionally, these medications have renoprotective effects and can slow the progression of DKD through various mechanisms, such as inhibiting the expression of hypoxia-inducible factor-1α, suppressing mesangial cell proliferation[18], and downregulating the expression of vascular endothelial growth factor in diabetic rats. In the management of coexisting conditions[19], it is important to address abnormal lipid metabolism, as PPARG mutations are often associated with it. Empirical evidence suggests an increased risk of neurological impairment owing to these mutations. Treatment plans should be tailored based on guideline-endorsed consensus to manage these complications. Studies[4] predict that PPARG-diabetes mellitus affects approximately 0.6% of Chinese patients with early-onset diabetes (EOD), with the prevalence of P.Tyr95Cys mutation-induced diabetes estimated to be approximately 0.3%. Therefore, in patients with familial EOD mellitus, it is crucial to consider the possibility of MODY and promptly conduct examinations on family members, especially those in the patrilineal lineage and their siblings. These assessments aim to identify individuals with gene mutations at the earliest possible stage, thereby reducing the risk of misdiagnosis and incorrect treatment.
An undiscovered subtype of MODY may emerge due to a mutation in the PPARG gene, which holds significant ramifications within the domain of medical investigation. Hyperlipidemia, inconspicuous onset, and gradual progression are distinguishing features of this unique variant of diabetes. Compared to patients with other forms of diabetes, those with PPARG mutation-induced MODY are more susceptible to DKD. Thiazolidinediones are the most effective treatment option. Familial inheritance is a notable trait of this distinct form of diabetes, and early genetic testing can substantially reduce the occurrence of misdiagnosis.
| 1. | Tattersall RB, Fajans SS. A difference between the inheritance of classical juvenile-onset and maturity-onset type diabetes of young people. Diabetes. 1975;24:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 208] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Gong S, Han X, Li M, Cai X, Liu W, Luo Y, Zhang SM, Zhou L, Ma Y, Huang X, Li Y, Zhou X, Zhu Y, Wang Q, Chen L, Ren Q, Zhang P, Ji L. Genetics and Clinical Characteristics of PPARγ Variant-Induced Diabetes in a Chinese Han Population. Front Endocrinol (Lausanne). 2021;12:677130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Ristow M, Müller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 353] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811-10816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 355] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Hegele RA, Cao H, Harris SB, Hanley AJ, Zinman B. The hepatic nuclear factor-1alpha G319S variant is associated with early-onset type 2 diabetes in Canadian Oji-Cree. J Clin Endocrinol Metab. 1999;84:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | SIGMA Type 2 Diabetes Consortium; Estrada K, Aukrust I, Bjørkhaug L, Burtt NP, Mercader JM, García-Ortiz H, Huerta-Chagoya A, Moreno-Macías H, Walford G, Flannick J, Williams AL, Gómez-Vázquez MJ, Fernandez-Lopez JC, Martínez-Hernández A, Jiménez-Morales S, Centeno-Cruz F, Mendoza-Caamal E, Revilla-Monsalve C, Islas-Andrade S, Córdova EJ, Soberón X, González-Villalpando ME, Henderson E, Wilkens LR, Le Marchand L, Arellano-Campos O, Ordóñez-Sánchez ML, Rodríguez-Torres M, Rodríguez-Guillén R, Riba L, Najmi LA, Jacobs SB, Fennell T, Gabriel S, Fontanillas P, Hanis CL, Lehman DM, Jenkinson CP, Abboud HE, Bell GI, Cortes ML, Boehnke M, González-Villalpando C, Orozco L, Haiman CA, Tusié-Luna T, Aguilar-Salinas CA, Altshuler D, Njølstad PR, Florez JC, MacArthur DG. Association of a low-frequency variant in HNF1A with type 2 diabetes in a Latino population. JAMA. 2014;311:2305-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 7. | Baier LJ, Muller YL, Remedi MS, Traurig M, Piaggi P, Wiessner G, Huang K, Stacy A, Kobes S, Krakoff J, Bennett PH, Nelson RG, Knowler WC, Hanson RL, Nichols CG, Bogardus C. ABCC8 R1420H Loss-of-Function Variant in a Southwest American Indian Community: Association With Increased Birth Weight and Doubled Risk of Type 2 Diabetes. Diabetes. 2015;64:4322-4332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 834] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 9. | Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150:620-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 650] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 10. | Ji S, Park SY, Roth J, Kim HS, Cho JW. O-GlcNAc modification of PPARγ reduces its transcriptional activity. Biochem Biophys Res Commun. 2012;417:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Diezko R, Suske G. Ligand binding reduces SUMOylation of the peroxisome proliferator-activated receptor γ (PPARγ) activation function 1 (AF1) domain. PLoS One. 2013;8:e66947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Katafuchi T, Holland WL, Kollipara RK, Kittler R, Mangelsdorf DJ, Kliewer SA. PPARγ-K107 SUMOylation regulates insulin sensitivity but not adiposity in mice. Proc Natl Acad Sci U S A. 2018;115:12102-12111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Keshet R, Bryansker Kraitshtein Z, Shanzer M, Adler J, Reuven N, Shaul Y. c-Abl tyrosine kinase promotes adipocyte differentiation by targeting PPAR-gamma 2. Proc Natl Acad Sci U S A. 2014;111:16365-16370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Blüher M, Lübben G, Paschke R. Analysis of the relationship between the Pro12Ala variant in the PPAR-gamma2 gene and the response rate to therapy with pioglitazone in patients with type 2 diabetes. Diabetes Care. 2003;26:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Bonofiglio D, Gabriele S, Aquila S, Catalano S, Gentile M, Middea E, Giordano F, Andò S. Estrogen receptor alpha binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor gamma signaling in breast cancer cells. Clin Cancer Res. 2005;11:6139-6147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Yan R, Wang Y, Shi M, Xiao Y, Liu L, Guo B. Regulation of PTEN/AKT/FAK pathways by PPARγ impacts on fibrosis in diabetic nephropathy. J Cell Biochem. 2019;120:6998-7014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Lin J, Shi Y, Peng H, Shen X, Thomas S, Wang Y, Truong LD, Dryer SE, Hu Z, Xu J. Loss of PTEN promotes podocyte cytoskeletal rearrangement, aggravating diabetic nephropathy. J Pathol. 2015;236:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Guan Y, Zhang Y, Schneider A, Davis L, Breyer RM, Breyer MD. Peroxisome proliferator-activated receptor-gamma activity is associated with renal microvasculature. Am J Physiol Renal Physiol. 2001;281:F1036-F1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Xu X, Chen P, Zheng Q, Wang Y, Chen W. Effect of pioglitazone on diabetic nephropathy and expression of HIF-1α and VEGF in the renal tissues of type 2 diabetic rats. Diabetes Res Clin Pract. 2011;93:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |