Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.3936
Revised: April 24, 2024
Accepted: May 17, 2024
Published online: July 6, 2024
Processing time: 129 Days and 7.2 Hours
Pancreatic cancer presents a challenge with its low early diagnosis and treatment rates, leading to high metastasis and mortality rates. The median survival time for advanced pancreatic cancer is a mere 3 months. However, there's hope: small pancreatic cancers diagnosed at an early stage (T1) or those less than or equal to 1 cm in diameter boast an impressive 5-year survival rate of nearly 100%. This underscores the critical importance of early pancreatic cancer detection for significantly improving prognosis.
Pancreatic cancer, a malignant tumor of the digestive tract, poses challenges in both diagnosis and treatment due to its occult and atypical clinical symptoms. Clinically, patients with recurrent pancreatitis should be vigilant, as it may be indicative of pancreatic cancer, particularly in middle-aged and elderly patients. Here, we presented the case of a patient who experienced recurrent acute pancreatitis within a span of 2 months. During the initial episode of pancreatitis, routine imaging failed to identify the cause of pancreatic cancer. However, upon recurrence of acute pancreatitis, endoscopic ultrasonography (EUS) revealed a space-occupying lesion approximately 1 cm in size in the pancreatic body. Subsequent EUS coupled with fine-needle aspiration examination demonstrated atypical pancreatic gland epithelium. Ultimately, the patient underwent surgery and was diagnosed with an intraductal papillary mucinous tumor of the pancreas (severe epithelial dysplasia, focal cancer).
We recommend EUS for patients with recurrent pancreatitis of unknown etiology to exclude early pancreatic cancer.
Core Tip: Pancreatic cancer is notorious for its low early diagnosis rate and high mortality. With a tendency for nerve invasion, local lymph node, and distant metastasis, it poses a significant challenge. The 5-year survival rate is less than 5%, and the median survival time for advanced pancreatic cancer is a mere 3 months. For patients experiencing recurrent pancreatitis of unclear origin during routine examination, we highly recommend the enhanced use of endoscopic ultrasonography. This approach is of paramount importance for early detection and exclusion of pancreatic cancer.
- Citation: Wei C, Li YC, Jin HT, Li DF, Wang LS, Yao J. Early detection of pancreatic cancer in patients with recurrent pancreatitis: A case report. World J Clin Cases 2024; 12(19): 3936-3941
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/3936.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.3936
Pancreatic cancer stands as one of the prevalent malignant tumors of the digestive tract, often referred to as the "king of cancer" within the realm of oncology[1]. Currently, tumor markers such as CA19-9 and CEA are frequently employed as screening tools in clinical practice. Diagnostic modalities such as abdominal color ultrasound, enhanced computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiopancreatography are commonly utilized for the detection of pancreatic tumors[2]. However, the aforementioned diagnostic methods may exhibit a certain rate of false negative results for pancreatic tumors smaller than 2 cm, particularly those smaller than 1 cm. In cases where clinical suspicion of pancreatic tumor persists despite negative findings on conventional imaging, endoscopic ultrasonography (EUS) coupled with fine-needle aspiration (FNA) for cytological examination represents a pivotal diagnostic approach[3].
Recently, within our department, a 1-cm hypoechoic lesion was identified in the pancreas of a patient experiencing recurrent pancreatitis of unexplained etiology, using EUS. However, routine CT and MRI examinations failed to reveal any abnormalities. Subsequent pathological examination following surgical intervention confirmed the lesion as early-stage T1a pancreatic cancer.
The patient was admitted to our hospital due to recurrent upper abdominal pain for more than 2 months.
The patient was admitted to our department 2 months ago due to sudden epigastric colic following dinner. Upon admission, laboratory investigations revealed a white blood cell count of 14.7 × 109 /L, a serum amylase level of 1304 U/L, and a serum lipase level of 1809 U/L. Aminotransferase, bilirubin, and blood lipid levels were within normal ranges.
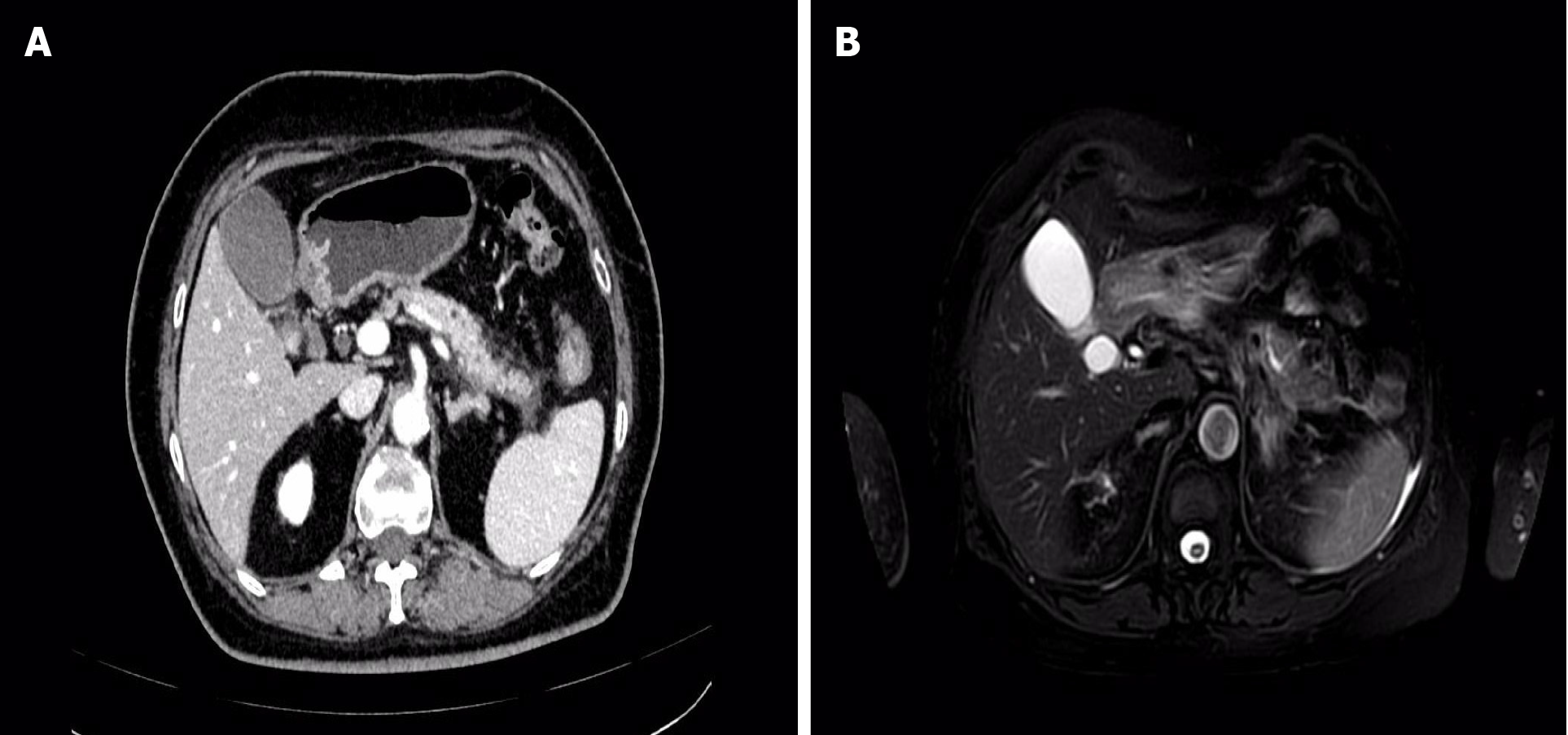
On May 28, 2023, an enhanced MRI + MRCP examination of the upper abdomen revealed no discernible abnormalities in the pancreatic duct or bile duct. However, there was noticeable fullness in the pancreatic body and tail, accompanied by a small amount of peripheral exudation, indicative of acute pancreatitis (Figure 1). Subsequently, on May 29, 2023, an enhanced CT scan of the upper abdomen confirmed the diagnosis of acute pancreatitis, showing fullness in the body and tail of the pancreas with surrounding exudation. Following symptomatic and supportive treatments, including fasting, fluid infusion, and inhibition of pancreatic enzymes, the patient experienced significant relief of symptoms and was discharged (Figure 1).
However, the patient returned to the emergency department of our hospital 1 day prior to the current admission, presenting with recurrent paroxysmal upper abdominal pain following a meal. Laboratory investigations at this time revealed a serum amylase level of 1657 U/L and a white blood cell count of 11.4 × 109 /L.
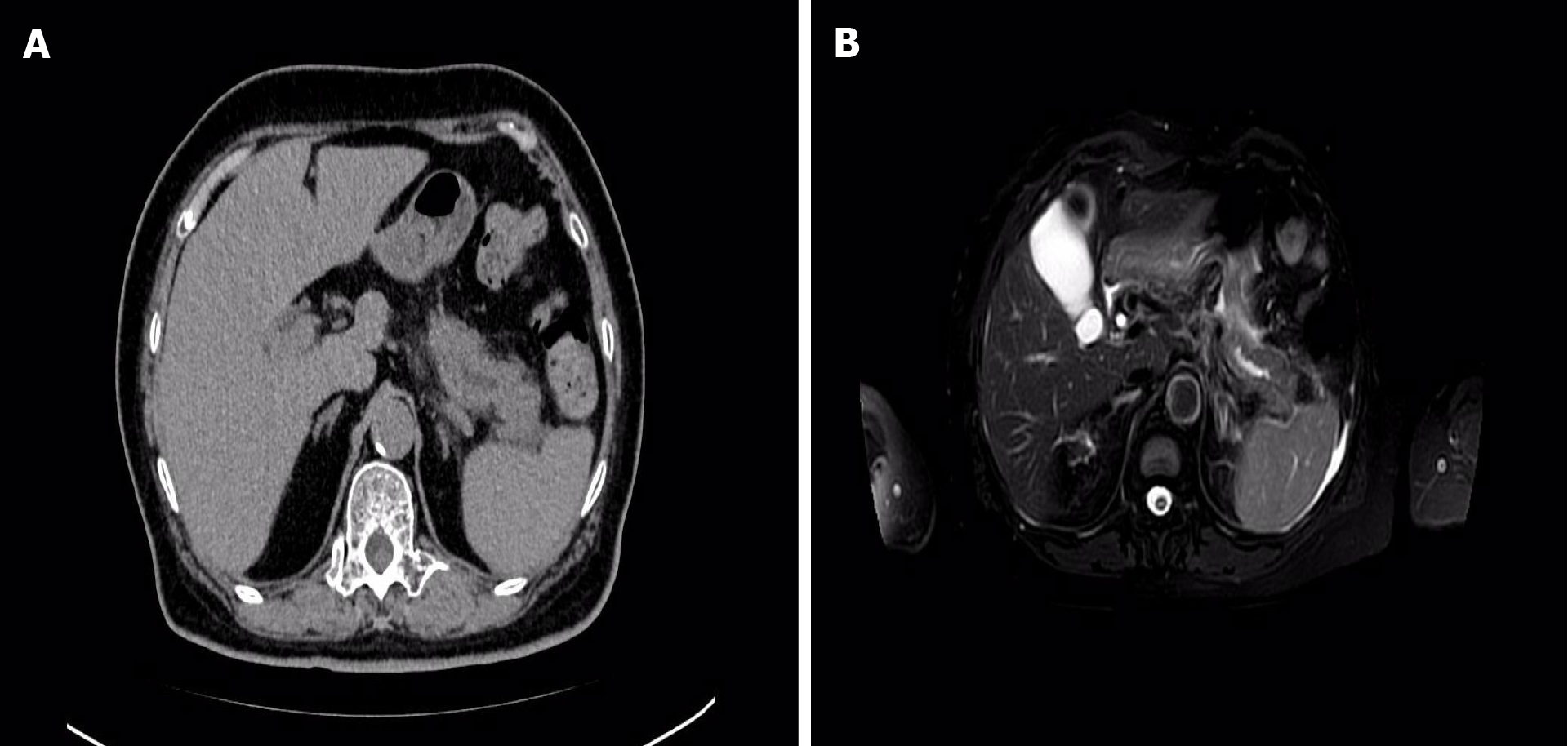
On July 24, 2023, an abdominal CT scan demonstrated pancreatitis, with more pronounced pancreatic duct dilatation compared to previous imaging (Figure 2). Consequently, the patient was readmitted to our department for acute pancreatitis.
The patient underwent surgery and postoperative radiotherapy for breast cancer over 20 years ago. She denied a history of chronic conditions such as hypertension, diabetes, cerebral infarction, and coronary heart disease. Additionally, there was no history of hepatitis, tuberculosis, blood transfusions, or major trauma. The patient reported allergies to sulfonamide and penicillin. However, her vaccination history was unknown.
The patient denied a history of alcohol consumption or smoking. She is married with children and postmenopausal, with no reported history of abnormal vaginal bleeding after menopause. Additionally, there is no family history of genetic or similar medical conditions.
The patient presented with stable vital signs, and examination revealed normal skin and mucous membrane coloration without jaundice. Superficial lymph nodes were not palpably swollen. Cardiopulmonary examination did not reveal any abnormalities.
Abdominal examination indicated soft abdomen with evident tenderness in the upper abdomen, although rebound pain was absent. Neither the liver nor the spleen showed signs of enlargement. Mobility dullness was negative, and there was no evidence of lower extremity edema.
The patient's laboratory results revealed a blood amylase level of 1657 U/L, a white blood cell count of 11.4 × 109/L, and a C-reactive protein level of 73.1 mg/L. Liver function tests including transaminases, bilirubin, alkaline phosphatase, and gamma-glutamyl transferase, as well as blood lipid and renal function tests, were within normal limits. Tumor marker levels, including CA19-9, CA125, CEA, and AFP, were also normal.
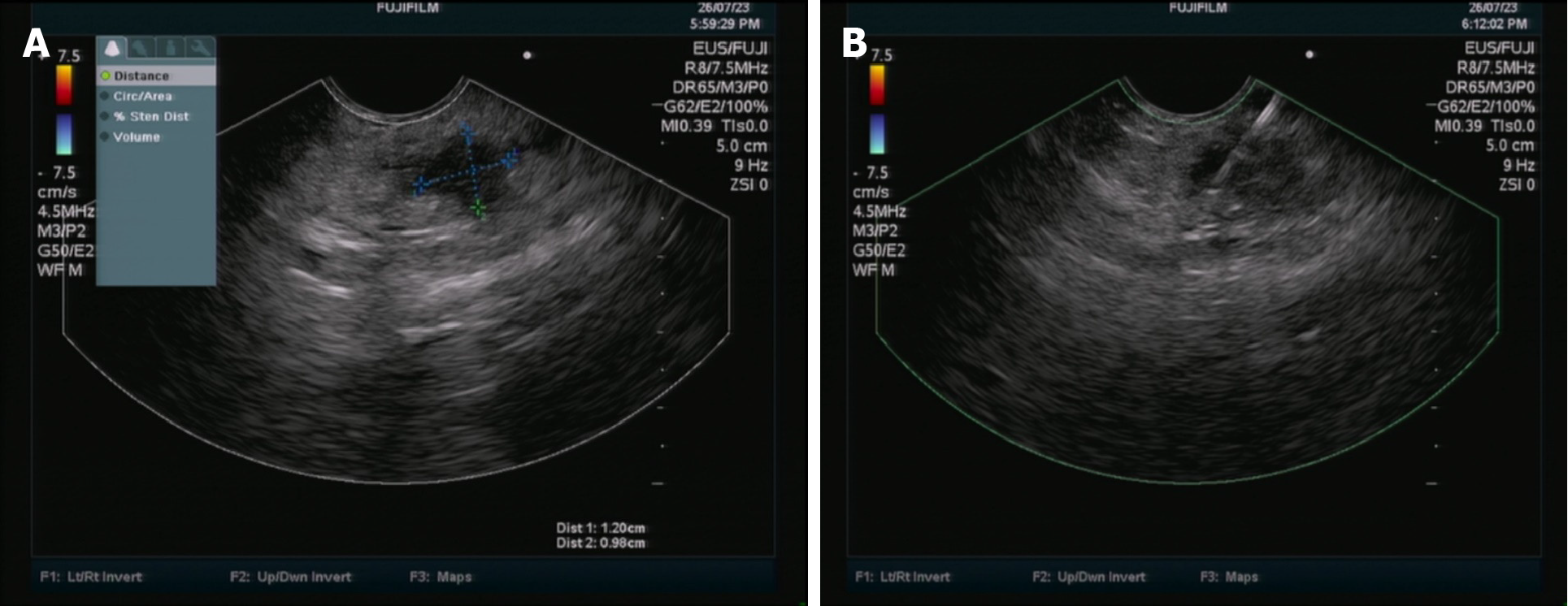
The patient underwent a repeat abdominal enhanced MRI + MRCP examination, which revealed findings consistent with pancreatitis and mild dilatation of the middle and distal pancreatic duct (Figure 2). Subsequent EUS identified a hypoechoic lesion measuring 12 mm × 9.8 mm in the body of the pancreas, accompanied by mild dilatation of the pancreatic duct behind the lesion, measuring 4.2 mm (Figure 3). Tissue samples were obtained for pathological analysis through EUS-FNA (Figure 3). Pathological analysis of the obtained tissue strips revealed atypical epithelial changes in the pancreatic gland.
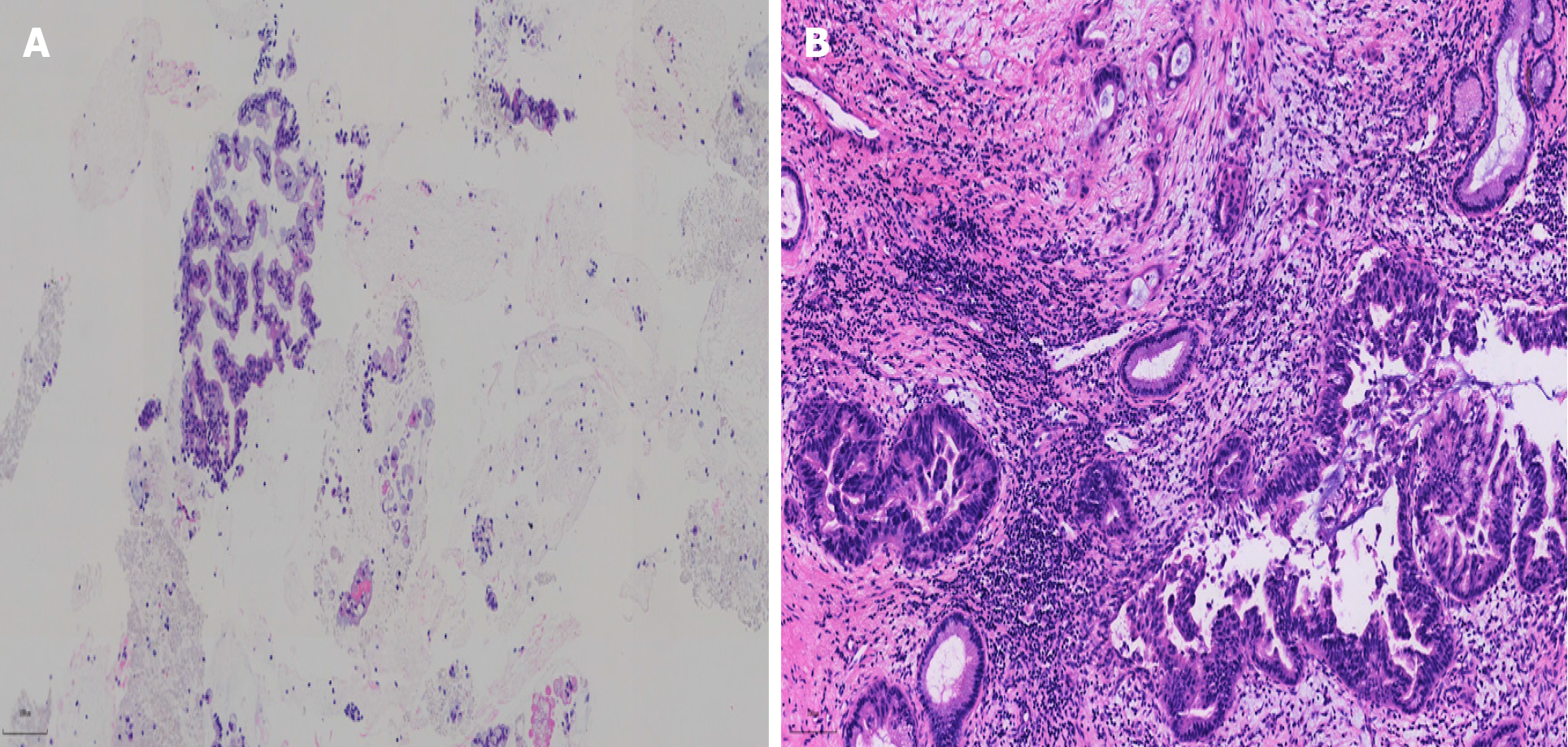
Following comprehensive discussion with the patient and her family, and after obtaining informed consent, surgical resection of the pancreatic lesion was performed. Postoperative pathology confirmed the diagnosis of pancreatic intraductal papillary mucinous neoplasm with severe epithelial dysplasia and focal cancerous invasion (T1a stage). Importantly, there was no evidence of vascular tumor thrombus, lymph node involvement, or nerve involvement (Figure 4).
The patient was diagnosed with early-stage pancreatic cancer, specifically stage T1a.
Following surgical resection, the patient recovered well, and no further adjuvant treatments such as radiotherapy or chemotherapy were deemed necessary.
One week post-surgery, the patient returned to the hospital for a CT examination, which revealed postoperative changes in the pancreas.
The etiology of pancreatic cancer remains poorly understood, although its occurrence has been associated with several factors, including smoking, alcohol consumption, high-fat and high-protein diets, excessive coffee consumption, environmental pollution, and genetic predisposition[4]. Recent studies have shed light on a significantly higher incidence of pancreatic cancer among individuals with diabetes and chronic pancreatitis compared to the general population[5].
Pancreatic cancer typically presents insidiously with atypical clinical manifestations, making it challenging to diagnose early. The most common symptoms include upper abdominal fullness, discomfort, and pain. Additionally, patients may experience jaundice, emaciation, fatigue, diarrhea, and ascites. As a result, early pancreatic cancer is often challenging to identify through routine laboratory tests and imaging methods[2].
Currently, pancreatic masses are primarily detected using tumor markers such as CA19-9, as well as imaging modalities like abdominal CT and MRI. However, these routine tests and imaging methods have a certain rate of missed diagnosis, particularly for small pancreatic masses ranging from 1-2 cm. This challenge is exacerbated in cases where patients also present with pancreatitis, as the inflammatory exudation background of the pancreas can interfere with radiological diagnosis.
Early diagnosis of pancreatic cancer is crucial for improving patient prognosis. The 5-year survival rate for early-stage T1a pancreatic cancer is nearly 100%. Moreover, patients with early-stage disease typically do not require additional treatments such as radiotherapy or chemotherapy following surgery[6].
The characteristics of this case can be summarized as follows. First, the patient presented with recurrent pancreatitis symptoms within a short period of time. However, CA19-9 and other tumor indicators were within normal ranges, and no pancreatic cancer was detected during the initial enhanced CT and enhanced MRI+MRCP examinations.
Second, despite the absence of pancreatic cancer detection in both CT and MRI + MRCP scans after the second episode of pancreatitis, the subsequent examination revealed a slight dilation of the pancreatic duct.
Third, in order to ascertain the cause of the patient's recurrent pancreatitis and pancreatic duct dilation, EUS identified a hypoechoic lesion of approximately 1 cm in the pancreatic body.
Finally, based on the pathological results obtained through EUS, the surgeon performed pancreatic mass resection. Postoperative pathology revealed an intraductal papillary mucinous tumor (focal cancer), staged as T1a. The patient exhibited a favorable clinical outcome.
For middle-aged and elderly patients with high-risk factors, such as short-term recurrent pancreatitis and the absence of pancreatic cancer lesions detected by routine examinations, EUS is recommended. This diagnostic approach offers high sensitivity and specificity for the early detection of pancreatic cancer. Additionally, if necessary, EUS allows for the collection of pathological specimens.
| 1. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 680] [Article Influence: 170.0] [Reference Citation Analysis (0)] |
| 2. | Loveday BPT, Lipton L, Thomson BN. Pancreatic cancer: An update on diagnosis and management. Aust J Gen Pract. 2019;48:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Tonini V, Zanni M. Early diagnosis of pancreatic cancer: What strategies to avoid a foretold catastrophe. World J Gastroenterol. 2022;28:4235-4248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 4. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1254] [Article Influence: 179.1] [Reference Citation Analysis (39)] |
| 5. | Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, Zhang T, Dai M, Zhao Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 6. | Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP, Huang BW, Sun ZH, Zhang CZ, Tang YQ, Hou BH. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol. 2020;26:2305-2322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (5)] |