Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3596
Revised: April 23, 2024
Accepted: May 7, 2024
Published online: June 26, 2024
Processing time: 107 Days and 23.2 Hours
The bacterium Eikenella, classified as a gram-negative member of the phylum Proteobacteria, is distinguished by its rarity, corrosive nature, facultative ana
Here we presented a case of empyema associated with E. corrodens infection in a 67-year-old male patient without any previous history of infectious diseases in our primary hospital in Dongguan district of China. The patient was admitted due to recurrent worsening cough, sputum production, and dyspnea for 3 d, which had persisted for over 20 years. Moreover, the patient experienced a one-hour episode of unconsciousness. Upon admission, immediate comprehensive examinations were conducted on the patient which subsequently led to his admission to the intensive care unit. Meanwhile, the patient presented with drowsiness and profuse sweating along with bilateral conjunctival edema ob
In this report, we presented a case of empyema associated with E. corrodens infection. Multiple courses of morphological examination, viable culture analysis, and biochemical identification revealed its difficulties in detecting distinctive characteristics, as well as a detection model worth promoting. It’s just that there were still certain deficiencies in terms of morphological assessment, biochemical identification, and drug susceptibility testing.
Core Tip: When encountering pleural effusion infection. In clinical laboratory practice, prioritizing attention towards less common bacteria such as Actinobacillus or Streptobacillus species within the HACEK group is crucial. By extending culture time and enhancing culture conditions to improve the isolation and identification of pathogens, detection rates can be significantly augmented. For laboratories, expediting and accurately identifying pathogens plays a pivotal role in optimizing patient treatment outcomes.
- Citation: Liang ZC, Ouyang H, Song XJ, Liang JX, Zheng WH, Chen JJ, Yin ZG, Chen SY. Eikenella corrodens isolated from pleural effusion: A case report. World J Clin Cases 2024; 12(18): 3596-3602
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3596.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3596
The genus Eikenella, classified under the phylum Bacteroidetes, class β-proteobacteria, order Neisseriales, and family Neisseriaceae, is primarily represented by the sole species Eikenella corrodens (E. corrodens)[1]. This Gram-negative bacterium commonly colonizes the human oral and upper respiratory tract as a commensal organism. Risk factors for E. corrodens infection include immunosuppression, mucosal damage, animal bites, dental procedures, and substance abuse[2]. Infections caused by E. corrodens can affect various anatomical sites such as the upper respiratory tract, pleura, abdominal cavity, joints, bone wounds, and endocardium; occasionally leading to infections in other locations (e.g., gangrenous stomatitis)[3-6].
As well known, the occurrence of E. corrodens invading the pleural cavity without forming a draining sinus is considered rare[7], as shown in Table 1. Consequently, a better understanding of the characteristics of E. corrodens is urgently needed. In this case report, we presented an instance where E. corrodens was isolated from pleural effusion in our hospital. The bacteriological and testing characteristics of E. corrodens were described.
| Ref. | Sole bacteria | Type of study | Location | Forming a draining sinus | Spontaneous | Antibiotics |
| Hu et al[16], 2020 | No | Case report | Lung | Yes | No | Yes |
| Abellán Martínez et al[17], 2000 | Yes | Case report and review | Lung | Yes | No | Yes |
| Hironaka et al[18], 1998 | Yes | Case report | Lung | Unknown | Yes | Yes |
| Salem et al[19], 1996 | Yes | Case report | Lung | Unknown | No | Yes |
| Fujii et al[20], 1995 | Yes | Case report | Lung | Unknown | No | Yes |
| Hsu et al[21], 1989 | Yes | Case report | Lung | Unknown | No | Yes |
| Moro et al[22], 1988 | Yes | Case report | Lung | Unknown | No | Yes |
| Joshi et al[23], 1991 | Yes | Case report | Lung | Unknown | Unknown | Yes |
The patient, a 67-year-old male with no previous medical infection history, was admitted due to recurrent exacerbation of cough, increased sputum production, and dyspnea over the past 3 d. Besides, the patient encountered a period of unconsciousness lasting for one hour, meanwhile, the patient presented with somnolence and profuse diaphoresis.
Symptoms, such as recurrent exacerbation of cough, increased sputum production, and dyspnea, started over 3 d. Moreover, the patient encountered a period of unconsciousness lasting for one hour. In addition, the patient presented with somnolence and profuse diaphoresis.
These exacerbated symptoms have lasted for more than two decades.
The patient denied any family history of illness.
On physical examination, the vital signs were as follows: Body temperature, 35.6 °C; blood pressure, 110/80 mmHg; heart rate, 120 beats per min; respiratory rate, 35 breaths per min. Besides the patient had poor overall nutrition, a thin body, a chronic Sick look, and a dull mind. Furthermore, bilateral conjunctival edema was observed.
Upon admission, peripheral blood gas analysis revealed a PCO2 level of 67.6 mmHg. Blood tests showed white blood cells 47.42 × 109/L (neutrophils 91%), hemoglobin 110 g/L, platelets 301.00 × 109 /L, CRP > 200.00 mg/L, and PCT 0.32 ng/mL. Renal and liver function indices were within normal range.
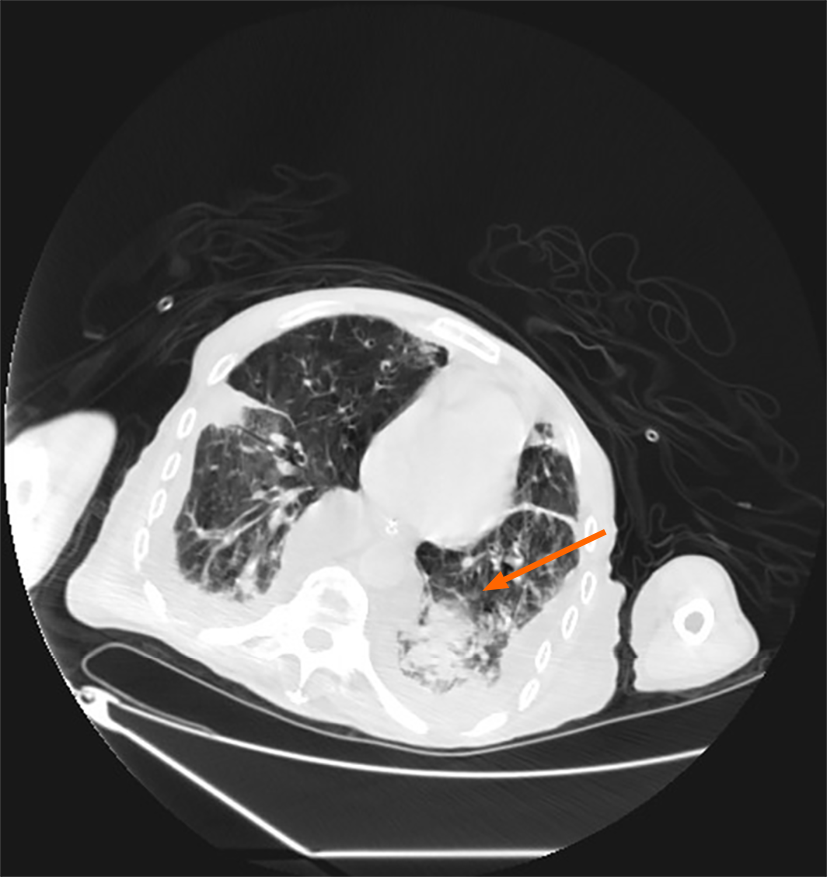
After admission, a computerized tomography (CT) scan taken revealed developed symptoms suggestive of infection, as shown in Figure 1.
Bilateral conjunctival edema was observed, indicating the presence of carbon dioxide retention and hypercapnia encephalopathy. Comprehensive examinations were promptly conducted and subsequently transferred to the intensive care unit.
Non-invasive ventilation was promptly initiated. Considering the significant right pleural effusion, a bedside ultrasound-guided procedure was performed to alleviate lung compression and mitigate the patient’s respiratory distress. A substantial volume of coffee-colored foul-smelling pleural fluid was drained during the procedure, suggestive of empyema, leading to the initiation of empirical antibiotic therapy for potential co-existing bacterial infection. Routine biochemical analysis confirmed empyema in the pleural fluid sample. The smear and culture of the clinical sample were difficultly established. In the meantime, pathogen culture identification and drug sensitivity testing were conducted.
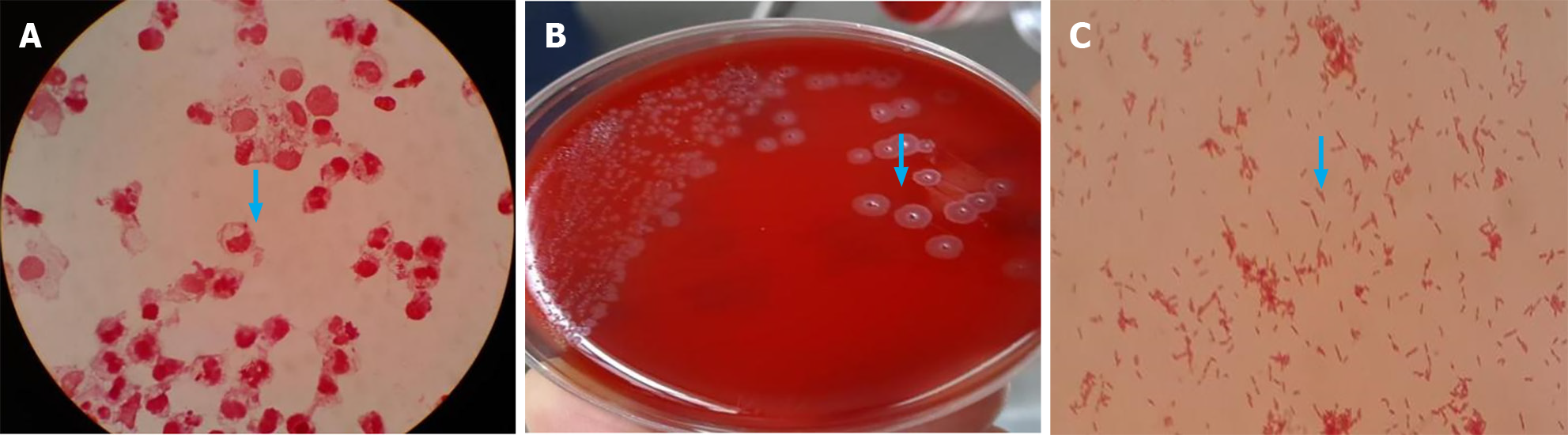
The specimens were collected via closed drainage after the surgical puncture of the patient, and subsequently, a small portion of which was smeared onto a glass slide for Gram staining. Microscopic examination revealed a significant presence of leukocytes and a few Gram-negative bacteria (Figure 2A).
The sample was cultured on sheep blood agar plates and incubated at a temperature range of 35 °C-37 °C in a CO2-enriched environment. After 24 h, pinpoint-sized colonies were observed on the blood agar plates. Continuing the culture for an additional 24 h revealed the characteristic colony morphology of this bacterium, which exhibited a dry, flat appearance with a centrally raised, radiating structure resembling either a mushroom or helmet shape. Prolonged incubation led to the development of greenish discoloration surrounding the colonies, while gentle scraping with a cotton swab resulted in light yellow coloration. Pure cultures of this bacterium emitted an unmistakable odor reminiscent of fungal oral flora (Figure 2B)[8]. Under microscopic observation (Figure 2C), numerous red and straight Gram-negative bacteria were detected.
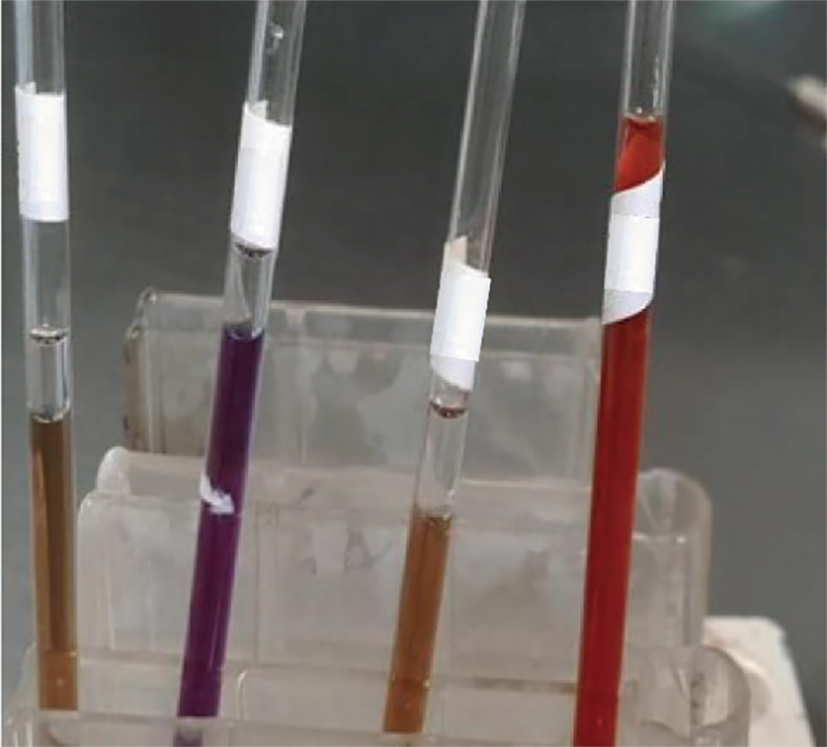
When pathogen biochemical tests of E. corrodens were taken, the E. corrodens strain exhibited negative results for catalase, urease, arginine hydrolase, and indole examinations[9]. However, the typical isolates demonstrated positive outcomes for ornithine decarboxylase and nitrate reduction tests, as well as lysine decarboxylase and oxidase detections (Figure 3), despite their inability to ferment sugars in a medium without additives.
As suggested by the infectiologist, empiric intravenous antibiotic treatment was started with imipenem cilastatin sodium (1 g every 6 h)[8], and meropenem (2 g every 8 h) was performed for more than 10 d after confirming infection of E. corrodens. While the patient started to worsen 18 d after antibiotic therapy, and then vancomycin (2 g/d) was added, and continued the antibiotic treatment until discharge with death.
Combined with the patient’s medical history, the final diagnosis was empyema.
The patient died on day 22 in hospital. After the diagnosis of empyema, the patient received antimicrobial therapy until his death.
On day 22 after admission, the patient died.
E. corrodens, a member of the HACEK group and part of the commensal flora on human mucosal surfaces is frequently isolated from upper respiratory tract specimens and can also be found in gastrointestinal or urogenital specimens as well as those associated with animal bite infections[10,11]. However, it typically exhibits non-pathogenic behavior when the immune defense system is normal. In situations where the body’s immune defense system is compromised or there is mucosal damage, E. corrodens can invade surrounding tissues and cause severe infections.
In this case report, when compared to the articles in the literature, the reports of E. corrodens invading the pleural cavity were not many, only 8 cases and most of they were non-spontaneous and sole pathogen bacterial, two cases with forming a draining sinus were confirmed and 6 ones were unknown because of the time of publication was too early, as shown in the Table 1. So, our case report was valuable enough.
This type of case report of E. corrodens invading the pleural cavity was rare may be for reason that detecting and culturing this bacterium presents a challenge due to its high nutritional requirements and facultative anaerobic nature that thrives in environments containing 5% to 10% CO2 and hemin-containing media. Therefore, the bacterium exhibits a slow growth rate, typically requiring 48-72 h for the development of characteristic colonies. In the course of laboratory work, it is often disregarded due to inadequate incubation time[1,8] and its classification as a contaminant. Consequently, the clinical examination and analysis of this bacterium face certain challenges, particularly in basic hospitals within China. Thus, extending the culture duration or improving culture conditions has the potential to facilitate pathogen isolation and identification, thereby enhancing patient care and rehabilitation.
Furthermore, meticulous consideration should be given to the selection of an appropriate specimen type, prioritizing samples collected from sterile sites, and strictly adhering to aseptic procedures during the sample collection. Moreover, timely clinical communication is indispensable for prompt reporting of identification results and providing empirical guidance for medication, particularly after culturing E. corrodens in sterile specimens where microbiology staff should immediately notify clinics about bacterial characteristics and drug recommendations.
The CLSI M45-A2 guidelines offer a comprehensive range of information on antimicrobial susceptibility. Preferred antimicrobial agents for E. corrodens include ampicillin-sulbactam, amoxicillin/clavulanic acid, cefotaxime, cefepime, imipenem, penicillin, and other options. Certain strains of E. corrodens may exhibit resistance to clindamycin and produce beta-lactamases that can be inhibited by enzyme inhibitors. Ampicillin-sulbactam or third-generation cephalosporins are recommended for soft tissue infections while third-generation cephalosporins are the optimal choice for infective endocarditis[1,12,13].
Despite the formidable challenges associated with conducting drug susceptibility testing for E. corrodens under its highly demanding culture conditions, we diligently adhere to the guidelines outlined by CLSI-M45 in our earnest endeavors to perform such assessments. In cases where trial or failure arises during drug sensitivity evaluations, it becomes imperative to actively consult relevant literature sources for extracting valuable insights, engage in effective communication with clinical personnel, and submit a comprehensive treatment proposal. Although the range of antibacterial drugs tested may be limited, they can still serve as invaluable tools for guiding clinical treatment and management.
Fortunately, with the advancement of microbiological detecting techniques, mass spectrometry technology, and molecular biological methods can be utilized for rapid and accurate identification of bacteria. It’s just there is an uneven distribution of construction and diagnostic capacity among the microbiology laboratories of the basic hospitals, resulting in insufficient identification capabilities for rare or difficult-to-identify bacteria as well as certain pathogenic bacteria causing special infections, and leading to delayed clinical treatment or inappropriate management. Therefore, it is crucial to strengthen the establishment of basic laboratories, enhance the diagnostic abilities of grassroots laboratories, and improve the professional competence of staff[14].
In summary, our case revealed its difficulties in detecting distinctive characteristics, as well as a detection model worth promoting. E. corrodens, a rare bacterium in the HACEK group, possesses the potential to induce infections across diverse anatomical sites. When it is identified as the sole pathogen causing pleural effusion infection, it should be regarded with utmost seriousness. In clinical laboratory practice, prioritizing attention towards less common bacteria such as Actinobacillus or Streptobacillus species within the HACEK group is crucial[15]. By extending culture time and enhancing culture conditions to improve the isolation and identification of pathogens, detection rates can be significantly augmented. For laboratories, expediting and accurately identifying pathogens plays a pivotal role in optimizing patient treatment outcomes.
We thank doctors for the provision of clinical data.
| 1. | Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Whitman WB, Goodfellow M, Kämpfer P, Busse HJ. Bergey’s Manual® of Systematic Bacteriology. Springer Nature Switzerland AG, 2005. [DOI] [Full Text] |
| 3. | Erci E, Avcu G, Ozer EC, Bal ZS, Ozkinay F, Kurugol Z, Gode S, Aydemir SS. A Rare Cause of Orbital Cellulitis: Eikenella corrodens. Pediatr Infect Dis J. 2023;42:e257. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Zhang X, Zhu G, Ma J, Wang G. A Case of Abdominal Subcutaneous Abscess Caused by Eikenella corrodens without Wound. Surg Infect (Larchmt). 2020;21:75-76. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Gonçalves RJ, Murinello A, Gomes da Silva S, Coelho JS, Lopes Santos A, Sá Damásio H. Hepatic Abscess due to Streptococcus anginosus and Eikenella corrodens, Secondary to Gastric Perforation by a Fish Bone. GE Port J Gastroenterol. 2019;26:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Lin YS, Juhasz ES. Eikenella corrodens retroperitoneal necrotising fasciitis post-endoscopic retrograde cholangio-pancreatography. N Z Med J. 2018;131:69-73. [PubMed] |
| 7. | Tanaka M, Araki K, Higuchi H, Fukuoka-Araki K, Horikoshi Y, Hataya H. Pediatric acute dacryocystitis due to Eikenella corrodens: A case report. J Infect Chemother. 2020;26:510-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Lewis J, Bush K. Manual of clinical microbiology. 11th ed. Washington DC: ASM Press, 2015: 653-662. [DOI] [Full Text] |
| 9. | Akhanlı P, Bayır Ö, Bayram SM, Hepşen S, Badirshaev M, Çakal E, Saylam G, Korkmaz MH. Acute spontaneous suppurative thyroiditis caused by Eikenella corrodens presented with thyrotoxicosis. Einstein (Sao Paulo). 2020;18:eRC5273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Angulo López I, Aguirre Quiñonero A, Fernández Torres M, Alegría Echauri E. Chorioamnionitis and neonatal septicaemia due to Eikenella corrodens. Enferm Infecc Microbiol Clin. 2017;35:266-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Correa Martínez L, González Velasco C, Gaona Álvarez CE, Sánchez Castañón J. Tuboovarian abscess due to Eikenella corrodens. Enferm Infecc Microbiol Clin (Engl Ed). 2018;36:319-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Schminke B, Kauffmann P, Brockmeyer P, Miosge N, Lenz C, Schubert A. The Proteomes of Oral Cells Change during Co-Cultivation with Aggregatibacter actinomycetemcomitans and Eikenella corrodens. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Roche Matheus MP, Fernández Vecilla D, Urrutikoetxea Gutiérrez MJ, Calvo Muro FE, Iglesias Hidalgo G, Díaz de Tuesta Del Arco JL. Bacteremia caused by Eikenella corrodens in a patient with pelvic inflammatory disease. Rev Esp Quimioter. 2023;36:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Adondakis MG, Skedros JG, Lopansri BK, Merrell SC. Subcutaneous Eikenella corrodens, Actinomyces sp., and α-Hemolytic Streptococcus Abscess of the Thigh following a Vitamin B12 Injection. Case Rep Infect Dis. 2018;2018:4650637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Salas Olortegui D, Moreno Felici O, Calvó Gómez J, Colomina Rodríguez J. [Eikenella corrodens brain abscess after frontal sinusitis in immunocompetent patient]. Rev Esp Quimioter. 2023;36:211-213. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Hu L, Lin J, Li J, Cao Y, Lin L. Lung abscess secondary to lung cancer with Eikenella corrodens and Streptococcus anginosus: a case report. BMC Infect Dis. 2020;20:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Abellán Martínez MC, Méndez Martínez P, Sánchez Gascón F, Hernández Martínez J, Ruiz López FJ. [Repeated hemoptysis for foreign body bronchial aspiration: presentation of a case and review of literature]. An Med Interna. 2000;17:652-654. [PubMed] [DOI] [Full Text] |
| 18. | Hironaka M, Ohta M, Oda T, Kamei T, Kitamura S. [A case of lung abscess caused by Eikenella corrodens]. Nihon Kokyuki Gakkai Zasshi. 1998;36:164-170. [PubMed] |
| 19. | Salem M, Dabbech R, Saffari H, Joudene M, Kammoun A, Ben Rejeb S, Najjar T, Meknini B, Amamou M, Yacoub M. [Eikenella corrodens pleuro-pulmonary infection. Apropos of a case]. Rev Pneumol Clin. 1996;52:192-195. [PubMed] |
| 20. | Fujii H, Fujimura T, Ariizumi K, Minami T, Odagiri S, Koide S. [A case of secondary valve replacement caused by pulmonary bioprosthetic valve endocarditis]. Nihon Kyobu Geka Gakkai Zasshi. 1995;43:1081-1085. [PubMed] |
| 21. | Hsu CY, Liu LL, Luh KT. Lung abscess caused by Eikenella corrodens: report of a case. Taiwan Yi Xue Hui Za Zhi. 1989;88:828-831. [PubMed] |
| 22. | Moro D, Pin I, Manquat G, Croize J, Paramelle B. [A case of pleuropneumopathy due to Eikenella corrodens]. Rev Mal Respir. 1988;5:523-524. [PubMed] |
| 23. | Joshi N, O’Bryan T, Appelbaum PC. Pleuropulmonary infections caused by Eikenella corrodens. Rev Infect Dis. 1991;13:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |