Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3589
Revised: April 14, 2024
Accepted: April 22, 2024
Published online: June 26, 2024
Processing time: 110 Days and 6.6 Hours
Extracorporeal membrane oxygenation (ECMO) is a new type of extracorporeal respiratory and circulatory assistance device. It can drain venous blood out of the body and inject it into veins or arteries after being oxygenated by an oxygenator (membrane lung) to replace lung and heart functions in a short time. ECMO can provide tissue blood perfusion and gas exchange almost equivalent to cardiac output and extend the effective treatment time window for patients with acute circulatory failure to restore cardiopulmonary function.
We report a case of an 81-year-old woman who underwent whole cerebral angiography, basilar artery thrombectomy and stent thrombectomy in the pos
Although the patient eventually died of circulatory failure, the result of this case verifies the feasibility of cerebral angiography and thrombectomy in patients with implanted ECMO in the intubated state.
Core Tip: Extracorporeal membrane oxygenation (ECMO)-supported total cerebral angiography, catheter thrombectomy and stent thrombectomy are very rare, and no similar cases have been reported on PubMed. We report a case of an 81-year-old woman who underwent whole cerebral angiography, basilar artery thrombectomy and stent thrombectomy in the posterior artery of the left brain after implantation of ECMO. This article describes in detail the preoperative evaluation of the patient's condition, the difficult operation and anticoagulation regimen during the operation, as well as the postoperative management. The results of this case verify the feasibility of cerebral angiography and thrombectomy in patients with ECMO implantation under intubation.
- Citation: Ding LS, Liang H, Zheng M, Shen M, Li ZJ, Song RP, Chen QL. Extracorporeal membrane oxygenation states basilar artery thrombectomy and left posterior cerebral artery stent thrombectomy: A case report. World J Clin Cases 2024; 12(18): 3589-3595
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3589.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3589
Extracorporeal membrane oxygenation (ECMO) is a new type of extracorporeal respiratory circulation assist device. It can drain venous blood outside the body, oxygenate it through an oxygenator (membrane lung) and inject it into a vein or artery, replacing lung and heart function in a short period of time, providing life support for critically ill patients. Since its launch in 1972, the national trend shows that the number of peripheral VA-ECMO used in refractory cardiogenic shock has increased significantly. According to the registration of extracorporeal life support, more than 15000 adult patients have been supported by VA-ECMO since 1990, with an approximate survival rate of 40% at discharge. However, there is no case report of neurological intervention in patients with ECMO. At present, its development in China is still not popular, and it is mostly used in clinical resuscitation and rescue of cardiac arrest patients and has achieved certain results[1-3]. However, ECMO implantation can only maintain the patient's vital signs[4]. Clinically, there is a greater need to remove the cause of treatment, and in patients receiving ECMO implantation, neurological complications are common. Paralysis and hypoxic states also limit the evaluation of neurological function. At present, ECMO combined with interventional surgery is rarely carried out, especially ECMO combined with nerve intervention therapy, and no relevant cases have been reported. The difficulty is that if the patient has acute circulatory failure, the heart cannot provide effective perfusion pressure and insufficient blood supply to the brain, which will greatly affect the implementation of nerve intervention. If ECMO can be established early, the patient's systemic blood circulation and blood oxygen supply can be quickly restored so that the hemodynamics during nerve intervention surgery can be stabilized and the blood pressure is stable, which can make it possible to carry out nerve intervention surgery under ECMO. Henan No. 3 Provincial People’s Hospital applies ECMO equipment, ECMO combined with emergency percutaneous coronary intervention, whole cerebral angiography with basilar artery thrombectomy and left posterior cerebral artery stent thrombectomy, and rescue of 1 patient with cardiac arrest caused by acute myocardial infarction and acute cerebral infarction, which is reported as follows.
Sudden chest tightness with dyspnea for 2 h.
The patient is an 81-year-old female. She was accompanied by sweating, nausea, vomiting, and fatigue. The family gave 7 "quick-acting heart-saving pills" to be taken sublingually, and the symptoms were not significantly alleviated. Electrocardiogram illustration: II, III, augmented unipolar limb lead ST-segment elevation oblique upward elevation (HR: 46/min), III. Degree atrioventricular block. Nasal cannula oxygen inhalation, establishment of venous access, injection of 500 mL of normal saline + 100 mg of dopamine injection and 0.5 mg of atropine sulfate injection. After the onset of the disease, consciousness gradually changes from awake to vague. The patient was admitted to the intensive care unit with "acute coronary syndrome".
The patient had a history of diabetes for more than 10 years, had not been formally treated, and had poor blood sugar control. She had no history of hypertension or hyperlipidemia.
The patient has no bad habits such as tobacco, alcohol and drugs. And there are no further contributing factors in the remaining personal and family history.
T: 36.1 °C; P: 48/min; R: 10 times/min; BP: 75/48 mmHg; peripheral oxygen saturation 94%. Lethargy, dysarthria, no pathological reflex. The National Institutes of Health Stroke Scale score was 15 points (2 point for consciousness, 1 point for gazing, 2 points for dysarthria, 2 points for partial facial paralysis, and 8 points for limb weakness). The modified Rankin Scale score was 4.
Finger blood glucose 15.6 mmol/L, other laboratory test results are in the normal range.
The patient was implanted with ECMO in an emergency with limited activity due to a comatose state. The results of magnetic resonance imaging and computed tomography were not obtained, therefore. The results of digital subtraction angiography were presented in detail during the operation.
In this case, ECMO was implanted to restore circulation in the case of complete cerebral ischemia. And selective coronary angiography (SCA), percutaneous coronary angiography (PTCA), cerebral angiography, basilar artery catheter thrombectomy, and posterior left cerebral artery stent embolectomy were performed.
Considering that the patient's blood pressure and heart rate could not be maintained after the application of a large number of vasoactive drugs and that severe hypoxemia and metabolic acidosis were combined, ECMO was placed at the bedside after communicating with the patient's family, informed consent was given and was placed through the right femoral artery/femoral vein route. After the ECMO operation, the patient's vital signs gradually stabilized, and after transfer to the catheter lab, SCA PTCA was performed; ECMO, temporary pacing, ventilators, and high-dose vasoactive drugs continued to maintain vital signs after surgery.
Implantation of ECMO: The patient was treated with V-AECMO auxiliary mode and puncture of femoral artery and femoral vein. 21F ECMO catheter was inserted into the right femoral vein and 17F ECMO ductus arteriosus was placed through the right femoral artery. The circulation began as soon as the placement of the pipe was completed. ECMO flow and ventilator parameters were adjusted according to urine volume and blood pressure. Before intubation, the saline of 1000 mL was selected as the prefilling fluid, and the initial flow velocity of ECMO was set to 3.0-4.0 L/min. Attention should be paid to the intermittent application of low-dose heparin during bypass, keeping the activated clotting time (ACT) about 180-220s. The activated partial thromboplastin time prolonged 1.5 times, and the flow rate was adjusted according to the changes of hemodynamics and blood oxygen saturation.
Combined with electrocardiogram and auxiliary examination for hospital admission diagnosis: (1) Coronary heart disease acute inferior ST-segment elevation myocardial infarction, cardiac function grade IV (Killip grade); (2) cardiogenic shock (CS); and (3) the cause of the consciousness disorder needs to be investigated Ischemic: hypoxic-ischemic cerebrovascular disease.
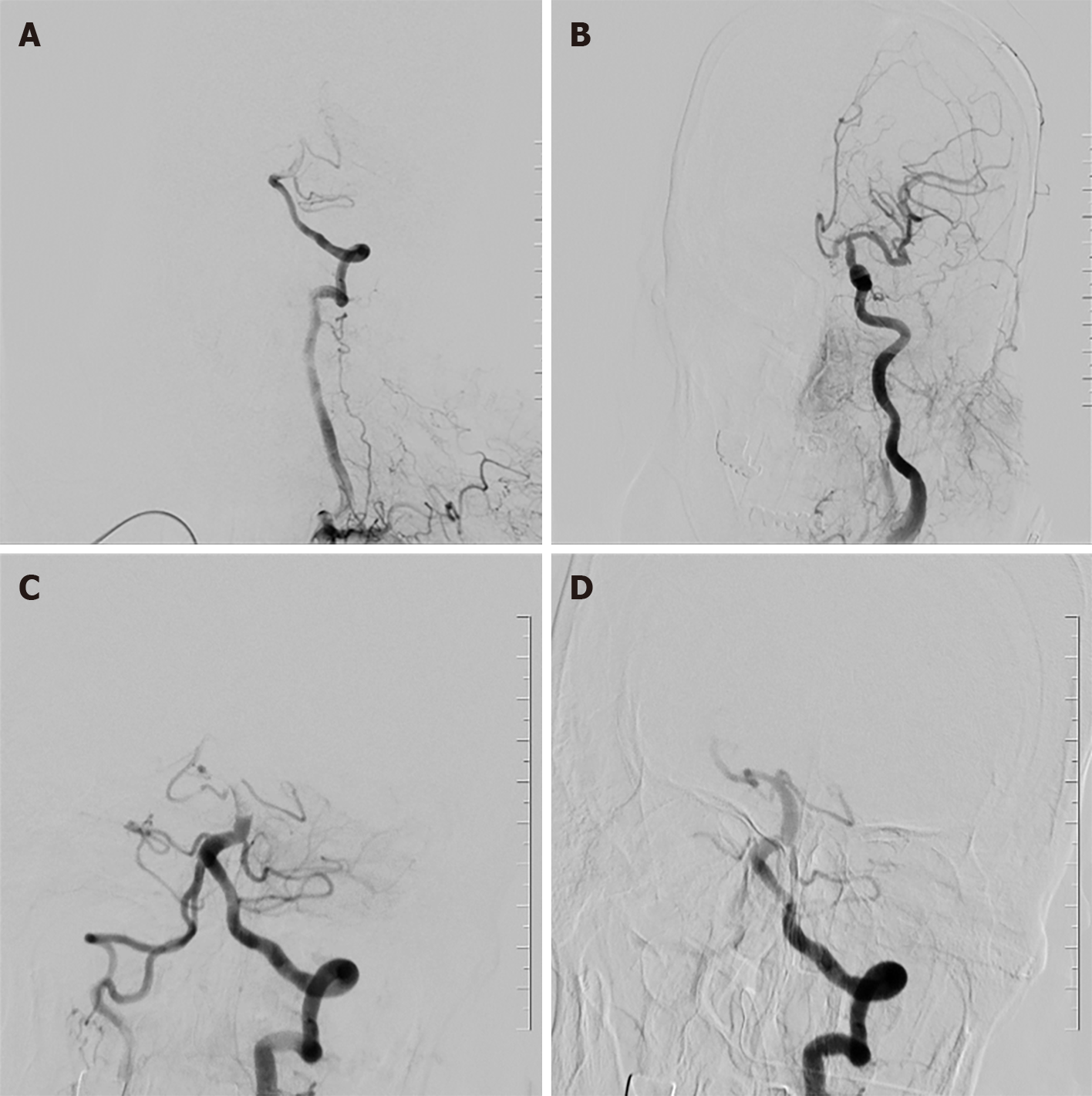
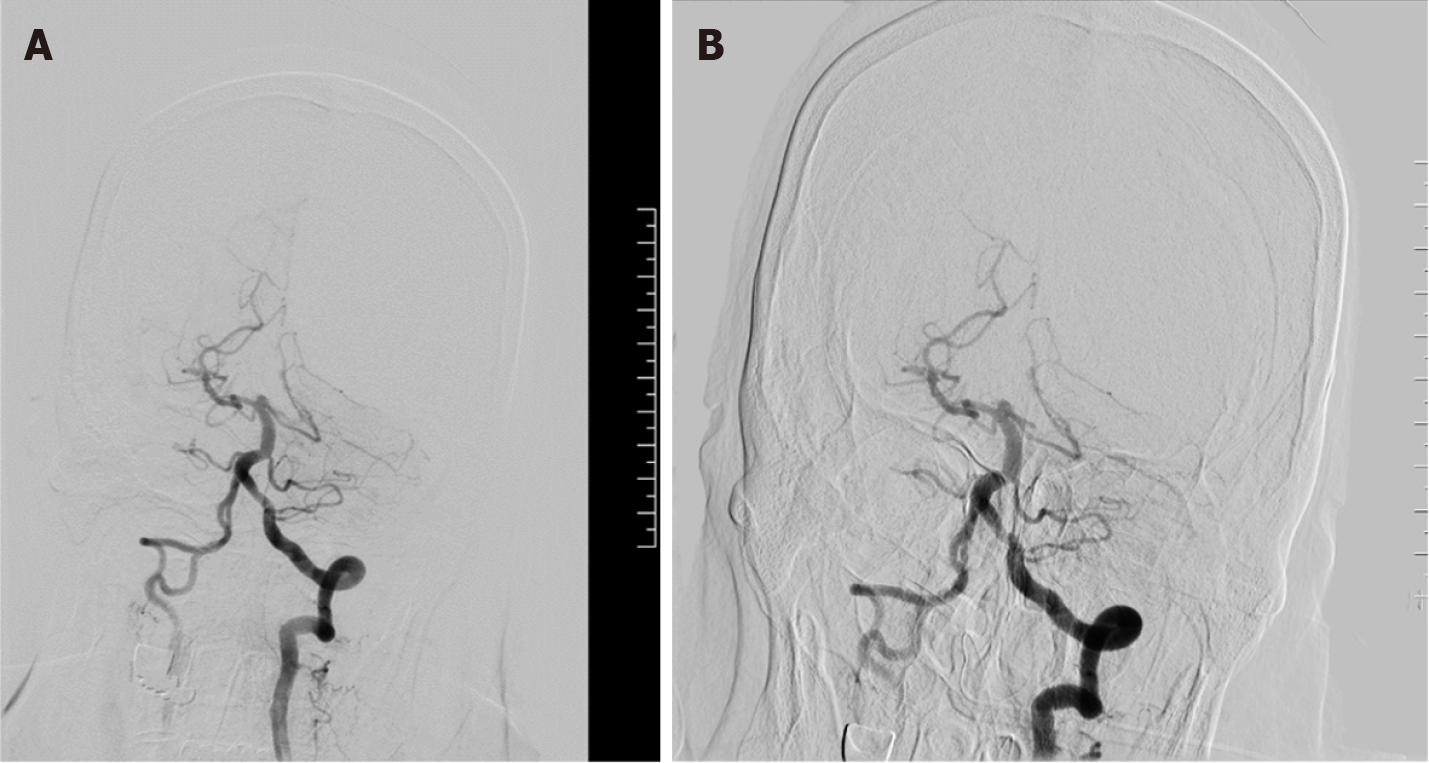
The patient remains impaired, and cerebrovascular accidents cannot be ruled out. Digital subtract angiography, puncture of the left femoral artery into the catheter sheath (COOK), successful insertion of super-slippery guidewire (COOK) and spinal catheter (COOK). Bilateral common carotid artery and left vertebral artery angiography showed that filling defects were visible in the middle and distal basilar arteries, and bilateral posterior cerebral arteries were not developed (Figure 1). The 8F guide tube (COOK) and 6F intermediate catheter (Navien) were introduced into the left vertebral artery, and heparin water was added to the TEE and Y valve. The guidewire cooperated with the suction catheter (catalyst 6) superselectively and entered the basilar artery, aspirated under negative pressure at the filling defect, and withdrawn under negative pressure suction. A strip-shaped dark red thrombus was extracted in the catheter. Repeated angiography showed that the basilar artery filling defect improved, but the distal vessel remained unseen. In the same way, the suction catheter was used again for thrombus aspiration, and the dark red thrombus was withdrawn again (Figure 2). Re-examination of the angiography showed that the basilar artery was opened, the right posterior cerebral artery had smooth blood flow, the posterior proximal segment of the left brain was occluded, and the distal blood vessel had not developed. The introduction of microguidewire (Synchro-14 200 cm) with microcatheter (Rebar-18) superselective slow passage through occlusion segment, contrast confirmed that the distal branch arterial blood flow is acceptable, the stent (Solitaire 4 mm × 20 mm) was sent to the obstruction site along the microguidewire and slowly released, and after 5 min, the angiography showed that blood flow in the P2 segment of the posterior cerebral artery was restored; simultaneous stent embolectomy was performed with a 50 mL syringe to retract the middle catheter, and a small number of emboli were attached to the stent; repeat angiography showed that the basilar artery trunk, superior cerebellar artery, inferior cerebellar artery, and posterior cerebral artery were all opened, a small amount of local filling defect in the lumen of the distal blood vessel (Figure 3), no other occluded blood vessels were seen, the catheter guidewire was withdrawn, the sheath canal was retained, the intraoperative process was smooth, and there was no bleeding at the puncture site.
Anticoagulation condition of patient: 300 mg of aspirin and 600 mg of clopidogrel were given routinely to inhibit platelet aggregation before surgery. 3000 units of heparin sodium were injected routinely through the sheath 3 minutes after the surgery began. At the 10th minute, 2000 units of heparin sodium were injected through the sheath to complete the angiographic examination. After the diseased vessels were further determined at the 30th minute, 1000 units of heparin sodium were injected through the sheath, and the measured whole blood ACT was 170 seconds. 10 ml of tirofiban injection was given through the sheath, and the measure value of ACT was 217 seconds. After the above operation, emergency thrombectomy was given. The measured ACT was 197 seconds after 120 min, and 2000 units of heparin were injected intravenously afterwards. The patient returned to the intensive care unit to continue treatment after surgery. The patient used anticoagulant and antiplatelet drugs before and during the operation, and no bleeding symptoms such as skin purpura and gum bleeding were observed.
The patient was successfully weaned and hemodynamically good. There were no obvious complications of bleeding, infection and ischemia, but the family gave up treatment because the cardiac function never recovered. The patient eventually died of circulatory failure. Nevertheless, the entire surgical process has demonstrated the feasibility of ECMO in combination with emergency nerve intervention in patients with circulatory failure.
Patients with cardiac arrest due to acute myocardial infarction are prone to severe ischemia and hypoxia in vital organs, especially the brain. After cardiac arrest, when hemodynamics deteriorate significantly and surgical treatment is difficult, ECMO can increase hemoperfusion, provide short-term circulatory support, stabilize the patient's blood flow status, and provide conditions for interventional treatment. In other studies, cerebral blood perfusion has been shown to remain relatively stable despite reduced cardiac output and cephalic vascular supply[5]. This means that in extreme cases of cardiac arrest in patients, ECMO provides effective circulatory support, maintains hemodynamic stability, raises blood pressure, and makes nerve intervention surgery under ECMO possible. At the same time, it has been reported that ECMO can increase the blood flow of arterial vessels, which helps to carry out thrombolysis and unclog blood vessels to restore blood circulation in brain tissue[6]; it also helps the patient's postoperative weaning and recovery. In addition, the ECMO puncture site of this patient was the right femoral artery, whole-brain angiography and embolectomy were selected from the left femoral artery, and the ECMO cannula was parallel to the intraoperative catheter guidewire so that the operation could proceed smoothly. Nerve intervention via radial artery approach has few complications, but the surgical failure rate is relatively high. The femoral artery approach is mature and easy to operate. So, we finally chose the left femoral artery approach. Although the intraoperative contrast medium is slower than that of normal patients and there is obvious contrast agent retention and development that is out of synchronization, it does not affect the identification of the filling defect site or the success of thrombectomy.
In addition, the patient had thrombus in the basilar artery and left posterior cerebral artery, and the source of the thrombus needs to be further clarified. Thrombosis in patients with ECMO includes thrombosis in the ECMO circuit in vitro and thrombosis in vivo. Thrombus is more likely to occur in the areas of slow blood flow, bifurcation and swirl in ECMO, such as oxygenator, blood pump, drainage catheter, return catheter and pipeline. This is most likely to happen when bubbles are entrapped in the negative pressure part of the circuit, but this type of thrombus is generally less likely to cause cerebral embolism. Furthermore, patients with acute heart failure lead to low circulation flow, easy to produce blood stasis, but also lead to local thrombosis. As time goes on, the chance of developing thrombosis will increase exponentially. But there is no exact data to confirm the conjecture. Nevertheless, the use of ECMO in the catheter lab remains controversial. The application of ECMO can cause adverse reactions such as bleeding, blood clots, infection, lower limb ischemia, and renal failure, which are mainly caused by intraoperative heparin and platelet loss in extracorporeal blood circulation, and the most dreaded complication is intracranial hemorrhage. Therefore, in nerve intervention, it is necessary to closely observe the changes in cerebral hemodynamic indicators, operate gently, avoid cerebral ischemia or congestion caused by cerebral perfusion pressure disorder and affect the surgical process, reduce the risk of secondary brain injury, and improve the prognosis of patients.
In summary, the complexity of ECMO, the high demand for perfusion expertise, and the high cost limit its use in the catheter lab[7]. Choosing when to use ECMO, when to wean after surgery, how to improve the success rate of rescue, minimize the time of patients in bed, reduce mortality and complication rates, and improve the safety and effectiveness of this technology in clinical practice, to promote popularization, are still problems that need to be explored and solved in future work.
We would like to thank the patient and her family for their informed cooperation and support in our work.
| 1. | Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ Heart Fail. 2018;11:e004905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 352] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 2. | Hutin A, Abu-Habsa M, Burns B, Bernard S, Bellezzo J, Shinar Z, Torres EC, Gueugniaud PY, Carli P, Lamhaut L. Early ECPR for out-of-hospital cardiac arrest: Best practice in 2018. Resuscitation. 2018;130:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | MacLaren G, Masoumi A, Brodie D. ECPR for out-of-hospital cardiac arrest: more evidence is needed. Crit Care. 2020;24:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Aboul Nour H, Poyiadji N, Mohamed G, Alsrouji OK, Ramadan AR, Griffith B, Marin H, Chebl AB. Challenges of acute phase neuroimaging in VA-ECMO, pitfalls and alternative imaging options. Interv Neuroradiol. 2021;27:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 5. | Bhagavan D, Padovano WM, Kovács SJ. Alternative diastolic function models of ventricular longitudinal filling velocity are mathematically identical. Am J Physiol Heart Circ Physiol. 2020;318:H1059-H1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Mu TS, Becker AM, Clark AJ, Batts SG, Murata LM, Uyehara CFT. ECMO with vasopressor use during early endotoxic shock: Can it improve circulatory support and regional microcirculatory blood flow? PLoS One. 2019;14:e0223604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Li Y, Yan S, Gao S, Liu M, Lou S, Liu G, Ji B, Gao B. Effect of an intra-aortic balloon pump with venoarterial extracorporeal membrane oxygenation on mortality of patients with cardiogenic shock: a systematic review and meta-analysis†. Eur J Cardiothorac Surg. 2019;55:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |