Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3582
Revised: April 13, 2024
Accepted: April 22, 2024
Published online: June 26, 2024
Processing time: 115 Days and 19.7 Hours
The aim of this study was to investigate the complex heterozygous mutations of ANK1 and SPTA1 in the same individual and improve our understanding of hereditary spherocytosis (HS) in children. We also hope to promote the appli
A 1-year and 5-month-old patient presented jaundice during the neonatal period, mild anemia 8 months later, splenic enlargement at 1 year and 5 months, and brittle red blood cell permeability. Genetic testing was performed on the patient, their parents, and sister. Swiss Model software was used to predict the protein structure of complex heterozygous mutations in ANK1 and SPTA1. Genetic testing revealed that the patient harbored a new mutation in the ANK1 gene from the father and a mutation in the SPTA1 gene from the mother. Combined with the clinical symptoms of the children, it is suggested that the newly discovered complex heterozygous mutations of ANK1 and SPTA1 may be the cause, provi
This case involves an unreported complex heterozygous mutation of ANK1 and SPTA1, which provides a reference for exploring HS.
Core Tip: The patient was a 1-year and 5-month-old child with hereditary spherocytosis (HS) whose diagnosis was confirmed by genetic testing. The patient had complex heterozygous mutations in ANK1 and SPTA1 that have not been previously reported. Combined with the relevant clinical manifestations in the children, it is suggested that the newly discovered complex heterozygous mutation of ANK1 gene and SPTA1 gene may be the cause of this disease and provide more molecular genetic information revealing the pathogenesis of HS in children. In addition, we hope to promote the application of genetic testing technology in children with HS and provide guidance for its diagnosis, treatment, and prevention.
- Citation: He M, Lv YC, Wei YH, Liu LQ, Guo L, Li C. Complex heterozygous mutations in hereditary spherocytosis: A case report. World J Clin Cases 2024; 12(18): 3582-3588
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3582.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3582
Hereditary spherocytosis (HS) is a heterogeneous disease of red blood cells (RBC). Currently, there is no accurate epidemiological survey data on HS in China, and its incidence in Europe and the United States is about 1/2000[1]. Approximately 70% of HS cases arise from autosomal dominant inheritance, about 15% are autosomal recessive, and some have new genetic mutations[2]. Abnormalities in the ANK1 gene, which encodes erythrocyte membrane proteins, are the most common causes of erythrocyte membrane protein abnormalities[3].
HS is the third most common underlying hemolytic disorder in the neonatal period, with the main clinical manifestations being anemia and jaundice[4]. There is no clear link between its severity and its associated genetic mutations[5]. Guidelines state that a reasonable diagnosis of HS can be based on typical clinical symptoms and signs such as anemia, jaundice, and splenomegaly, combined with laboratory tests and a family history of HS[6,7]. However, because HS patients often lack typical symptoms or changes in test indicators, the sensitivity of traditional laboratory indicators is poor. Neonates have 1% spherocytes[8], which is expected to provide a basis for the early diagnosis and intervention of HS[9].
A 1-year and 5-month-old patient presented with anemia for over one year and cough for 5 d.
The patient had a hemoglobin level of 95 g/L during routine childcare at the age of 8 months. However, no yellow coloration was observed on the face or sclera. After treatment with iron supplementation, anemia persisted.
The patient received blue light treatment for jaundice during the neonatal period.
Routine blood tests of the mother and 8-month-old sister of the patient showed no anemia; however, the sister's RBC osmotic fragility test was negative, as shown in Table 1. The child’s father refused routine blood tests and other related tests.
| Items | Patient | Mother of patient | Sister of patient |
| Blood routine | |||
| WBC/(× 109/L) | 8.64 | 6.59 | 8.82 |
| RBC/(× 1012/L) | 3.04 | 4.32 | 4.63 |
| HGB/(g/L) | 87 | 129 | 124 |
| PLT/(g/L9/L) | 376 | 306 | 367 |
| MCH/pg | 28.50 | 29.9 | 26.8 |
| MCV/fl | 85.90 | 91.7 | 76 |
| MCHC/(g/L) | 332 | 326 | 352 |
| Reticulocyte rate/% | 11.98 | ||
| RBC osmotic fragility | Heighten | Feminine | |
| Spherocytes/% | 3 | None | None |
| Bone marrow cell analysis | G/E inversion |
It was found that the patient had a mild anemia appearance, no yellow staining on the skin or sclera, and the liver was not palpable under the ribs. The spleen was 1 cm in line I and 1.5 cm in line II.
The auxiliary inspection results are shown in Table 1.
Genetic testing: With the informed consent of the family, 2 mL of peripheral venous blood of the patient, his parents, and sister was taken, genomic DNA was extracted, and Beijing Mackinol was entrusted to perform gene sequencing. In this study, whole exon gene V4 was tested by Trios. The sequencing target gene number was approximately 2300, the length of the target region was 49.11 Mb, and the average sequencing depths were 178.90, 186.55, and 161.01, respectively. The coverage at 10X and 20X were all greater than 98% and 96.9%, respectively. The average sequencing depth was greater than 200X magnification.
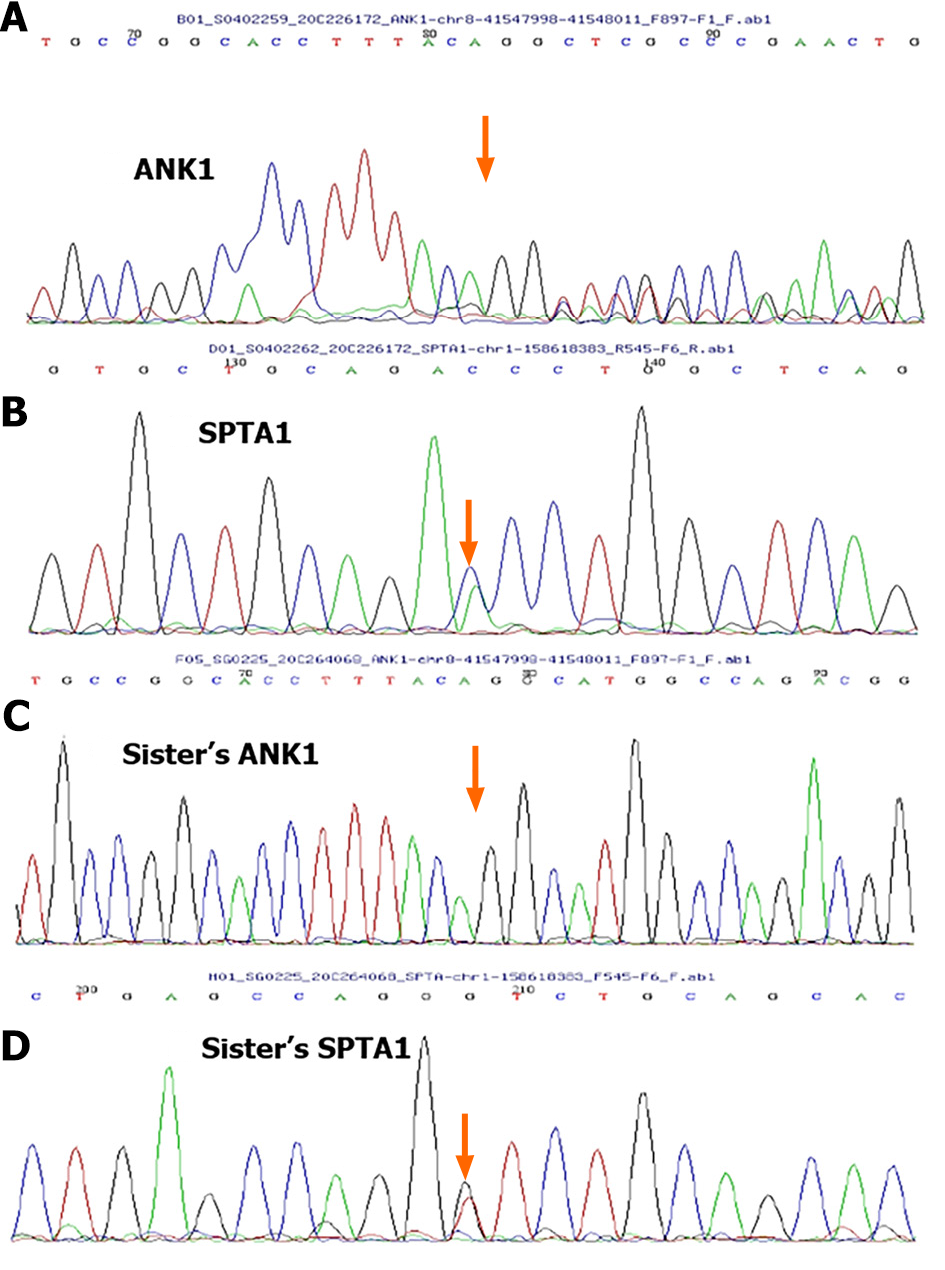
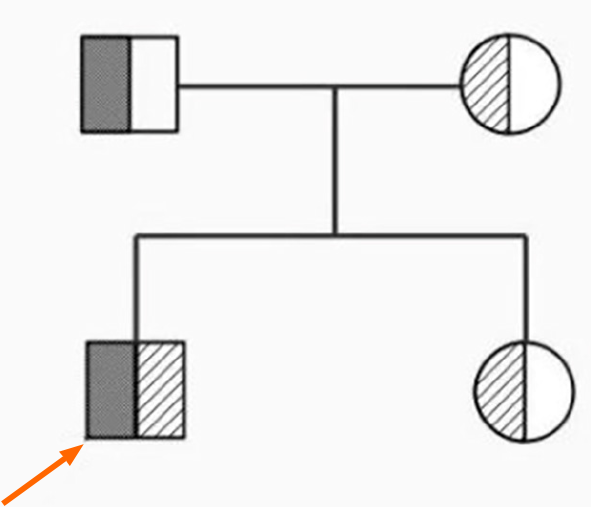
A new mutation, c.3965_3977delGTCTGGCCATGCC (p.R1322Lfs *2), was detected in ANK1 gene of the patient. The patient's father harbored a heterozygous variant at this site, whereas the patient's mother harbored a normal variant. A new mutation, c.3630C>A (p.D1210E), was detected in the SPTA1 gene of the patient. The mother had a heterozygous mutation at this locus, whereas the father had a normal mutation. A new mutation in the SPTA1 gene was detected with his sister, c.3630C>A (p.D1210E), which was derived from his mother (Figure 1). A pedigree diagram is shown in Figure 2.
Bioinformatics analysis: REVEL, SIFT, and PolyPhen_2 were used to predict the pathogenicity. The Swiss Model was used to predict the three-dimensional structure of proteins after genetic mutation.
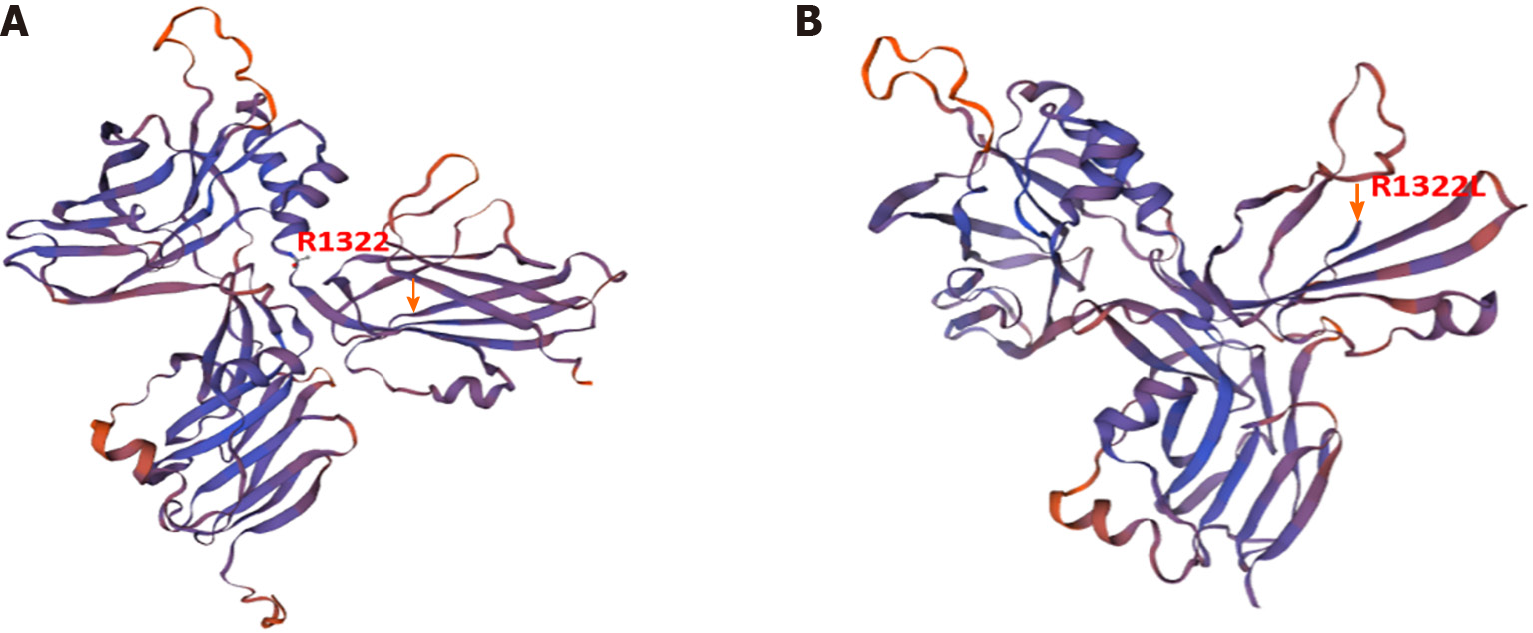
According to ACMG guidelines, the ANK1 variant in the patient was preliminarily considered a suspected pathogenic mutation, PVS1 + PM2, and the PVS1 mutation that may lead to the loss of gene function was a frameshift mutation. Swiss Model analysis revealed that arginine at the three-dimensional structure of the protein encoded by ANK1 at site 1322 became leucine, a stop code appeared in two digits of the frameshift (Figure 3), and translation was stopped. The frequency of SPTA1 gene PM2 in the normal population database shows a low-frequency variation, the prediction results of the BP4 bioinformatics protein function comprehensive prediction software REVEL were benign, and the prediction results of SIFT and PolyPen_2 were benign.
Combined with clinical symptoms, laboratory examination, and genetic test results, it was suggested that the patient may have inherited spherocytosis caused by complex heterozygous mutations in ANK1 and SPTA1.
HS is a congenital disease; there is no drug that can reduce the number of spherical RBC in the blood circulation, so the main treatment remains surgical treatment, with anti-infection, electrolyte, and acid-base balance, and other symptomatic treatments. RBC destruction primarily occurs in the spleen. The removal of the spleen cannot cure birth defects in RBC but can stop pathological hemolysis and prolong the life cycle of RBC; thus, splenectomy is still the only effective treatment. Considering the patient's condition and related test results, after communicating with the family about the treatment plan, prognosis, and possible related risks, the family members temporarily did not consider surgical treatment and performed clinical follow-ups.
The patient's daily activities and life were normal; however, long-term follow-up is still needed. Since spleen embolization and excision were not performed and repeated iron treatment was ineffective, it is necessary to remain alert to acute hemolysis due to infection and other factors that may result in severe anemia and even life-threatening conditions. Family members were advised to dynamically monitor laboratory indicators such as hemoglobin and bilirubin levels. RBC transfusion was considered necessary[10].
Spherocytosis is a congenital hemolytic anemia that can occur at any age, though presentation in childhood is the most common[11]. At present, the main laboratory indicators related to the diagnosis of HS are blood smears and osmotic tests. However, because the results of a single test may be impacted by irrelevant factors and have limited specificity and sensitivity[12], it is recommended that blood cell smears and osmotic tests are combined for differential diagnosis. In general, traditional laboratory tests are less sensitive and responsive for the diagnosis of HS; therefore, genetic testing and other methods are required to confirm the diagnosis[13].
ANK1, SPTA1, SPTB, SLC4A1, and EPB42 are the five main pathogenic genes of HS, encoding ankyrin α-hemoshadow protein, β-hemoyin protein, band 3 protein, and protein 4.2[14]. Among them, ANK1 and SPTB mutations are the main causes of HS in China. The ANK1 gene, located on chromosome 8 p11.21, contains 43 exons and encodes the ankyrin responsible for the structure of the erythrocyte membrane[15], weakening the interaction between the RBC membrane skeleton and the lipid bipolar layer, which makes the bipolar layer unstable and reduces the surface area of the membrane, resulting in a spherical shape. A literature review shows that the pathogenesis of ANK1 mutations remains unclear, with many reports on mutations in different regions of ANK1. At least 60 mutations in ANK1 have been identified; most reported neonatal mutations in ANK1 are frameshift and nonsense mutations, whereas splicing mutations are rare. In sporadic cases, many nascent mutations appear in maternal germ cells. However, the mother of this patient did not have clinical manifestations related to HS and showed normal laboratory blood test results. The patient's father did not provide a local history or related examinations, and it is challenging to preemptively diagnose HS in children without a sufficient family history[16-18]. The clinical features of this patient were consistent with the HS phenotype, and gene sequencing revealed that the ANK1 base deletion caused the conserved site R1322 arginine to become leucine and a stop code to appear, which contained a highly conserved central spectrin-binding domain that changed protein function after mutation[19]. According to the American College of Medical Genetics and Genomics guidelines, the variant is preliminarily judged to be a null mutation (frameshift mutation), and the mutation type has not been reported. Subsequently, we used the Swiss Model to predict the structural changes of realistic proteins, verifying that the stopcode protein no longer translates after the change in amino acid 1322 (Figure 3); therefore, it is considered a pathogenic mutation. Further RNA analysis and other experiments are needed to confirm the pathogenicity of the ANK1 mutation in this patient[20].
In addition, 5% of HS cases are caused by α-hemoshadow protein defects caused by SPTA1 gene mutations, but heterozygous mutations do not cause hemolysis and disease manifestations[21], and obvious symptoms occur only when the SPTA1 allele is homozygous or compound heterozygous mutations[22]. Once the α-hemoshadow protein as a cytoskeletal protein is defective, it will affect the[23,24] α-β heterodimer formed by binding to β-hemo shadow protein, so that the interaction between α-β heterodimer, actin and, protein 4.2 changes and finally leads to membrane instability such as weakened deformation ability of RBC membrane. In addition to the ANK1 mutation, the patient also inherited the SPTA1 mutation c.3630C > A (P.D1210E) from the mother; however, the mutation at this site was not reported in the literature and the bioinformatics prediction results were benign. Combined with the fact that the mother and sister of the patient have SPTA1 mutations and no clinical manifestations, we speculate that the new mutation in ANK1 gene, forming a compound heterozygous mutation with SPTA1, is the reason why the patient has this phenotype.
Although the patient harbored a complex heterozygous mutation, the clinical symptoms were relatively mild. The literature review shows that studies on HS caused by ANK1 gene mutations primarily include reports of different mutation sites of ANK1 gene, but the same mutant gene and site may also show clinical phenotype variation. Some studies suggest that this may be related to age and race, whereas others suggest that it may be related to the expression of mutant gene alleles. That is, low allele expression may lead to mild clinical manifestations in patients[25].
The patient's mild clinical symptoms, physical signs, and laboratory examination results led the family to decline surgical treatment and opt for a clinical follow-up. Surgical treatment is the main approach for HS, with splenectomy chosen based on the patient's clinical symptoms and complications, such as gallstones. However, this should not be based solely on the diagnosis. Splenectomy is recommended for severe HS in children, considered for moderate cases, and may not be necessary for mild cases. Studies have supported splenectomy as the standard surgical treatment for moderate to severe HS. Nevertheless, total splenectomy exposes patients to a lifelong risk of potentially fatal infections, prompting reconsideration of its use. Therefore, partial splenectomy is a potential alternative for preserving the splenic immune function while reducing the hemolysis rate. Symptomatic treatment should also be included in the management of HS. Some reports suggest that folic acid supplementation is beneficial for severe and moderate HS but is unnecessary for mild cases[13]. Owing to the large range of disease severity, individualized planning of follow-up frequencies is required. Follow-up visits should assess the extent of anemia and monitor its growth and development. Iron overload should be closely monitored in children with HS who require continued blood transfusions.
In summary, the patient had a novel mutation in the ANK1 gene from the father, c.3965_3977delGTCTGGCCATGCC (p.R1322Lfs*2), accompanied by a mutation in the SPTA1 c.3630C >A (p.D1210E) gene from the mother, resulting in the transformation of arginine to leucine at the 1322 site of the three-dimensional structure of the ANK1 protein. The mutation type in this clinical case was the compound heterozygous mutation of ANK1 and SPTA1, which has not been reported, providing a reference for more in-depth exploration of HS. At present, the misdiagnosis and missed diagnosis rates of HS are relatively high. However, with the continuous popularization and development of genetic diagnostic technology, the diagnosis rate of HS is gradually improving. Many new mutations have been identified in genes associated with HS, including SPTA1, SPTB, ANK1, SLC4A1, etc. However, these sites are sporadic and diverse, which makes the study of HS constantly face new challenging. By summarizing the existing literature on the mutation sites and possible mechanisms of HS, we can provide more molecular genetic information for genetic counseling, prenatal and postnatal care, prenatal diagnosis, and further reveal the pathogenesis of HS.
We thank the patients’ parents for providing permission to share their information.
| 1. | Rosman CWK, Broens PMA, Trzpis M, Tamminga RYJ. A long-term follow-up study of subtotal splenectomy in children with hereditary spherocytosis. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Joshi P, Aggarwal A, Jamwal M, Sachdeva MU, Bansal D, Malhotra P, Sharma P, Das R. A comparative evaluation of Eosin-5'-maleimide flow cytometry reveals a high diagnostic efficacy for hereditary spherocytosis. Int J Lab Hematol. 2016;38:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Liao L, Qiu YL, Deng ZF, Huang J, Lin FQ. [The genetic mutation research of hereditary spherical red blood cells]. Zhongguo Fuyou Baojian. 2013;36:600-6004. |
| 4. | Christensen RD, Yaish HM, Gallagher PG. A pediatrician's practical guide to diagnosing and treating hereditary spherocytosis in neonates. Pediatrics. 2015;135:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Sun XJ, Li HY, Li DP, Liu YZ, Zhang JY, Yi YK, Su MH, Pan H, Li QL, Hu B. [The clinical characteristics of the hereditary spherical red blood cells enlarged the red blood cell membrane gene mutation]. Zhonghua Xueyexue Zazhi. 2018;39:912-916. |
| 6. | Manciu S, Matei E, Trandafir B. Hereditary Spherocytosis - Diagnosis, Surgical Treatment and Outcomes. A Literature Review. Chirurgia (Bucur). 2017;112:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 7. | Bianchi P, Fermo E, Vercellati C, Marcello AP, Porretti L, Cortelezzi A, Barcellini W, Zanella A. Diagnostic power of laboratory tests for hereditary spherocytosis: a comparison study in 150 patients grouped according to molecular and clinical characteristics. Haematologica. 2012;97:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Li JY. [Hereditary spherical red blood cells increased and treated the progress of the diagnosis and treatment]. Zhongguo Shiyong Neike Zazhi. 2012;32:347-350. |
| 9. | Delhommeau F, Cynober T, Schischmanoff PO, Rohrlich P, Delaunay J, Mohandas N, Tchernia G. Natural history of hereditary spherocytosis during the first year of life. Blood. 2000;95:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Shih YH, Huang YC, Lin CY, Lin HY, Kuo SF, Lin JS, Shen MC. A large family of hereditary spherocytosis and a rare case of hereditary elliptocytosis with a novel SPTA1 mutation underdiagnosed in Taiwan: A case report and literature review. Medicine (Baltimore). 2023;102:e32708. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Bolton-Maggs PH, Stobart K, Smyth RL. Evidence-based treatment of haemophilia. Haemophilia. 2004;10 Suppl 4:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Gao M, Li F, Huang ZW, Zhang KH, Lv YQ, Gai ZT, Liu Y. [The new mutation of the. ANK1 gene caused the genetic spherical red blood cells to analyze 1 family analysis]. Zhongguo Shiyong Erke Linchunag Zazhi. 2019;34:1826-1827. |
| 13. | Bolton-Maggs PH, Langer JC, Iolascon A, Tittensor P, King MJ; General Haematology Task Force of the British Committee for Standards in Haematology. Guidelines for the diagnosis and management of hereditary spherocytosis--2011 update. Br J Haematol. 2012;156:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Bolton-Maggs PH. Hereditary spherocytosis; new guidelines. Arch Dis Child. 2004;89:809-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Eber SW. [Disorders of the membrane skeleton of erythrocytes in hereditary spherocytosis and elliptocytosis: significance of the molecular defect for pathogenesis and clinical severity]. Klin Padiatr. 1991;203:284-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Barcellini W, Bianchi P, Fermo E, Imperiali FG, Marcello AP, Vercellati C, Zaninoni A, Zanella A. Hereditary red cell membrane defects: diagnostic and clinical aspects. Blood Transfus. 2011;9:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 17. | He Y, Jia S, Dewan RK, Liao N. Novel mutations in patients with hereditary red blood cell membrane disorders using next-generation sequencing. Gene. 2017;627:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Lin PC, Chiou SS, Lin CY, Wang SC, Huang HY, Chang YS, Tseng YH, Kan TM, Liao YM, Tsai SP, Peng CT, Chang JG. Whole-exome sequencing for the genetic diagnosis of congenital red blood cell membrane disorders in Taiwan. Clin Chim Acta. 2018;487:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Wang C, Yu C, Ye F, Wei Z, Zhang M. Structure of the ZU5-ZU5-UPA-DD tandem of ankyrin-B reveals interaction surfaces necessary for ankyrin function. Proc Natl Acad Sci U S A. 2012;109:4822-4827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Huang L, Zhu Y, An X, Li J, Zhen J, Yu J. De novo variations of ANK1 gene caused hereditary spherocytosis in two Chinese children by affecting pre-mRNA splicing. BMC Pediatr. 2023;23:23. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Chonat S, Risinger M, Sakthivel H, Niss O, Rothman JA, Hsieh L, Chou ST, Kwiatkowski JL, Khandros E, Gorman MF, Wells DT, Maghathe T, Dagaonkar N, Seu KG, Zhang K, Zhang W, Kalfa TA. The Spectrum of SPTA1-Associated Hereditary Spherocytosis. Front Physiol. 2019;10:815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Zhang YG, Xu ZL. [4 cases of clinical and genetic analysis of patients with genetic spherical red blood cells]. Zhongguo Dangdai Erke Zazhi. 2019;21:29-32. |
| 23. | Delaunay J, Nouyrigat V, Proust A, Schischmanoff PO, Cynober T, Yvart J, Gaillard C, Danos O, Tchernia G. Different impacts of alleles alphaLEPRA and alphaLELY as assessed versus a novel, virtually null allele of the SPTA1 gene in trans. Br J Haematol. 2004;127:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Hülsmeier J, Pielage J, Rickert C, Technau GM, Klämbt C, Stork T. Distinct functions of alpha-Spectrin and beta-Spectrin during axonal pathfinding. Development. 2007;134:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Xu L, Wei X, Liang G, Zhu D, Zhang Y, Shang X. A novel splicing mutation of ANK1 is associated with phenotypic heterogeneity of hereditary spherocytosis in a Chinese family. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |