Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3555
Revised: April 10, 2024
Accepted: May 8, 2024
Published online: June 26, 2024
Processing time: 147 Days and 19.9 Hours
In recent years, immune checkpoint inhibitors (ICIs) have demonstrated remarkable efficacy across diverse malignancies. Notably, in patients with advanced gastric cancer, the use of programmed death 1 (PD-1) blockade has significantly prolonged overall survival, marking a pivotal advancement com
This article presents the case of a patient with advanced gastric cancer and chronic plaque psoriasis. Following sintilimab therapy, the patient developed severe rashes accompanied by cytokine release syndrome (CRS). Fortunately, effective management was achieved through the administration of glucocorticoid, tocili
Glucocorticoid and tocilizumab therapy was effective in managing CRS after PD-1 blockade therapy for gastric cancer in a patient with chronic plaque psoriasis.
Core Tip: The increasing use of immunotherapy has led to an increase in immune-related adverse events. This article presents the case of a patient with advanced gastric cancer and chronic plaque psoriasis. Following sintilimab therapy, the patient developed severe rashes accompanied by cytokine release syndrome. Fortunately, effective management was achieved through the administration of glucocorticoids, tocilizumab, and acitretin, which resulted in favorable treatment outcomes.
- Citation: Zhou MH, Ye MF, Zhang ZX, Tao F, Zhang Y. Cytokine release syndrome triggered by programmed death 1 blockade (sintilimab) therapy in a psoriasis patient: A case report. World J Clin Cases 2024; 12(18): 3555-3560
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3555.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3555
Gastric cancer, a prevalent and heterogeneous malignancy, ranks fifth globally in incidence and fourth in China. Unfortunately, the majority of patients with gastric cancer are diagnosed at stage IV due to low early detection rates, thereby missing the window for initial surgery[1,2]. Limited effective chemotherapy options result in a short overall survival. The advent of immune checkpoint inhibitors (ICIs), such as programmed death 1 (PD-1) inhibitors, has highlighted the significant survival extension that can be achieved through PD-1 blockade in gastric cancer, as demonstrated in trials such as CheckMate-649 and Orient-16 (NCT03745170)[3]. This increased survival has propelled the combination of chemo
However, the widespread use of ICIs has led to a surge in diverse immune-related adverse events (irAEs). While most grade ≤ 2 irAEs can be managed with standard symptomatic treatment, a subset of irAEs, specifically grade ≥ 3 irAEs or life-threatening side effects, require precise and timely intervention[4]. Psoriasis, an immune-mediated disease, involves a delicate balance between autoimmunity and autoinflammation. ICIs have the potential to disrupt this balance, leading to exacerbations of psoriasis and other dermatological issues[5]. This article describes an elderly male who experienced severe skin toxicity and cytokine release syndrome (CRS) after receiving only one dose of sintilimab.
Fever with rash for 11 d.
A 75-year-old male patient had been recently diagnosed with advanced gastric cancer (stage T4NxM1 (IVB)). After the first cycle of treatment with oxaliplatin, capecitabine and sintilimab, he developed a low-grade exacerbated rash accompanied by fever, with a body temperature less than 38 °C. His daily life was not significantly affected. The patient came to our hospital for further treatment.
The patient had a medical history of infantile paralysis, hypertension, diabetes mellitus, and plaque psoriasis spanning several years.
The patient denied any family history of malignancy.
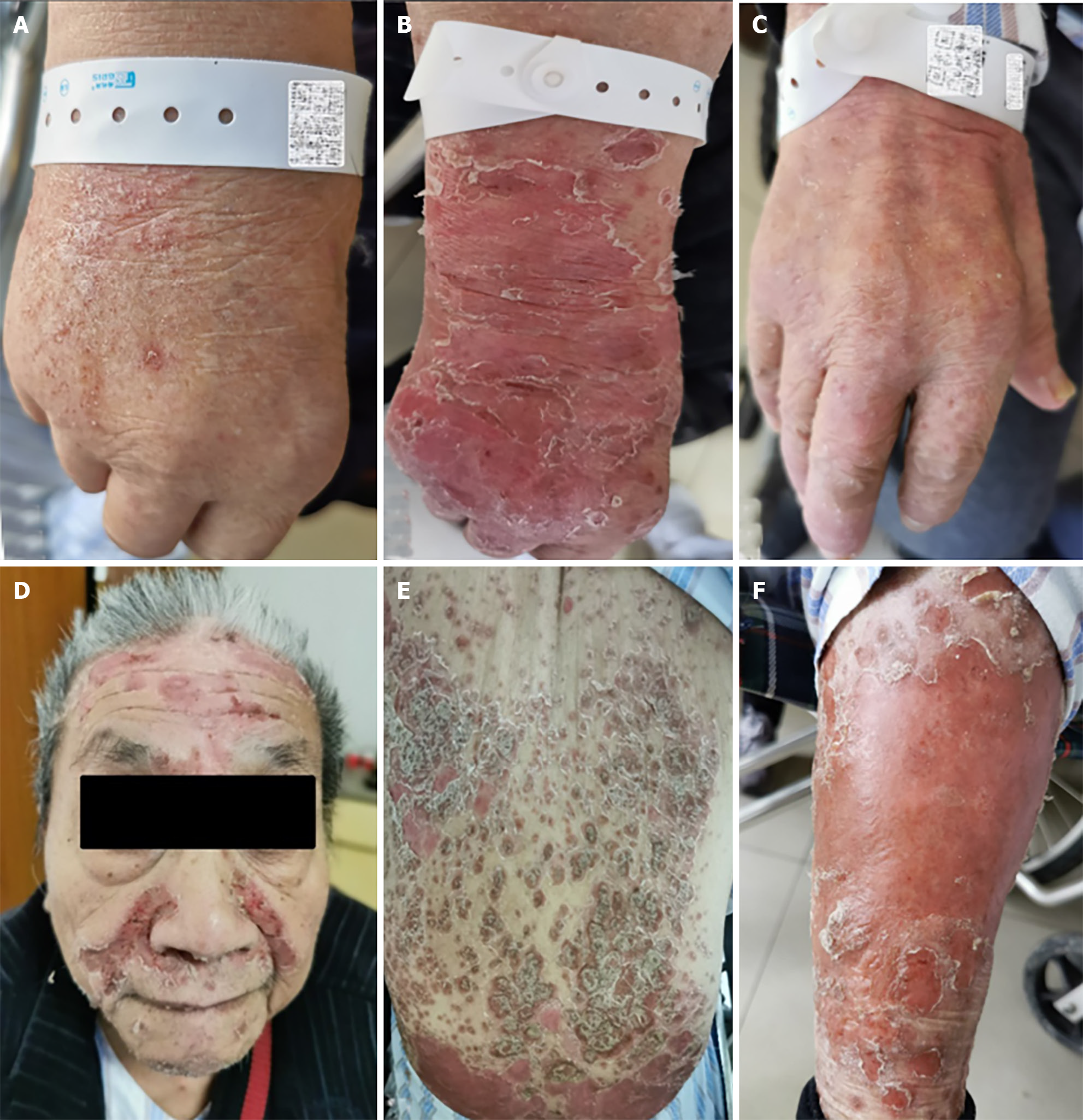
The patient's vital signs were as follows: body temperature, 38.7 °C; pulse oximetry, 92%; reported shortness of breath; and blood pressure and heart rate within normal ranges. The rash significantly worsened but without skin ulceration (Figure 1A).
The patient’s C-reactive protein level was 232.94 mg/L (reference: 0-7 mg/L), while his leukocyte and neutrophil counts remained within the normal ranges.
The final diagnosis of was CRS, hypertension, diabetes mellitus, infantile paralysis, and plaque psoriasis.
After admission to the hospital, immediate low-flow oxygen inhalation was initiated to address hypoxia, and antipyretic analgesics were administered to reduce body temperature. Although there was no evidence of infection, antibiotics were prescribed to mitigate any fatal complications that might develop.
While the patient's polypnea resolved, the fever recurred, with the body temperature increasing to 41 °C. His blood pressure decreased to 88/55 mmHg, and the rash intensified, resulting in skin damage on the forehead, face, ante
| Laboratory test | Value at presentation | Reference value |
| Interleukin-2 | 0.97 pg/mL | ≤ 9.80 pg/mL |
| Interleukin-4 | 1.98 pg/mL | ≤ 3.00 pg/mL |
| Interleukin-6 | 631.25 pg/mL | ≤ 16.60 pg/mL |
| Interleukin-10 | 12.93 pg/mL | ≤ 4.90 pg/mL |
| TFN-α | 2.36 pg/mL | ≤ 5.20 pg/mL |
| Interferon-γ | 66.89 pg/mL | ≤ 17.3 pg/mL |
| CRP | 232.94 mg/L | ≤ 7 mg/L |
| Procalcitonin | 0.25 ng/mL | ≤ 0.5 ng/mL |
| ESR | 60 mm/h | ≤ 15 mm/h |
| APTT | 49.4 s | 26.0-40.0 s |
| INR | 1.62 | 1.0-1.27 |
| Fibrinogen1 | 6.73 | 2.0-4.0 |
| Albumin | 23.5 g/L | 30-40 g/L |
| Rheumatoid factor | 6.25 IU/mL | ≤ 30 IU/mL |
| SARS-Co RNA | Negative | Negative |
| Influenza virus-A RNA | Negative | Negative |
| Influenza virus-B RNA | Negative | Negative |
| EB-Ab. IgM | Negative | Negative |
Over 4 wk, the patient's CLS, diffuse rash, and arthralgia ameliorated, leading to hospital discharge without recurrence or worsening of psoriasis symptoms (Figure 1C). A low maintenance dose of prednisolone (6 mg/d) was continued for an additional 2 wk during the follow-up period.
Studies indicate a pivotal role for Th1 and Th17 cells in the pathogenesis of psoriasis. By blocking the PD-1 pathway, PD-1 inhibitors may inadvertently increase the activity of Th1 and Th17 cells, leading to the onset, recurrence, or exacerbation of psoriasis[6,7]. Clinical investigations have established psoriasis as a significant risk factor for the accelerated development of psoriatic rash compared to patients without a prior history. A retrospective analysis by Halle et al[8] revealed a 57% incidence of relapsed or worsened psoriasis in cancer patients after ICI treatment, with an average onset time of 44 d. For many psoriasis patients, local or systemic treatments are effective, but discontinuation or suspension of drug therapy based on individual response is necessary for some patients. Additional options include oral administration of glucocorticoids or tretinoin in cases where external treatments prove ineffective.
CRS is clinically characterized by a spectrum of inflammatory symptoms and signs. Severe CRS is more commonly associated with CAR-T-cell therapy, while CRS occurring after PD-1 inhibitor therapy is relatively rare. The notable cases reported by Rotz et al[9] and Hu et al[10] involved alveolar soft part sarcoma and pulmonary fibrosis, respectively, with complicating CRS following nivolumab and sintilimab treatments. Distinguishing and diagnosing CRS after PD-1 treatment is challenging due to the variety of clinical manifestations. The patient whose case we present exhibited the recurrence and exacerbation of rash as the initial manifestation, followed by acute high fever, hypoxia, and severe skin lesions. The disease progressed rapidly, becoming life-threatening and leading to a grade 3 CRS according to the Common Terminology Criteria for Adverse Events grading system[11]. Treatment involving methylprednisolone and tocilizumab proved effective, aligning with the literature on CRS after ICI treatment[12]. Moreover, emerging evidence suggests that tocilizumab has potential for alleviating cancer-related cachexia and demonstrated latent effects in preclinical tumor models. Consequently, certain medical centers are exploring PD-1 plus tocilizumab therapy in patients at high risk of irAEs[13,14]. However, further analysis and discussion of the therapeutic efficacy are needed given the current insufficient clinical evidence.
Patients with psoriasis encounter a common challenge - the potential for psoriasis exacerbation upon discontinuation of glucocorticoid treatment for CRS. Our experience suggests the efficacy of a gradual reduction in the glucocorticoid dose over 1 wk when systemic symptoms are effectively controlled. Moreover, conventional methods such as acitretin, vitamin D3 derivatives, or ultraviolet irradiation can complement psoriasis treatment. It is advisable to continue medication for more than 2-3 wk when tapering to low doses of glucocorticoids rather than abruptly stopping the drug.
In summary, the use of immunotherapy in psoriasis patients is on the rise with the increasing prevalence of immunotherapy. It is crucial to be vigilant about the side effects of immunotherapy to promptly identify and manage serious complications. Our case underscores the therapeutic efficacy of glucocorticoids and tocilizumab in managing CRS after PD-1 blockade therapy for gastric cancer in a patient with chronic plaque psoriasis.
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11935] [Article Influence: 2983.8] [Reference Citation Analysis (4)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2857] [Article Influence: 571.4] [Reference Citation Analysis (5)] |
| 3. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1898] [Article Influence: 474.5] [Reference Citation Analysis (1)] |
| 4. | Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 882] [Article Influence: 176.4] [Reference Citation Analysis (36)] |
| 5. | Cutroneo P, Ingrasciotta Y, Isgrò V, Rullo EV, Berretta M, Fiorica F, Trifirò G, Guarneri C. Psoriasis and psoriasiform reactions secondary to immune checkpoint inhibitors. Dermatol Ther. 34:e14830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30:e89-e91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Murata S, Kaneko S, Harada Y, Aoi N, Morita E. Case of de novo psoriasis possibly triggered by nivolumab. J Dermatol. 2017;44:99-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Halle BR, Betof Warner A, Zaman FY, Haydon A, Bhave P, Dewan AK, Ye F, Irlmeier R, Mehta P, Kurtansky NR, Lacouture ME, Hassel JC, Choi JS, Sosman JA, Chandra S, Otto TS, Sullivan R, Mooradian MJ, Chen ST, Dimitriou F, Long G, Carlino M, Menzies A, Johnson DB, Rotemberg VM. Immune checkpoint inhibitors in patients with pre-existing psoriasis: safety and efficacy. J Immunother Cancer. 2021;9:; e003066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Hu J, Li Y, Chen X, Luo C, Zuo X. Pulmonary fibrosis and cytokine release syndrome after hyperactivation with sintilimab. J Clin Pharm Ther. 2020;45:1474-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, Go WY, Eldjerou L, Gardner RA, Frey N, Curran KJ, Peggs K, Pasquini M, DiPersio JF, van den Brink MRM, Komanduri KV, Grupp SA, Neelapu SS. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25:625-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2367] [Cited by in RCA: 2239] [Article Influence: 373.2] [Reference Citation Analysis (0)] |
| 12. | Ellis SR, Vierra AT, Millsop JW, Lacouture ME, Kiuru M. Dermatologic toxicities to immune checkpoint inhibitor therapy: A review of histopathologic features. J Am Acad Dermatol. 2020;83:1130-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, Cherukuri S, Parent T, Hardin J, Walker P. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019;25:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 14. | Li YH, Zhou Y, Liu YY, Zhang GJ, Xiao L, Li N, Qin HF, Wang JG, Zhang L. Severe immune-related hyperthermia followed by immune-related pneumonitis with PD-1 inhibitor (sintilimab) in small cell lung cancer: A case report. Thorac Cancer. 2021;12:1780-1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |