Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3505
Revised: April 23, 2024
Accepted: April 30, 2024
Published online: June 26, 2024
Processing time: 109 Days and 1.1 Hours
Hypertrophic scar (HTS) is dermal fibroproliferative disorder, which may cause physiological and psychological problems. Currently, the potential mechanism of WuFuYin (WFY) in the treatment of HTS remained to be elucidated.
To explore the potential mechanism of WFY in treating HTS.
Active components and corresponding targets were retrieved from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform. HTS-related genes were obtained from the GeneCards, DisGeNET, and National Center for Biotechnology Information. The function of targets was analyzed by performing Gene Ontology and Kyoto Encyclopaedia of Genes and Genome (KEGG) enrichment analysis. A protein + IBM-protein interaction (PPI) network was developed using STRING database and Cytoscape. To confirm the high affinity between compounds and targets, molecular docking was performed.
A total of 65 core genes, which were both related to compounds and HTS, were selected from multiple databases. PPI analysis showed that CKD2, ABCC1, MMP2, MMP9, glycogen synthase kinase 3 beta (GSK3B), PRARG, MMP3, and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma (PIK3CG) were the hub targets and MOL004941, MOL004935, MOL004866, MOL004993, and MOL004989 were the key compounds of WFY against HTS. The results of KEGG enrichment analysis demonstrated that the function of most genes were enriched in the PI3K-Akt pathway. Moreover, by performing molecular docking, we confirmed that GSK3B and 8-prenylated eriodictyol shared the highest affinity.
The current findings showed that the GSK3B and cyclin dependent kinase 2 were the potential targets and MOL004941, MOL004989, and MOL004993 were the main compounds of WFY in HTS treatment.
Core Tip: This study identified 8 hub genes [CKD2, ABCC1, MMP2, MMP9, glycogen synthase kinase 3 beta (GSK3B), PRARG, MMP3, and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma (PIK3CG)] and five key compounds (MOL004941, MOL004935, MOL004866, MOL004993, and MOL004989) in the WuFuYin (WFY) as an effective treatment for hypertrophic scar (HTS). We found that PI3K-Akt pathway played a crucial role in the WFY. The results of molecular docking showed that GSK3B and cyclin dependent kinase 2 affected HTS through targeting MOL004941, MOL004989, and MOL004993.
- Citation: Zhang SY, Guo SX, Chen LL, Zhu JY, Hou MS, Lu JK, Shen XX. Exploring the potential mechanism of WuFuYin against hypertrophic scar using network pharmacology and molecular docking. World J Clin Cases 2024; 12(18): 3505-3514
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3505.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3505
Hypertrophic scar (HTS) is dermal fibroproliferative disorder characterized by hyperfibrosis of the skin and persistent local histological inflammation that usually occurs after burns or trauma[1,2]. To date, therapies such as cryotherapy and drug injection for HTS are emerging[3]. Previous studies proved that some signal pathways, including Transforming growth factor beta (TGF-β), Wnt/β-catenin, and Notch signal pathways, played vital roles in the development of HTS[4]. At present, some drugs targeting signal pathways were applied in clinical practice to treat HTS. Poon et al[5] indicated that Nefopam could reduce HTS through inhibiting Wnt/β-catenin. Qiu et al[6] showed that the application of P144, an inhibitor of TGF-β1, promoted the improvement of HTS. These results suggested that targeting signal pathways could be a promising treatment for HTS. However, drugs targeting signal pathways in HTS are limited, which requires further exploration.
WuFuYin (WFY) is a traditional Chinese medicine prescription normally used for the deficiency of energy and state of the blood. It has been proven that WFY could enhance the immune response of patients and improve inflammation[7]. The prescription of WFY contains Ren-Shen (RS, Panax Ginseng C. A. Mey.), Shu-Di-Huang (SDH, Rehmanniae Radix Praeparata), Dang-Gui (DG, Angelicae Sinensis Radix), Bai-Zhu (BZ, Atractylodes Macrocephala Koidz), and Gan-Cao (GC, licorice). In vitro study showed that the extract of RS could reduce the production of inflammatory cytokines and improve inflammation[8]. Rehmanniae Radix-related herbal formula could promote wound healing in vitro, and under the use of herbal formula differentially expressed genes were enriched in inflammatory-related pathways[9]. Wu et al[10] demonstrated that DG-related herbal formula could reduce inflammation to inhibit the development of fibrosis through suppressing extracellular signal-regulated kinase 1/2 (ERK1/2)-protein kinase B (AKT) signaling pathways. Guo et al[11] demonstrated that Atractylodes macrocephala Koidz could enhance immunity and reduce inflammation through activating nuclear factor-kappaB (NF-κB) signal pathway. Additionally, it was also proven that licorice could improve the inflammation through NF-κB signal pathway[12]. Although the HTS is related to inflammation, the application of WFY in HTS has not been reported.
Hence, this study explored the molecular regulatory network using network pharmacology and performed enrichment analysis to determine the function and signal pathway involved in using WFY to treat HTS.
In this study, Genecard (https://www.genecards.org/), DisGeNET (https://www.disgenet.org/), and National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/) were used to obtain HTS-related genes using HTSs as the keyword.
All the compounds of WFY were collected from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database (https://old.tcmsp-e.com/tcmsp.php) using “Bai Shu”, “Ren Shen”, “Dang Gui”, “Gan Cao”, and “Shu Di Huang” as the input words. The compounds with drug-like properties ≥ 0.18 and oral bioavailability≥ 30% were filtered for further analysis.
The Gene Ontology (GO) and Kyoto Encyclopaedia of Genes and Genome (KEGG) enrichment analysis were performed in R software (version 4.3.0) package. The org.Hs.eg.db package was used for the GO analysis, with biological process (BP), cell composition, and molecular function (MF) as gene annotation information. Additionally, the relation between gene and GO or KEGG pathway was computed by cnetplot package.
The protein-protein interaction (PPI) was analyzed in STRING database (https://cn.string-db.org/) with minimum required interaction score ≥ 0.4. Next, the optical network was constructed in Cytoscape (version 3.9.1). Additionally, some applications in Cytoscape such as MCODE and cytohubba were used for the selection of subnetworks and calculation of degrees.
The 2D structures of the primary compounds in WFY were downloaded from the TCMSP database and the 3D structures of the targets were downloaded from the Worldwide Protein Data Bank database (https://www.rcsb.org/). They were imported to AutoDockTools for hydrogenation, dehydration, and other pretreatments. Then, molecular docking of the receptors and ligands was conducted to analyze its binding activity. The docking results were visualized using the PyMol software.
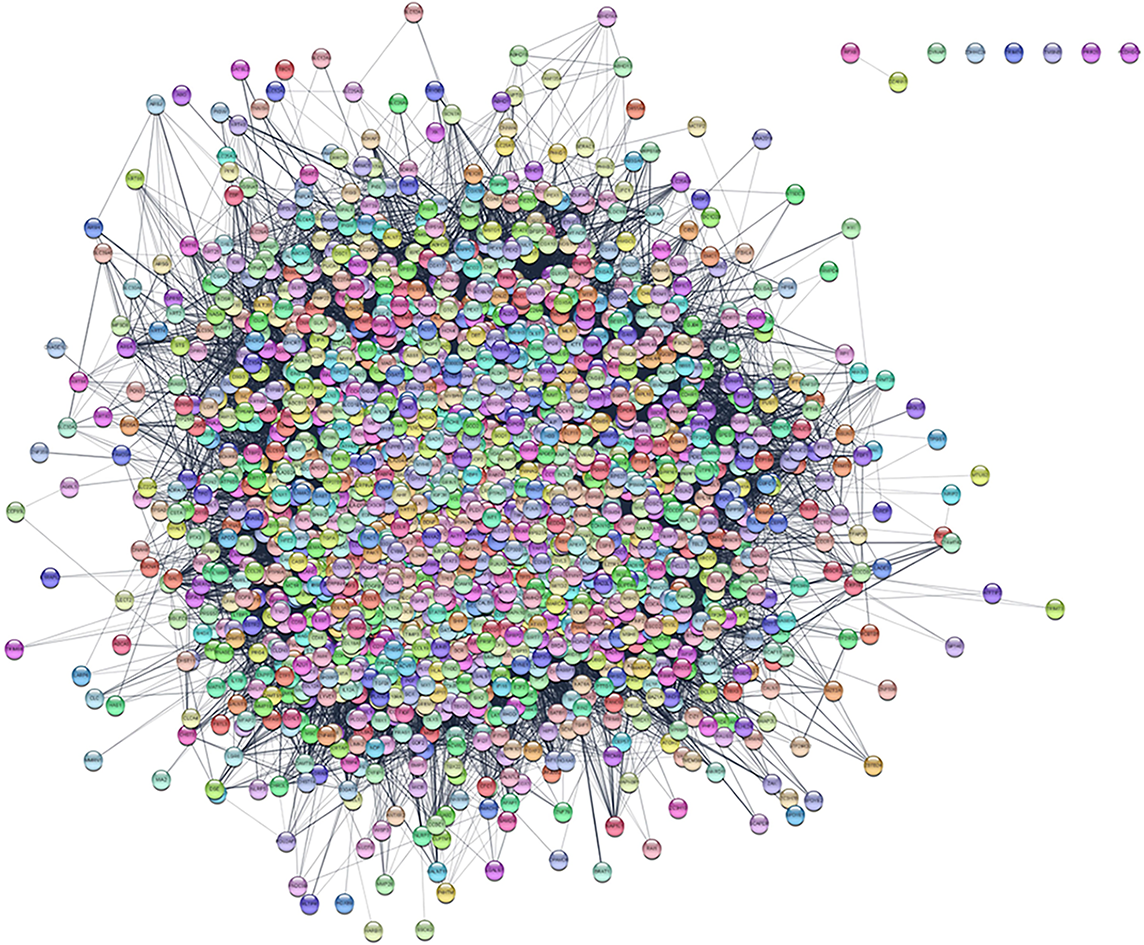
We collected 2405 HTS-related genes from GeneCard, NCBI, and DisGeNET databases. As shown in Figure 1, the PPI network contained most HTS-related genes. However, RFX8 was only related to DZANK1, while DYNAP, ZDHHC24, TRIM74, TMSNB, PRR25, and PCDHGC4 were not closely related to other genes.
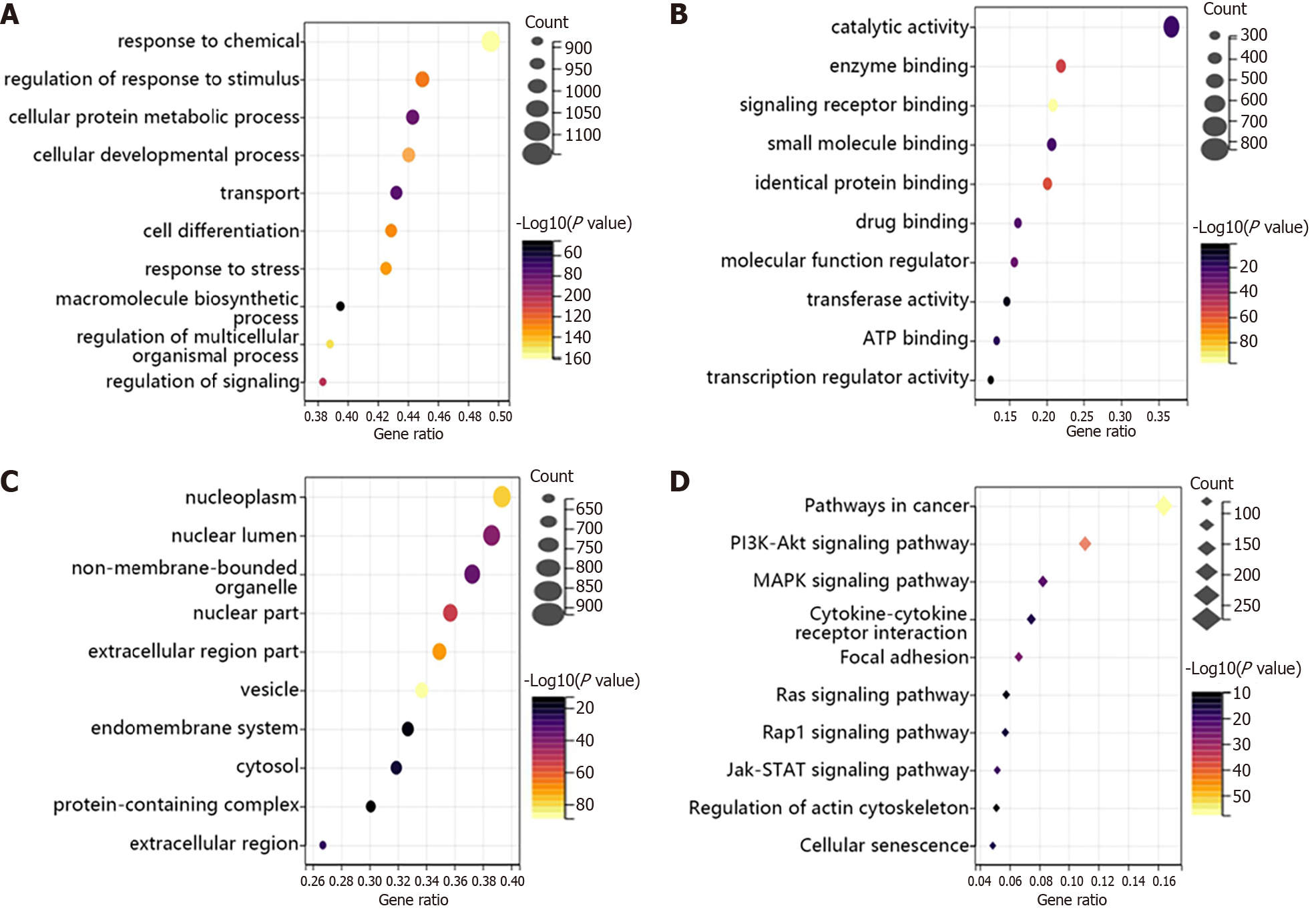
Then, we analyzed the function of these genes using GO and KEGG. From Figure 2A-C, we found that HTS-related genes were mainly involved in response to chemicals, regulation of response to stimulus and cellular protein metabolic process in BP terms (Figure 2A). In addition, the main functions of these genes were related to catalytic activity, enzyme binding and signaling receptor binding in MF terms (Figure 2B). Besides, these genes mainly existed in the nucleoplasm, nuclear lumen and non-membrane-bounded organelle (Figure 2C). Furthermore, the KEGG results showed that HTS-related genes were enriched in pathways in cancer, PI3K-Akt signaling pathway and mitogen-activated protein kinase (MAPK) signaling pathway (Figure 2D).
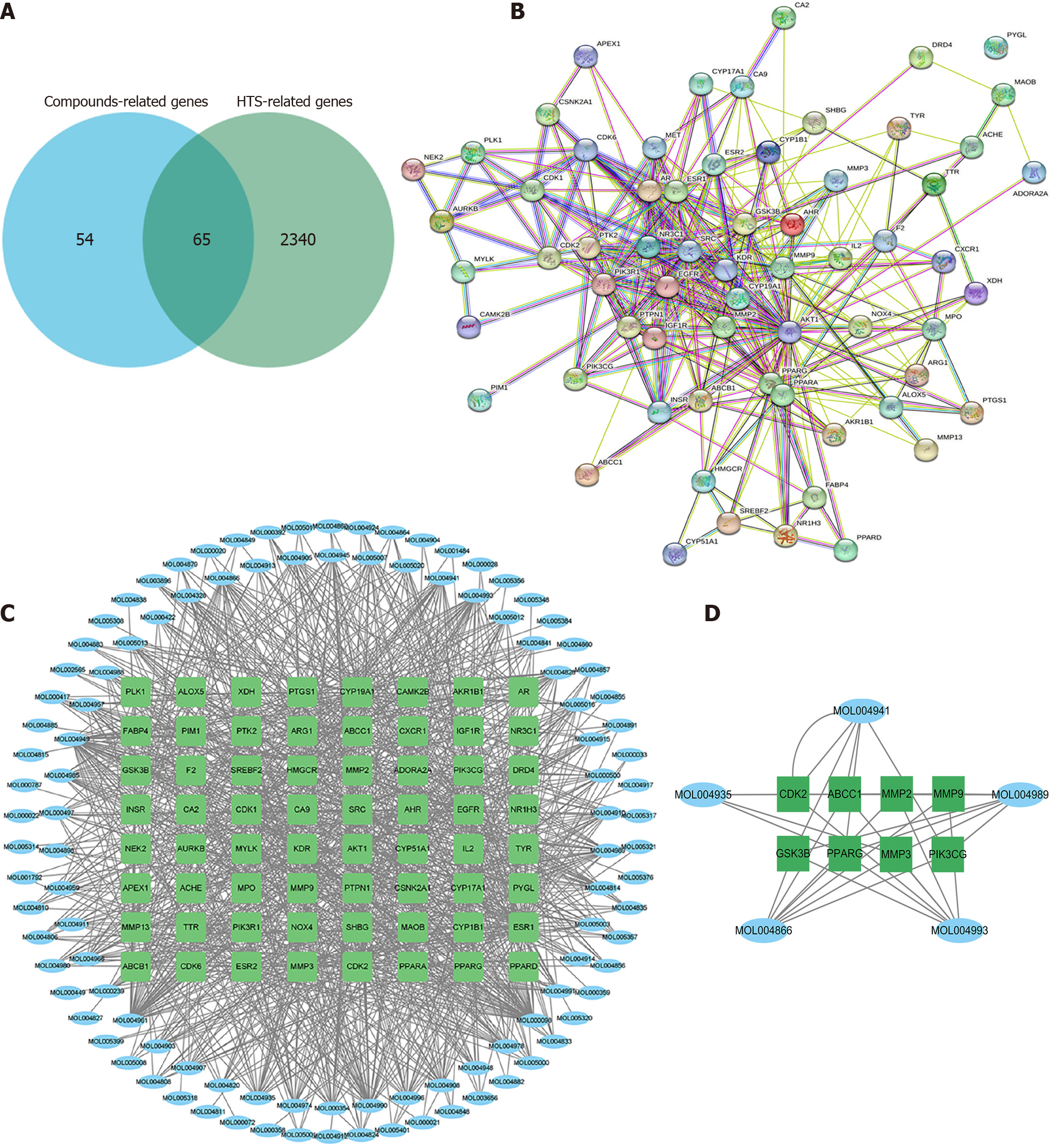
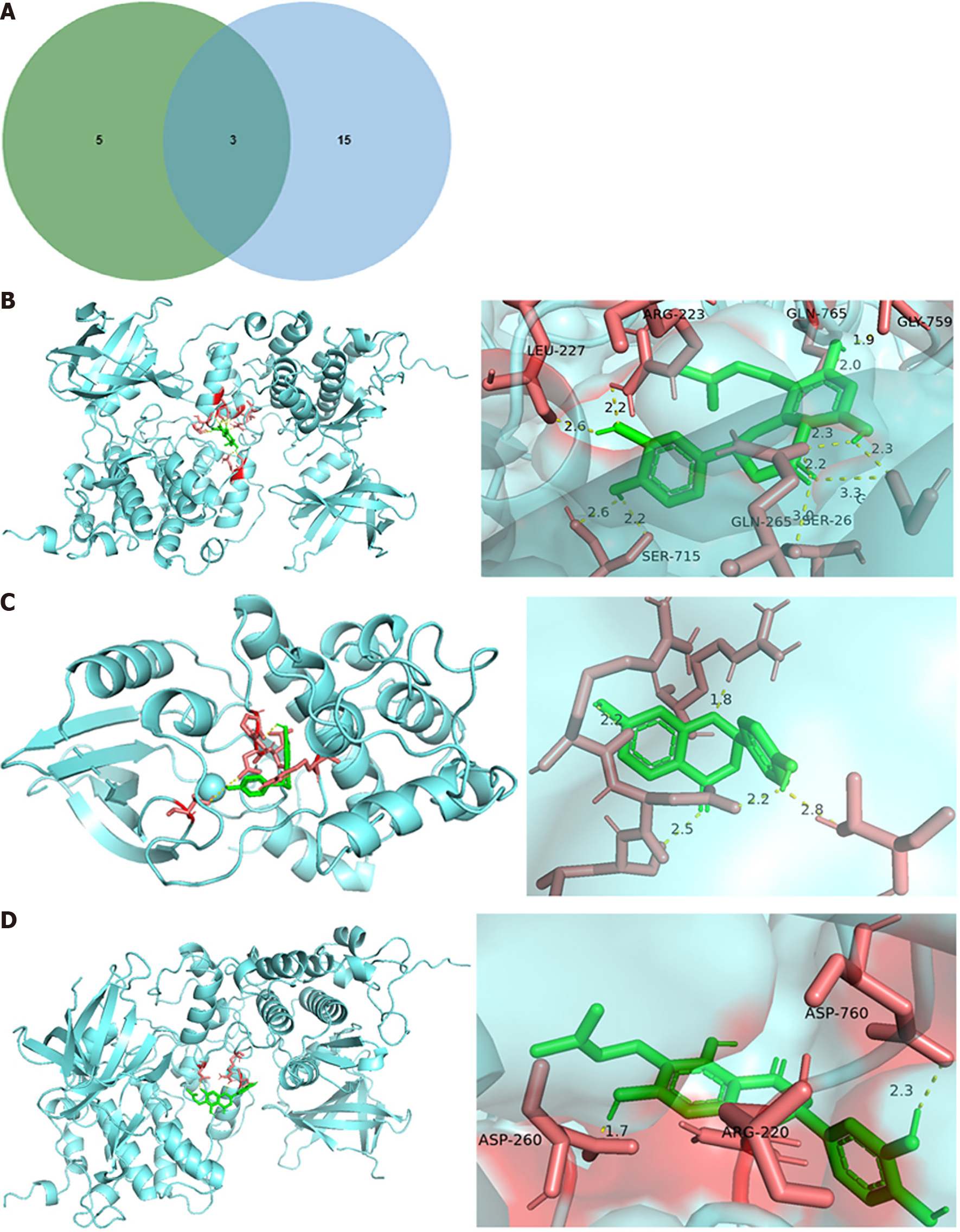
According to all HTS-related genes and WFY-related genes, we determined 65 targets existed in both two gene sets (Figure 3A). The PPI network showed that PYGL was not related to other genes. Therefore, 64 genes were used for further analysis (Figure 3B).
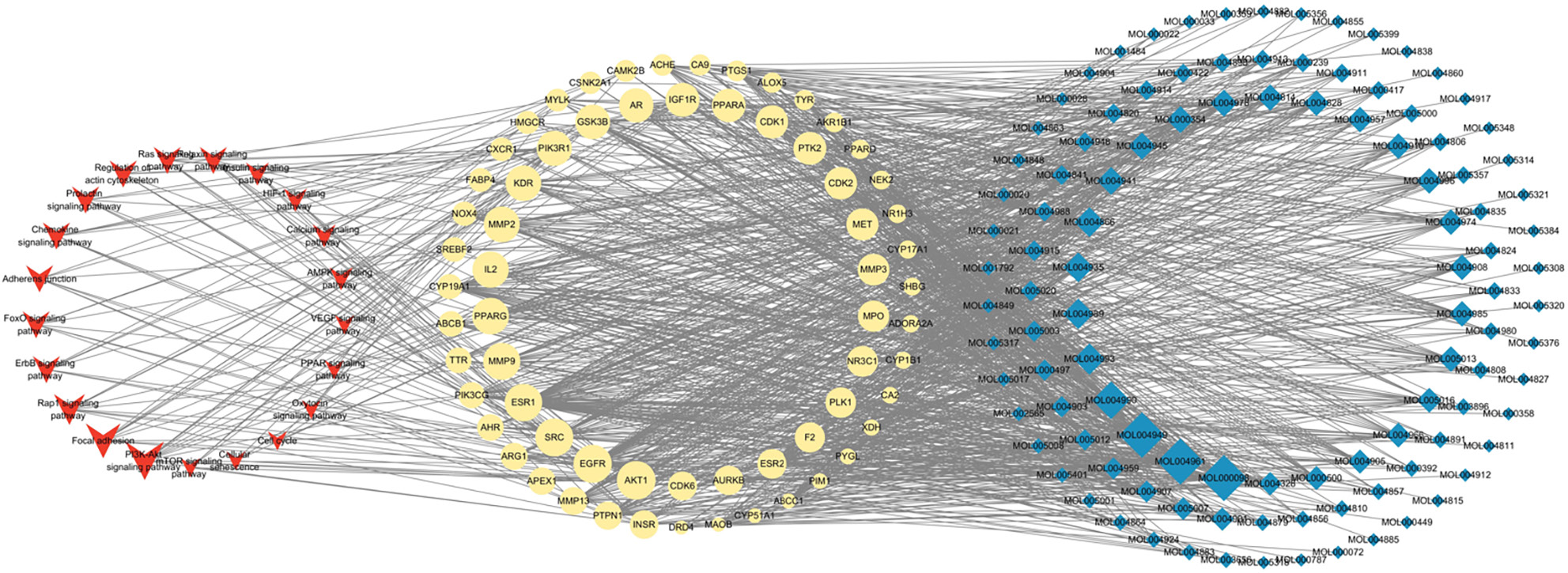
Using the TCSMP database, 118 candidate compounds were selected (Supplementary Table 1). Then, we developed a compound-gene network, as shown in Figure 3C. The network contained 172 nodes (108 compounds and 64 targets) and 1049 edges. Moreover, the degree of 88 compounds was greater than or equal to 2, suggesting that most compounds could target multiple genes. Moreover, we determined the key subnetwork using MCODE (Figure 3D), including 8 genes [CKD2, ABCC1, MMP2, MMP9, glycogen synthase kinase 3 beta (GSK3B), PRARG, MMP3, and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma (PIK3CG)] and 5 compounds (MOL004941, MOL004935, MOL004866, MOL004993, and MOL004989) involved in WFY therapy against HTS.
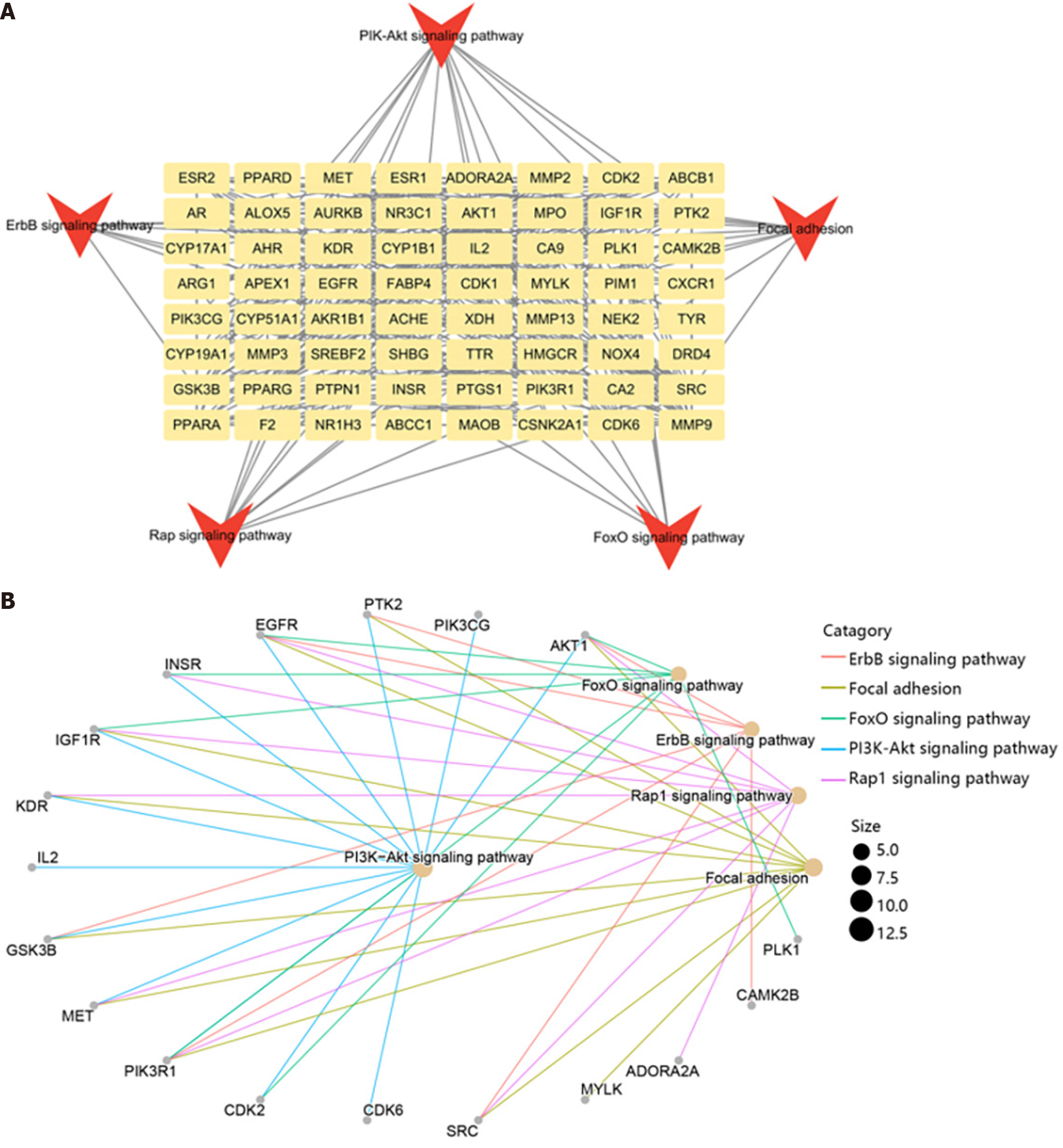
Furthermore, we explored the function of key genes using KEGG enrichment analysis. The results showed that these genes were mainly enriched in PI3K-Akt, ErbB, Rap, FoxO signaling pathway, and focal adhesion (Figure 4A). The genes enriched in the top 5 pathways were visualized in Figure 4B. It could be observed that AKT1 was the most important genes enriched in 5 pathways, while the PI3K-Akt signaling pathway was the most important pathway containing 13 genes.
To better understand the functions of compounds in the HTS, we established a compound-gene-pathway network (Figure 5). This network included 195 nodes, which contained the top 20 pathways (such as PI3K-Akt signal pathway, focal adhesion, and Rap1 signal pathway), 64 target genes (such as AKT1, EGFR, and SRC) and 108 compounds (such as MOL000098, MOL004961, and MOL4949), and 1195 edges.
According to the results above, we identified three target genes that all played crucial roles in top 5 pathways and the key subnetwork of the compound-gene network (Figure 6A). As a result, three hub genes, cyclin dependent kinase 2 (CDK2), GSK3B, and PIK3CG, were determined, and five compounds targeting the hub genes were also identified.
Molecular docking analysis in silico was performed to further validate the potential effect of the five main active compounds of WFY on the 3 hub targets. The binding free energies were shown in Table 1 and the results were visualized in Figure 6B-D. The three pairs of compounds and proteins with the highest binding affinity were GSK3B and 8-prenylated eriodictyol, while GSK3B and 6-prenylated eriodictyol was the pair with the lowest binding affinity.
| Targets | Mol ID | Molecule name | Docking score (kcal/mol) |
| CDK2 | MOL004941 | (2R)-7-hydroxy-2-(4-hydroxyphenyl) chroman-4-one | -2.89 |
| GSK3B | MOL004989 | 6-prenylated eriodictyol | -1.62 |
| GSK3B | MOL004993 | 8-prenylated eriodictyol | -3.12 |
HTS is a common disease in the progress of wound healing[13], and excessive scarring may cause some physical and psychological problems[14]. Currently, alleviating and repairing scars still remained a clinical challenge. This study predicted the potential targets and analyzed mechanisms of WFY against HTS using network pharmacology, enrichment analysis and molecular docking. We determined 5 compositions, 118 compounds and 64 overlapping potential targets and multiple biological progresses and signal pathways, which indicated that WFY affected the development of HTS through diverse pathways. According to the composition of WFY, we determined MOL004941, MOL004935, MOL004866, MOL004993 and MOL004989 as the key potential compounds. In addition, three genes (CDK2, GSK3B, and PIK3CG) targeted by the key compounds were found to play a vital role in the top 5 signal pathways.
CDK2 is a serine/threonine-protein kinase and is involved in the cell cycle, DNA replication and apoptosis[15]. A study demonstrated that the development of HTS was affected by the promotion of cell proliferation and the inhibition of apoptosis[16]. Additionally, Hsieh et al[17] demonstrated that the application of gallic acid inhibited the cell cycle and promoted the apoptosis of HTS fibroblasts accompanied with downregulated CDK2 expression. This indicated that CDK2 could regulate the development of HTS through mediating the process of cell cycle and apoptosis.
GSK3B is the serine/threonine kinase and downstream target genes of PI3K/AKT signal pathway[18]. Wang et al[18] found that the suppression of AKT/GSK3B axis reduced the production of TGF-β1 and other fibrogenic cytokines, therefore improving the condition of HST. Furthermore, GSK3B was also involved in the inflammation through promoting NF-κB activation to mediate immune response[19-21]. In vitro experiments found that the inhibition of PI3K-Akt caused the downregulation of GSK3B expression[22].
PIK3CG, alternatively known as PI3Kγ/p110γ, is a subtype of PI3K and a key regulatory molecule during inflammation[23]. A study found that PIK3CG affected inflammation through mediating TLR9, and that the inhibition of PIK3CG reduced phosphorylated Akt. Moreover, PIK3CG also inhibited the immune response through suppressing T cell activation[24]. On the other hand, the other study showed that the application of Eganelisib, an inhibitor of PIK3CG, improved the immune response and inhibited the cell growth of cancer stem cells[22].
However, the function of CDK2, GSK3B, and PIK3CG in HTS remained unclear. Therefore, network pharmacological analysis confirmed that they were the hub genes in WFY therapy against HTS. Moreover, enrichment analysis demonstrated that PI3K-Akt was the most important pathway in WFY treatment and was closely related to CDK2, GSK3B, and PIK3CG. Previous studies observed that PI3K/Akt played an essential role in the development of HTS through promoting cell proliferation and migration and inhibiting apoptosis[25-27]. Yang et al[28] verified that the activation of PI3K/Akt/FoXO axis induced the development of cell fibrosis. These results indicated that PI3K/Akt signal pathway could target genes including CDK2, GSK3B, and PIK3CG as well as other pathways to regulate cell proliferation, apoptosis and fibrosis.
Molecular docking analysis showed that GSK3B was targeted by 6-prenylated eriodictyol and 8-prenylated eriodictyol, and CDK2 was targeted by (2R)-7-hydroxy-2-(4-hydroxyphenyl) chroman-4-one. This indicated that these compounds could mediate cell cycle and apoptosis through targeting GSK3B and CDK2 to regulate the PI3K-Akt and other signal pathways.
In conclusion, we determined the potential active compounds of WFY and hub genes that played essential roles in the development of HTS. The PI3K-Akt signal pathway was identified as the most significant pathway in HTS treatment. However, there were still some limitations in the current study. Firstly, as the target and compounds were separately obtained from a database that may have data incompleteness, the interaction between PIK3CG and compounds was not determined. Moreover, our results relied on the bioinformatics analysis, which should be validated based on experiments.
| 1. | Wang J, Zhao M, Zhang H, Yang F, Luo L, Shen K, Wang X, Li Y, Zhang J, Wang K, Li J, Wang Y, Yang Y, Hu D. KLF4 Alleviates Hypertrophic Scar Fibrosis by Directly Activating BMP4 Transcription. Int J Biol Sci. 2022;18:3324-3336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 2. | Nischwitz SP, Fink J, Schellnegger M, Luze H, Bubalo V, Tetyczka C, Roblegg E, Holecek C, Zacharias M, Kamolz LP, Kotzbeck P. The Role of Local Inflammation and Hypoxia in the Formation of Hypertrophic Scars-A New Model in the Duroc Pig. Int J Mol Sci. 2022;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Bi M, Sun P, Li D, Dong Z, Chen Z. Intralesional Injection of Botulinum Toxin Type A Compared with Intralesional Injection of Corticosteroid for the Treatment of Hypertrophic Scar and Keloid: A Systematic Review and Meta-Analysis. Med Sci Monit. 2019;25:2950-2958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Amini-Nik S, Yousuf Y, Jeschke MG. Scar management in burn injuries using drug delivery and molecular signaling: Current treatments and future directions. Adv Drug Deliv Rev. 2018;123:135-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Poon R, Hong H, Wei X, Pan J, Alman BA. A high throughput screen identifies Nefopam as targeting cell proliferation in β-catenin driven neoplastic and reactive fibroproliferative disorders. PLoS One. 2012;7:e37940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Qiu SS, Dotor J, Hontanilla B. Effect of P144® (Anti-TGF-β) in an "In Vivo" Human Hypertrophic Scar Model in Nude Mice. PLoS One. 2015;10:e0144489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Ye ZC, Wang CF, Han L, Cao GP, Shen QR. Chondroprotective Effect of Wufu Decoction on Tumor Necrosis Factor-α-Induced Chondrocytes via the Extracellular Signal Regulated Kinase 1/2 Signaling Pathway. Orthop Surg. 2020;12:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Yang S, Li F, Lu S, Ren L, Bian S, Liu M, Zhao D, Wang S, Wang J. Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J Ethnopharmacol. 2022;283:114739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 9. | Zhang Q, Wei F, Fong CC, Yu WK, Chen Y, Koon CM, Lau KM, Leung PC, Lau CB, Fung KP, Yang M. Transcriptional profiling of human skin fibroblast cell line Hs27 induced by herbal formula Astragali Radix and Rehmanniae Radix. J Ethnopharmacol. 2011;138:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Wu JZ, Li YJ, Huang GR, Xu B, Zhou F, Liu RP, Gao F, Ge JD, Cai YJ, Zheng Q, Li XJ. Mechanisms exploration of Angelicae Sinensis Radix and Ligusticum Chuanxiong Rhizoma herb-pair for liver fibrosis prevention based on network pharmacology and experimental pharmacologylogy. Chin J Nat Med. 2021;19:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Guo S, Li W, Chen F, Yang S, Huang Y, Tian Y, Xu D, Cao N. Polysaccharide of Atractylodes macrocephala Koidz regulates LPS-mediated mouse hepatitis through the TLR4-MyD88-NFκB signaling pathway. Int Immunopharmacol. 2021;98:107692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Sun J, Zhang Q, Yang G, Li Y, Fu Y, Zheng Y, Jiang X. The licorice flavonoid isoliquiritigenin attenuates Mycobacterium tuberculosis-induced inflammation through Notch1/NF-κB and MAPK signaling pathways. J Ethnopharmacol. 2022;294:115368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 938] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 14. | Lee HJ, Jang YJ. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 15. | Liu YC, Feng N, Li WW, Tu PF, Chen JP, Han JY, Zeng KW. Costunolide Plays an Anti-Neuroinflammation Role in Lipopolysaccharide-Induced BV2 Microglial Activation by Targeting Cyclin-Dependent Kinase 2. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Yaundong L, Dongyan W, Lijun H, Zhibo X. Effects of downregulation of S100A8 protein expression on cell cycle and apoptosis of fibroblasts derived from hypertrophic scars. Aesthet Surg J. 2014;34:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Hsieh SC, Wu CC, Hsu SL, Yen JH. Molecular mechanisms of gallic acid-induced growth inhibition, apoptosis, and necrosis in hypertrophic scar fibroblasts. Life Sci. 2017;179:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Wang M, Chen L, Huang W, Jin M, Wang Q, Gao Z, Jin Z. Improving the anti-keloid outcomes through liposomes loading paclitaxel-cholesterol complexes. Int J Nanomedicine. 2019;14:1385-1400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Gianferrara T, Cescon E, Grieco I, Spalluto G, Federico S. Glycogen Synthase Kinase 3β Involvement in Neuroinflammation and Neurodegenerative Diseases. Curr Med Chem. 2022;29:4631-4697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Cortés-Vieyra R, Silva-García O, Gómez-García A, Gutiérrez-Castellanos S, Álvarez-Aguilar C, Baizabal-Aguirre VM. Glycogen Synthase Kinase 3β Modulates the Inflammatory Response Activated by Bacteria, Viruses, and Parasites. Front Immunol. 2021;12:675751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Grove LM, Mohan ML, Abraham S, Scheraga RG, Southern BD, Crish JF, Naga Prasad SV, Olman MA. Translocation of TRPV4-PI3Kγ complexes to the plasma membrane drives myofibroblast transdifferentiation. Sci Signal. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Jin N, Shi R, Jiang Y, Chu D, Gong CX, Iqbal K, Liu F. Glycogen synthase kinase-3β suppresses the expression of protein phosphatase methylesterase-1 through β-catenin. Aging (Albany NY). 2019;11:9672-9688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Liu Q, Dong Y, Escames G, Wu X, Ren J, Yang W, Zhang S, Zhu Y, Tian Y, Acuña-Castroviejo D, Yang Y. Identification of PIK3CG as a hub in septic myocardial injury using network pharmacology and weighted gene co-expression network analysis. Bioeng Transl Med. 2023;8:e10384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Chandrasekaran S, Funk CR, Kleber T, Paulos CM, Shanmugam M, Waller EK. Strategies to Overcome Failures in T-Cell Immunotherapies by Targeting PI3K-δ and -γ. Front Immunol. 2021;12:718621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Ma F, Shen J, Zhang H, Zhang Z, Yang A, Xiong J, Jiao Y, Bai Z, Ma S, Jiang Y. A novel lncRNA FPASL regulates fibroblast proliferation via the PI3K/AKT and MAPK signaling pathways in hypertrophic scar. Acta Biochim Biophys Sin (Shanghai). 2022;55:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Xiao Y. MiR-486-5p inhibits the hyperproliferation and production of collagen in hypertrophic scar fibroblasts via IGF1/PI3K/AKT pathway. J Dermatolog Treat. 2021;32:973-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Zhi Y, Wang H, Huang B, Yan G, Yan LZ, Zhang W, Zhang J. Panax Notoginseng Saponins suppresses TRPM7 via the PI3K/AKT pathway to inhibit hypertrophic scar formation in vitro. Burns. 2021;47:894-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Yang F, Chen E, Yang Y, Han F, Han S, Wu G, Zhang M, Zhang J, Han J, Su L, Hu D. The Akt/FoxO/p27(Kip1) axis contributes to the anti-proliferation of pentoxifylline in hypertrophic scars. J Cell Mol Med. 2019;23:6164-6172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |