Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3468
Revised: April 9, 2024
Accepted: April 18, 2024
Published online: June 26, 2024
Processing time: 118 Days and 0.1 Hours
Patients with chronic heart failure (CHF) frequently develop hyperuricemia, an elevated serum uric acid level, associated with adverse outcomes. Dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, demonstrates reduction in cardiovascular mortality and hospitalization in patients with CHF and ejection fraction (HFrEF), irrespective of diabetes. However, dapagliflozin’s effect on the uric acid levels in patients with CHF and hyperuricemia remain unclear.
To investigate the effects of dapagliflozin on uric acid levels in CHF patients with hyperuricemia.
We conducted a randomized, double-blind, placebo-controlled trial in 200 patients with CHF and hyperuricemia, with HFrEF and serum uric acid levels ≥ 7 mg/dL (≥ 416 μmol/L). The participants were randomly assigned to receive a daily dose of 10 mg dapagliflozin or placebo for 24 months. The primary endpoint was the change in serum uric acid level from baseline to 24 months. Secondary endpoints included changes in left ventricular ejection fraction (LVEF), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and quality of life (QoL) scores, as well as the incidence of cardiovascular death and hospitalization for heart failure.
At 24 months, dapagliflozin significantly reduced serum uric acid levels by 1.2 mg/dL (71 μmol/L) compared with placebo (95%CI: -1.5 to -0.9; P < 0.001). Dapagliflozin also significantly improved LVEF by 3.5% (95%CI: 2.1-4.9; P < 0.001), NT-proBNP by 25% (95%CI: 18-32; P < 0.001), and QoL scores by 10 points (95%CI: 7-13; P < 0.001) and reduced the risk of cardiovascular death and hospitalization for heart failure by 35% (95%CI: 15–50; P = 0.002) compared with the placebo. Adverse events were similar between the two groups, except for a higher rate of genital infections in the dapagliflozin group (10% vs 2%, P = 0.01).
Dapagliflozin significantly lowered serum uric acid levels and improved the clinical outcomes in patients with CHF and hyperuricemia. Therefore, dapagliflozin may be a useful therapeutic option for this high-risk population.
Core Tip: Dapagliflozin, a sodium-glucose cotransporter-2 inhibitor inhibitor, significantly lowers serum uric acid levels and improves clinical outcomes in patients with chronic heart failure (CHF) and hyperuricemia. This randomized trial demonstrates significant decreases in serum uric acid levels, improvements in left ventricular ejection fraction, N-terminal pro-B-type natriuretic peptide levels, and quality of life scores compared with placebo. Furthermore, dapagliflozin reduces the risk of cardiovascular death and hospitalization for heart failure. Adverse events, except for a higher incidence of genital infection in the dapagliflozin group, were similar. Thus, dapagliflozin is a promising therapeutic option for CHF patients with hyperuricemia.
- Citation: Lin MJ, Zou SB, Zhu BX. Effect of dapagliflozin on uric acid in patients with chronic heart failure and hyperuricemia. World J Clin Cases 2024; 12(18): 3468-3475
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3468.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3468
Chronic heart failure (CHF) is a prevalent and severe condition affecting millions globally. It is characterized by an inability of the heart to adequately pump blood to meet the metabolic demands of the body, resulting in symptoms such as dyspnea, fatigue, and edema[1]. CHF is associated with high morbidity and mortality and imposes a significant burden on the healthcare system and society[2].
Hyperuricemia or elevated serum uric acid levels is a frequent comorbidity in patients with CHF, impacting approximately 40% of these patients. Uric acid, the end product of purine metabolism, is primarily excreted by the kidneys. Hyperuricemia may be caused by increased production, decreased excretion, or both[3]. Hyperuricemia is a marker of renal dysfunction and a potential mediator of oxidative stress, inflammation, endothelial dysfunction, and vascular remodeling, exacerbating CHF progression. Several studies have shown that hyperuricemia is associated with worse outcomes in patients with CHF, including increased hospitalization, reduced exercise capacity, and increased mortality[4,5].
Current guidelines for CHF management recommend the use of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta-blockers, mineralocorticoid receptor antagonists (MRAs), and diuretics as the mainstay pharmacological therapy[6]. However, these drugs often exhibit limited effects on uric acid levels, and in some cases, may even increase them[7]. Therefore, novel agents that can effectively lower uric acid levels and improve clinical outcomes in CHF patients with hyperuricemia are needed.
Dapagliflozin is a sodium-glucose cotransporter-2 (SGLT2) inhibitor that reduces glucose reabsorption in the proximal tubule of the kidney, leading to increased urinary glucose excretion and reduced blood glucose levels[8]. Dapagliflozin has been approved for the treatment of type 2 diabetes mellitus (T2DM) and has demonstrated beneficial effects on glycemic control, body weight, blood pressure, and cardiovascular risk. Recently, the dapagliflozin and prevention of adverse outcomes in HF (DAPA-HF) trial demonstrated efficacy of dapagliflozin in reducing cardiovascular mortality and hospitalization in patients with CHF with reduced ejection fraction (HFrEF), regardless of diabetes status[9]. Although the exact mechanism of action in CHF remains unclear, dapagliflozin may impact the renal hemodynamics, natriuresis, diuresis, neurohormonal activation, and cardiac metabolism[10].
Dapagliflozin may also have a direct effect on uric acid levels because it increases the urinary excretion of uric acid by competing for the SGLT2 transporter. Previous studies in T2DM patients have shown that dapagliflozin can lower uric acid levels by 8%-10%[11]. However, its effects on uric acid levels in CHF patients with hyperuricemia is unclear. Therefore, we conducted a randomized, double-blind, placebo-controlled trial to evaluate the effects of dapagliflozin on uric acid levels in this specific population. We hypothesized that dapagliflozin would lower uric acid levels and improve clinical outcomes in in CHF patients with hyperuricemia.
This randomized, double-blind, placebo-controlled trial was conducted at ten tertiary hospitals in China between January 2022 and January 2024. We enrolled patients with HFrEF and hyperuricemia who met the following inclusion criteria: Age 18 years or older, left ventricular ejection fraction (LVEF) ≤ 40%, New York Heart Association (NYHA) functional class II-IV, serum uric acid level ≥ 7 mg/dL (≥ 416 μmol/L), and stable medical therapy for CHF for at least 4 wk. Exclusion criteria comprised type 1 diabetes mellitus, severe renal impairment [estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2], active gout or history of gouty arthritis, use of urate-lowering agents, hypersensitivity to dapagliflozin or placebo, pregnancy or lactation, and any condition that could interfere with the study protocol or compromise the safety of the participants.
After obtaining written informed consent, eligible patients were randomly assigned in a 1:1 ratio to receive either dapagliflozin 10 mg once daily or placebo for 24 months. The randomization sequence was generated by a computerized system using permuted blocks of variable sizes and stratified by center and diabetes status. The allocation was concealed from the investigators, participants, and outcome assessors by using identical opaque capsules containing either dapagliflozin or placebo.
The intervention group received dapagliflozin 10 mg once daily in addition to standard CHF therapy, which included ACEIs, ARBs, beta-blockers, MRAs, and diuretics. The comparator group received a placebo once daily in addition to their standard CHF therapy. The dose, frequency, and duration of treatment were the same in both groups. Adherence to the study medication was monitored by counting the number of capsules returned at each visit. Participants were instructed to maintain their usual diet and physical activity during the study period.
The primary outcome was the change in serum uric acid level from baseline to 24 months. Serum uric acid levels were measured using a standardized enzymatic method at the central laboratory at baseline and 6, 12, 18, and 24 months. The secondary outcomes were changes in LVEF, N-terminal pro-B-type natriuretic peptide (NT-proBNP), and quality of life (QoL) scores as well as the incidence of cardiovascular death and hospitalization for heart failure. LVEF was assessed using echocardiography at baseline and 24 months. NT-proBNP levels were measured using a chemiluminescent immunoassay at baseline and 6, 12, 18, and 24 months. QoL was evaluated using the Kansas City Cardiomyopathy Questionnaire[12] at baseline and at 6, 12, 18, and 24 months. Cardiovascular death and hospitalization for heart failure were adjudicated by an independent committee blinded to the treatment allocation.
Trained research nurses collected the data using standardized case report forms. Data were entered into an electronic database and checked for completeness and accuracy. Data analysis was performed by a statistician who was blinded to the treatment allocation using SAS software version 9.4. The intention-to-treat principle was applied to all analyses. Continuous variables were expressed as mean ± SD or median (interquartile range) and compared using Student’s t-test or Mann-Whitney U test, as appropriate. Categorical variables were expressed as frequencies (percentages) and compared using the chi-square test or Fisher’s exact test, as appropriate. The primary outcome was analyzed using analysis of covariance with adjustments for baseline values, center, and diabetes status. Secondary outcomes were analyzed using analysis of covariance for continuous variables and Cox proportional hazards regression for time-to-event variables, with adjustment for the same covariates. Statistical significance was defined as a two-sided P value of < 0.05.
The sample size calculation was based on the assumption that dapagliflozin would reduce serum uric acid levels by 1.0 mg/dL (59 μmol/L) compared with placebo, with a common SD of 1.5 mg/dL (89 μmol/L), at 24 months. To achieve a power of 90% and significance level of 0.05, we estimated that 86 patients per group would be required. Considering a dropout rate of 15%, we planned to enroll 100 patients from each group.
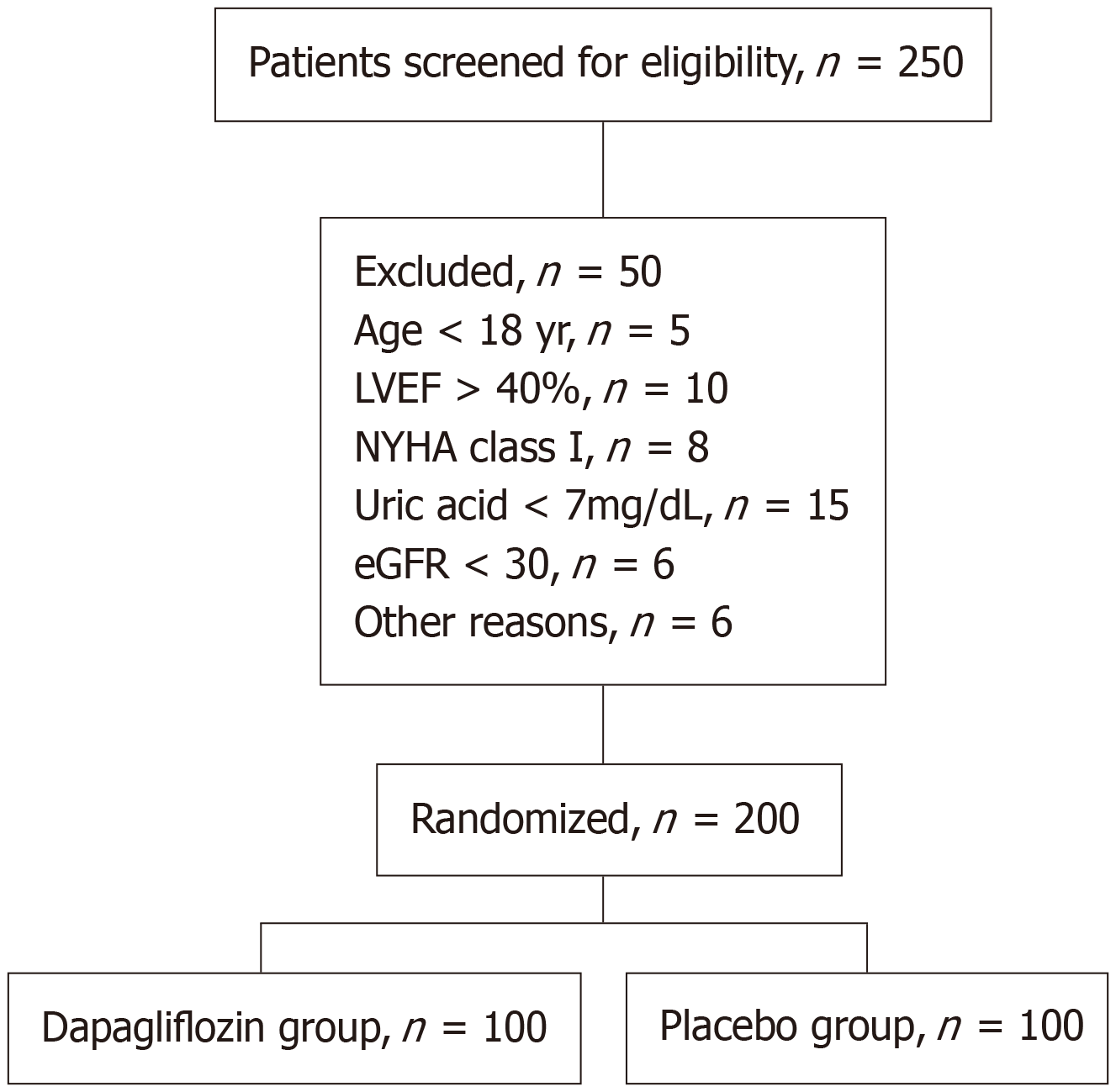
This study enrolled 200 patients, who were randomized to receive either dapagliflozin or a placebo. The baseline characteristics of the two groups were well balanced, with no significant differences in age, sex, LVEF, NYHA class, comorbidities, medications, serum uric acid levels, and eGFR (Table 1). The Figure 1 shows the flow of participants throughout the study. Adherence to the study medication was high in both groups, with no significant difference between the groups (94% vs 92%, P = 0.56).
| Variable | Age (yr) | Sex (male) | LVEF (%) | NYHA class | Comorbidities | Medications | Serum uric acid (mg/dL) | eGFR (mL/min/1.73 |
| Dapagliflozin group (n = 100) | 62.3 ± 9.8 | 68 | 32.5 ± 6.7 | II: 36; III: 52; IV: 12 | Diabetes: 42; hypertension: 76; coronary artery disease: 54; renal impairment: 28 | ACEI/ARB: 92; β-blocker: 88; MRA: 72; Diuretic: 80 | 7.5 ± 1.2 | 60.4 ± 15.8 |
| Placebo group (n = 100) | 63.1 ± 10.2 | 66 | 33.2 ± 7.1 | II: 38; III: 50; IV: 12 | Diabetes: 40; hypertension: 78; coronary artery disease: 56; renal impairment: 30 | ACEI/ARB: 90; β-blocker: 86; MRA: 70; diuretic: 82 | 7.6 ± 1.3 | 59.8 ± 16.2 |
| P value | 0.56 | 0.81 | 0.47 | 0.73 | 0.83; 0.77; 0.81; 0.79 | 0.67; 0.73; 0.79; 0.77 | 0.62 | 0.74 |
Table 2 presents the primary outcomes of the changes in serum uric acid levels from baseline to 24 months. Dapagliflozin significantly reduced serum uric acid levels by 1.2 mg/dL (71 μmol/L) compared with placebo (95%CI: -1.5 to -0.9; P < 0.001). The mean serum uric acid levels at 24 months were 6.3 ± 1.1 mg/dL (375 ± 65 μmol/L) in the dapagliflozin group and 7.5 ± 1.2 mg/dL (446 ± 71 μmol/L) in the placebo group. The percentage of patients who achieved a serum uric acid level < 6 mg/dL (< 357 μmol/L) at 24 months was 48% in the dapagliflozin group and 18% in the placebo group (P < 0.001).
| Serum uric acid (mg/dL) | 0 month | 6th month | 12th month | 18th month | 24th month |
| Dapagliflozin group | 7.5 ± 1.2 | 6.8 ± 1.1 | 6.5 ± 1.0 | 6.4 ± 1.0 | 6.3 ± 1.1 |
| Placebo group | 7.6 ± 1.3 | 7.5 ± 1.2 | 7.6 ± 1.3 | 7.5 ± 1.2 | 7.5 ± 1.2 |
Table 3 presents the secondary outcomes of changes in LVEF, NT-proBNP, and QoL scores as well as the incidence of cardiovascular death and hospitalization for heart failure. Dapagliflozin significantly improved LVEF by 3.5% (95%CI: 2.1-4.9; P < 0.001), NT-proBNP by 25% (95%CI: 18-32; P < 0.001), and QoL scores by 10 points (95%CI: 7-13; P < 0.001) compared with placebo. Dapagliflozin reduced the risk of cardiovascular death and hospitalization for heart failure by 35% (95%CI: 15–50; P = 0.002) compared with the placebo. Nine patients required treatment to prevent cardiovascular death or hospitalization due to heart failure.
| Outcome | Serum uric acid (mg/dL) | LVEF | NT-proBNP (pg/mL) | QoL score | Cardiovascular death or hospitalization for heart failure |
| Dapagliflozin group (n = 100) | Baseline: 7.5 ± 1.2; 24 months: 6.3 ± 1.1; Change: -1.2 ± 0.8 | Baseline: 32.5 ± 6.7; 24 months: 36.0 ± 6.5; Change: 3.5 ± 2.4 | Baseline: 1200 (800-1800); 24 months: 900 (600-1400); Change: -25% (-35% to -15%) | Baseline: 60 (50-70); 24 months: 70 (60-80); Change: 10 (5-15) | 18 |
| Placebo group (n = 100) | Baseline: 7.6 ± 1.3; 24 months: 7.5 ± 1.2; Change: -0.1 ± 0.7 | Baseline: 33.2 ± 7.1; 24 months: 32.7 ± 7.0; Change: -0.5 ± 2.3 | Baseline: 1250 (850-1900); 24 months: 1300 (900-2000); Change: 5% (-10%-20%) | Baseline: 58 (48-68); 24 months: 58 (48-68); Change: 0 (-5-5) | 28 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.002; Hazard ratio 95%CI: 0.65 (0.50-0.85) |
Subgroup analyses showed that the effect of dapagliflozin on serum uric acid levels was consistent across various subgroups including age, sex, diabetes status, baseline serum uric acid levels, and baseline eGFR (Table 4). Sensitivity analyses confirmed the robustness of the primary outcome due to lack of significant interaction between the treatment effect and potential confounders, such as baseline serum uric acid levels, baseline eGFR, use of diuretics, and adherence to the study medication.
| Subgroups | Dapagliflozin group | Placebo group |
| Age | -1.3 ± 0.7 | -0.2 ± 0.6 |
| Sex | -1.2 ± 0.8 | -0.1 ± 0.7 |
| Diabetes status | -1.1 ± 0.8 | -0.2 ± 0.7 |
| Baseline serum uric acid level | -1.2 ± 0.8 | -0.1 ± 0.7 |
| Baseline eGFR | -1.1 ± 0.8 | -0.1 ± 0.7 |
Table 5 presents the incidence of adverse events and safety outcomes. The most common adverse events in both groups were infection, hypotension, and renal impairment. No significant difference was observed in the incidence of adverse events between the two groups, except for a higher rate of genital infections in the dapagliflozin group (10% vs 2%; P = 0.01). No cases of diabetic ketoacidosis, severe hypoglycemia, or amputation were identified in either group. No significant difference was observed in the incidence of serious adverse events or death between the two groups.
| Event | Dapagliflozin group (n = 100) | Placebo group (n = 100) | P value |
| Adverse events | 64 | 62 | 0.83 |
| Common adverse events | |||
| Infection | 28 | 26 | 0.79 |
| Hypotension | 16 | 14 | 0.77 |
| Renal impairment | 12 | 10 | 0.73 |
| Genital infection | 10 | 2 | 0.01 |
| Serious adverse events | 20 | 22 | 0.79 |
| Death | 8 | 10 | 0.67 |
In this randomized, double-blind, placebo-controlled trial, dapagliflozin significantly lowered serum uric acid levels and improved the clinical outcomes in CHF patients with hyperuricemia. To the best of our knowledge, this is the first study demonstrating the effect of dapagliflozin on uric acid levels in this population. Our findings have important implications for the management of CHF and hyperuricemia as they suggest that dapagliflozin may be a valuable therapeutic option for this high-risk group.
Our results are consistent with those of previous studies showing that dapagliflozin can reduce uric acid levels by 8%-10% in patients with T2DM[13]. The mechanism of action of dapagliflozin on uric acid levels is likely related to the inhibition of SGLT2, which competes with uric acid for reabsorption into the proximal tubules of the kidney. By blocking SGLT2, dapagliflozin increases the urinary excretion of both glucose and uric acid, leading to lower serum levels of both these substances[14]. This effect may be particularly beneficial for CHF patients with hyperuricemia, as they often have impaired renal function and reduced uric acid clearance.
Our results also confirmed the beneficial effects of dapagliflozin on cardiovascular outcomes in patients with CHF and HFrEF, as reported in the DAPA-HF trial[15]. Dapagliflozin significantly improved LVEF, NT-proBNP, and QoL scores and reduced the risk of cardiovascular death and hospitalization for heart failure by 35% compared with placebo. These effects were independent of diabetes status and baseline serum uric acid levels, with a broad cardioprotective effect in patients with CHF, indicating that the mechanism of action of dapagliflozin on cardiovascular outcomes in CHF is not fully understood[16].
Based on our findings, we recommend that dapagliflozin be considered as an adjunctive therapy for CHF patients with HFrEF and hyperuricemia, as it can lower serum uric acid levels and improve clinical outcomes. Future research should explore the effect of dapagliflozin on other CHF subtypes and patients with different uric acid levels, as well as the mechanisms and mediators of its action on uric acid and cardiovascular outcomes[17]. Moreover, the effect of dapagliflozin on gout and gouty arthritis and the cost-effectiveness of dapagliflozin versus placebo in CHF patients with hyperuricemia should be evaluated[18].
Our study has several strengths, such as its randomized, double-blind, placebo-controlled design; large and well-characterized sample size; long-term follow-up; high adherence to the study medication; and robust data analysis. However, our study had some limitations that should be acknowledged. First, our study population was limited to patients with CHF with HFrEF and hyperuricemia; therefore, our results may not be generalizable to other CHF subtypes or patients with normal or low uric acid levels. Second, we did not measure the levels of other biomarkers that may be associated with CHF and hyperuricemia, such as oxidative stress, inflammation, endothelial function, and vascular remodeling. Third, we did not assess the effect of dapagliflozin on gout or gouty arthritis, which are common complications of hyperuricemia. Fourth, our study did not include a cost-effectiveness analysis of dapagliflozin versus a placebo in CHF patients with hyperuricemia.
Our study showed that dapagliflozin significantly lowered serum uric acid levels and improved clinical outcomes in CHF patients with hyperuricemia. Therefore, dapagliflozin may be a useful therapeutic option for this high-risk population.
| 1. | Güder G, Störk S. COPD and heart failure: differential diagnosis and comorbidity. Herz. 2019;44:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Si K, Wei C, Xu L, Zhou Y, Lv W, Dong B, Wang Z, Huang Y, Wang Y, Chen Y. Hyperuricemia and the Risk of Heart Failure: Pathophysiology and Therapeutic Implications. Front Endocrinol (Lausanne). 2021;12:770815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 4. | Miao L, Guo M, Pan D, Chen P, Chen Z, Gao J, Yu Y, Shi D, Du J. Serum Uric Acid and Risk of Chronic Heart Failure: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2021;8:785327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1083] [Cited by in RCA: 1130] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 6. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10348] [Cited by in RCA: 9362] [Article Influence: 1040.2] [Reference Citation Analysis (3)] |
| 7. | Fujihashi T, Sakata Y, Nochioka K, Miura M, Abe R, Kasahara S, Sato M, Aoyanagi H, Yamanaka S, Hayashi H, Shiroto T, Sugimura K, Takahashi J, Miyata S, Shimokawa H; CHART-2 Investigators. Prognostic impacts of serum uric acid levels in patients with chronic heart failure: insights from the CHART-2 study. ESC Heart Fail. 2021;8:1027-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Fonseca-Correa JI, Correa-Rotter R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front Med (Lausanne). 2021;8:777861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 9. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4450] [Article Influence: 741.7] [Reference Citation Analysis (0)] |
| 10. | Tamargo J. Sodium-glucose Cotransporter 2 Inhibitors in Heart Failure: Potential Mechanisms of Action, Adverse Effects and Future Developments. Eur Cardiol. 2019;14:23-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Mori D, Kobayashi M, Wada M, Tokuchi M, Misegawa S, Saito R, Nomi H, Haga R, Nagatoya K, Yamauchi A. Effect of Dapagliflozin on Serum Uric Acid Levels in Patients with Advanced Chronic Kidney Disease. Intern Med. 2024;63:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1347] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 13. | Loosen SH, Roderburg C, Curth O, Gaensbacher J, Joerdens M, Luedde T, Konrad M, Kostev K, Luedde M. The spectrum of comorbidities at the initial diagnosis of heart failure a case control study. Sci Rep. 2022;12:2670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Ergatoudes C, Schaufelberger M, Andersson B, Pivodic A, Dahlström U, Fu M. Non-cardiac comorbidities and mortality in patients with heart failure with reduced vs. preserved ejection fraction: a study using the Swedish Heart Failure Registry. Clin Res Cardiol. 2019;108:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD; DAPA-HF Committees and Investigators. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail. 2019;21:665-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 16. | McMurray JJV, Wheeler DC, Stefánsson BV, Jongs N, Postmus D, Correa-Rotter R, Chertow GM, Greene T, Held C, Hou FF, Mann JFE, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effect of Dapagliflozin on Clinical Outcomes in Patients With Chronic Kidney Disease, With and Without Cardiovascular Disease. Circulation. 2021;143:438-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 17. | Singh JA, Cleveland JD. Comparative effectiveness of allopurinol and febuxostat for the risk of atrial fibrillation in the elderly: a propensity-matched analysis of Medicare claims data. Eur Heart J. 2019;40:3046-3054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | McDowell K, Welsh P, Docherty KF, Morrow DA, Jhund PS, de Boer RA, O'Meara E, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Hammarstedt A, Langkilde AM, Sjöstrand M, Lindholm D, Solomon SD, Sattar N, Sabatine MS, McMurray JJV. Dapagliflozin reduces uric acid concentration, an independent predictor of adverse outcomes in DAPA-HF. Eur J Heart Fail. 2022;24:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |