Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3360
Revised: April 23, 2024
Accepted: May 10, 2024
Published online: June 26, 2024
Processing time: 110 Days and 22.7 Hours
Although chemotherapy is effective for treating advanced gastric carcinoma (aGC), it may lead to an adverse prognosis. Establishing a highly effective and low-toxicity chemotherapy regimen is necessary for improving efficacy and outcomes in aGC patients.
To determine the efficacy and safety of cetuximab (CET) combined with the FOLFOX4 regimen (infusional fluorouracil, folinic acid, and oxaliplatin) as first-line therapy for patients with aGC, who received evidence-based care (EBC).
A total of 117 aGC patients who received EBC from March 2019 to March 2022 were enrolled. Of these, 60 in the research group (RG) received CET + FOLFOX4 as first-line therapy, whereas 57 in the control group (CG) received FOLFOX4. The efficacy [clinical response rate (RR) and disease control rate (DCR)], safety (liver and kidney dysfunction, leukopenia, thrombocytopenia, rash, and diarrhea), serum tumor marker expression [STMs; carbohydrate antigen (CA) 19-9, CA72-4, and carcinoembryonic antigen (CEA)], inflammatory indicators [interleukin (IL)-2 and IL-10], and quality of life (QOL) of the two groups were compared.
A markedly higher RR and DCR were observed in the RG compared with the CG, with an equivalent safety profile between the two groups. RG exhibited notably reduced CA19-9, CA72-4, CEA, and IL-2 levels following treatment, which were lower than the pre-treatment levels and those in the CG. Post-treatment IL-10 was statistically increased in RG, higher than the pre-treatment level and the CG. Moreover, a significantly improved QOL was evident in the RG.
The CET + FOLFOX4 regimen is highly effective as first-line treatment for aGC patients receiving EBC. It facilitates the suppression of STMs, ameliorates the serum inflammatory microenvironment, and enhances QOL, without increased adverse drug effects.
Core Tip: This study primarily analyzes the effect of cetuximab in combination with FOLFOX4 regimen (infusional fluorouracil, folinic acid, and oxaliplatin) as first-line therapy on the clinical outcomes of patients with advanced gastric carcinoma receiving evidence-based care. Based on the efficacy, serum tumor markers (STMs), inflammatory indicators, and improvement in quality of life (QOL), first-line treatment with cetuximab combined with the FOLFOX4 regimen has a profound effect on patients with advanced gastric carcinoma receiving evidence-based care, without increasing adverse drug effects, which is conducive to inhibiting STMs, ameliorating the serum inflammatory microenvironment, and enhancing patient QOL.
- Citation: Ying H, Huang RJ, Jing XM, Li Y, Tong QQ. Effect of cetuximab plus FOLFOX4 regimen on clinical outcomes in advanced gastric carcinoma patients receiving evidence-based care. World J Clin Cases 2024; 12(18): 3360-3367
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3360.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3360
Gastric carcinoma (GC) is a malignancy closely related to multiple factors, such as the environment and heredity. It is highly invasive and heterogeneous[1,2]. GC afflicts nearly one million people worldwide resulting in an estimated 740000 deaths each year, with most cases found in men and developing countries[3]. Advanced GC (aGC) represents a serious stage pathologically and is primarily characterized by tumor tissue invasion into the muscle layer of the gastric wall and the serosal layer. If the tumor continues to metastasize and spread, it is highly likely to further progress to aGC[4,5]. Difficulty in the diagnosis and treatment of GC lies in its atypical early clinical symptoms; therefore, most patients are diagnosed with aGC when they seek medical attention[6]. Chemotherapy is the mainstay for the treatment of aGC. Although it is effective, it may damage normal cells and affect immune function, further triggering a series of adverse events that impact therapeutic outcomes and patient prognosis[7-9]. Therefore, it is important to select an effective chemotherapy regimen with low toxicity to improve the curative effect and outcomes of aGC patients.
The FOLFOX4 regimen, which consists of oxaliplatin (OXA), folinic acid (FA), and fluorouracil (FU), is commonly used to treat aGC patients[10]. Of these agents, OXA is a third-generation platinum-based anti-tumor drug with efficacy superior to cisplatin, which mainly inhibits deoxyribonucleic acid (DNA) synthesis and forms intra- and inter-strand DNA crosslinks[11]. Although FA has no anticancer activity, it may enhance the inhibitory effects of FU on DNA synthesis; thus, playing a therapeutic role without increasing toxicity[12]. FU, as a thymidylate synthase inhibitor, disrupts cancer cell division and proliferation by interfering with nucleic acid metabolism in cancer cells[13]. In addition, it is necessary to consider strategies to reduce the toxic and side effects of the FOLFOX4 regimen while improving therapeutic efficacy[14]. Cetuximab (CET) targets the epidermal growth factor receptor (EGFR) and is essentially a recombinant human-mouse chimeric EGFR IgG1 monoclonal antibody that can be used for advanced gastric and gastroesophageal junction adenocarcinoma with reliable efficacy and safety[15]. CET plus tegafur, gimeracil, and oteracil potassium capsules (S-1) + OXA regimen for aGC have been shown to not only improve one-year survival, but is also well-tolerated[16].
At present, the research on the clinical effect of CET combined with FOLFOX4 regimen in patients with aGC receiving evidence-based care (EBC) is still limited. This study attempts to conduct relevant analysis to provide an optimal choice for the application of the FOLFOX4 regimen and to contribute to the management optimization of patients with aGC.
This study selected 117 aGC patients who received EBC in Yongkang First People’s Hospital between March 2019 and March 2022. Of these, 60 patients in the research group (RG) received the CET + FOLFOX4 regimen as first-line treatment, whereas 57 patients in the control group (CG) received the FOLFOX4 regimen only. RG and CG were clinically comparable with no marked difference in baseline data (P > 0.05).
Eligibility criteria: All participants were postoperative patients with aGC diagnosed by clinicopathological and imaging examinations. They exhibited a tolerance for chemotherapy, no distant organ metastasis, no contraindications for the drugs used, intact medical records, and a willingness to participate in this clinical trial.
Exclusion criteria: Patients with severe abdominal adhesion, diaphragmatic/inguinal hernia, gastrointestinal perforation, severe malnutrition, coagulation dysfunction, hepatic and renal insufficiency, blood system diseases, estimated survival < 1 month, other malignant tumors, or other stomach diseases were excluded.
Patients in both groups received EBC with the following nursing measures: (1) Established an EBC team, which consisted of attending doctors, head nurses, and nursing staff. The team was responsible for the formulation of the postoperative and post-discharge action plans for patients, and the head nurse supervised the entire treatment and care process; (2) Established evidence-based questions: With respect to aGC patient symptoms, the team members conducted a discussion based on research and established a preliminary plan. During implementation, the patient’s physical and psychological conditions were analyzed to reflect, summarize, and improve the plan; and (3) EBC intervention: Patients were informed in advance of the mode of surgical treatment, preoperative precautions, potential postoperative complications, and corresponding treatment measures. In addition, soothing music was played to divert the patient’s attention. To reduce pain, the patient was guided through deep breathing and psychological exercises. In terms of diet, the intake of sodium salt was strictly limited. Patients were advised to eat small meals frequently, including high-protein and crude fiber foods, drink more warm water, focus on fresh vegetables and fruits that are easy to digest, and avoid spicy, greasy, raw, and cold foods. Furthermore, the nurses understood the patients’ conditions, observed and paid attention to their quality of life (QOL) and psychological changes during treatment, and actively communicated with them. Moreover, the medical staff engaged in communication with the families of the patients, strengthened their education, and alleviated the potential anxiety, irritability, depression, and other adverse emotions experienced by patients and their families.
CG was treated with the following FOLFOX4 regimen: On the first day, 85 mg/m2 OXA was administered intra
Clinical efficacy: Complete response (CR) was defined as the disappearance of lesions with no new focus; partial response (PR) represented a ≥ 30% reduction in the lesions and a duration of ≥ 4 wk; stable disease (SD) corresponded to < 30% reduction of the lesions; progressive disease referred to the presence of new lesions or the increase of the lesions by ≥ 20%. The clinical response rate (RR) was the sum of the CP and PR cases as a percentage of the total number of cases, and the disease control rate (DCR) was calculated as the sum of the number of CP, PR, and SD patients as a percentage of the total number of cases.
Safety: We observed and recorded adverse reactions, such as liver and kidney dysfunction, leukopenia, thrombocytopenia, rash, and diarrhea in both groups, and calculated the incidence rate.
Serum tumor markers: Approximately 5 mL of fasting radial venous blood was collected from each patient before and after treatment. The serum was obtained after centrifugation and stored at -20 °C for detection. The expression of carbohydrate antigen (CA) 19-9, CA72-4, and carcinoembryonic antigen (CEA) were measured by chemiluminescence immunoassay.
Inflammatory factors: Enzyme-linked immunosorbent assays were used to quantify interleukin (IL)-2 and IL-10 in the serum.
QOL improvement: We used the Karnofsky Performance Status (KPS; score range: 0-100) to assess patient QOL. Scores increasing by ≥ 10 points after completing the treatment, increasing by < 10 points, or decreasing by < 10 points after 3 courses, and decreasing by ≥ 10 points after 3 courses indicated enhancement, stability, and decline in QOL, respectively.
The mean ± SE was used as the statistical description of the measured data, through which the inter-group and intra-group comparisons were made using an independent samples t-test and a paired t-test, respectively. The inter-group comparison of counting data represented by the ratio (percentage) was done by the χ2 test. The experimental data were analyzed by SPSS20.0, with P < 0.05 as the threshold of statistical significance.
The baseline data for RG and CG were similar, including age, sex, disease course, pathological stage, differentiated degree, and tumor diameter (P > 0.05; Table 1).
| Classification | Research group (n = 60) | Control group (n = 57) | χ2/t | P value |
| Age (yr) | 58.53 ± 7.49 | 56.84 ± 5.55 | 1.381 | 0.170 |
| Sex (male/female) | 40/20 | 34/23 | 0.619 | 0.431 |
| Disease course (yr) | 2.43 ± 1.01 | 2.23 ± 0.82 | 1.172 | 0.244 |
| Pathological stage (IIIa/IIIb) | 35/25 | 40/17 | 1.781 | 0.182 |
| Differentiation degree (low/moderate or high) | 21/39 | 22/35 | 0.163 | 0.687 |
| Tumor diameter (cm) | 3.71 ± 0.69 | 3.47 ± 0.79 | 1.753 | 0.082 |
The RR and DCR of the RG were 85.00% and 93.33% respectively, whereas those of the CG were 50.88% and 80.70% respectively. By comparison, both the RR and DCR were significantly higher in RG compared with that in the CG (P < 0.05; Table 2).
| Classification | Research group (n = 60) | Control group (n = 57) | χ2 | P value |
| CR | 12 (20.00) | 2 (3.51) | ||
| PR | 39 (65.00) | 27 (47.37) | ||
| SD | 5 (8.33) | 17 (29.82) | ||
| PD | 4 (6.67) | 11 (19.30) | ||
| RR | 51 (85.00) | 29 (50.88) | 15.740 | < 0.001 |
| DCR | 56 (93.33) | 46 (80.70) | 4.173 | 0.041 |
The number of cases of liver and kidney dysfunction, leukopenia, thrombocytopenia, rash, and diarrhea were counted and the incidence rate was calculated. These events occurred at 10.00% in the RG, which was lower, but not significantly different, compared with 14.04% the in CG (P > 0.05; Table 3).
| Classification | Research group (n = 60) | Control group (n = 57) | χ2 | P value |
| Liver and kidney dysfunction | 0 (0.00) | 0 (0.00) | ||
| Leukopenia | 1 (1.67) | 1 (1.75) | ||
| Thrombocytopenia | 1 (1.67) | 2 (3.51) | ||
| Rash | 3 (5.00) | 3 (5.26) | ||
| Diarrhea | 1 (1.67) | 2 (3.51) | ||
| Total | 6 (10.00) | 8 (14.04) | 0.452 | 0.502 |
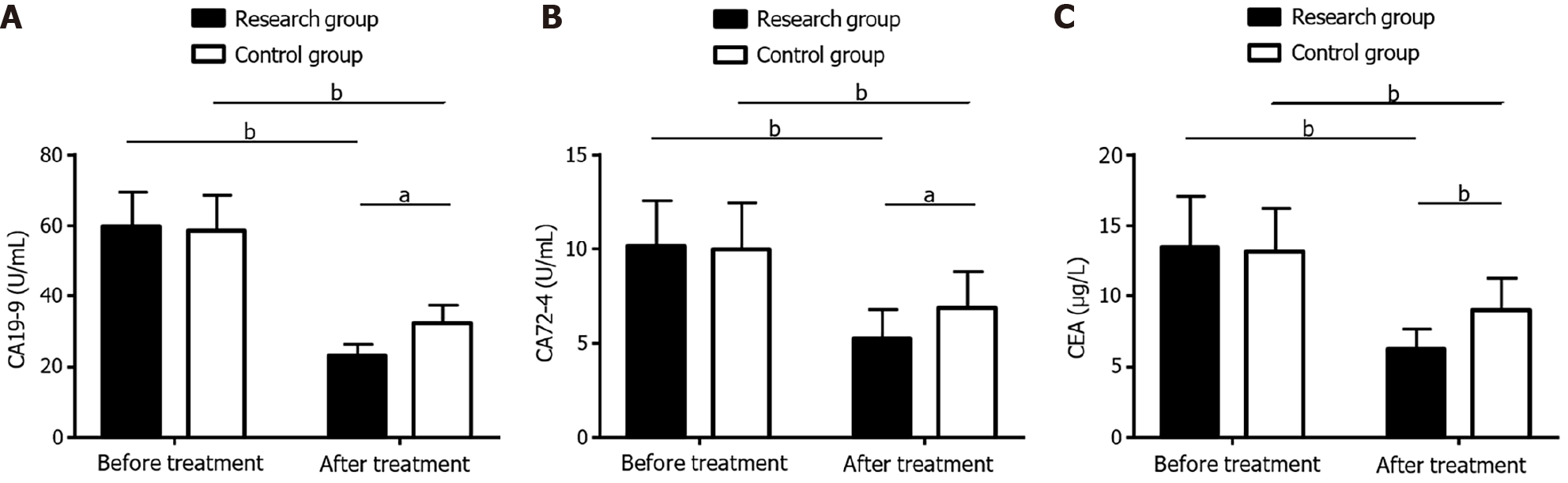
No differences were observed between the two groups in pre-treatment CA19-9, CA72-4, or CEA levels (P > 0.05). These indices decreased statistically following treatment (P < 0.05), with a more prominent decrease in RG compared with CG (P < 0.05; Figure 1).
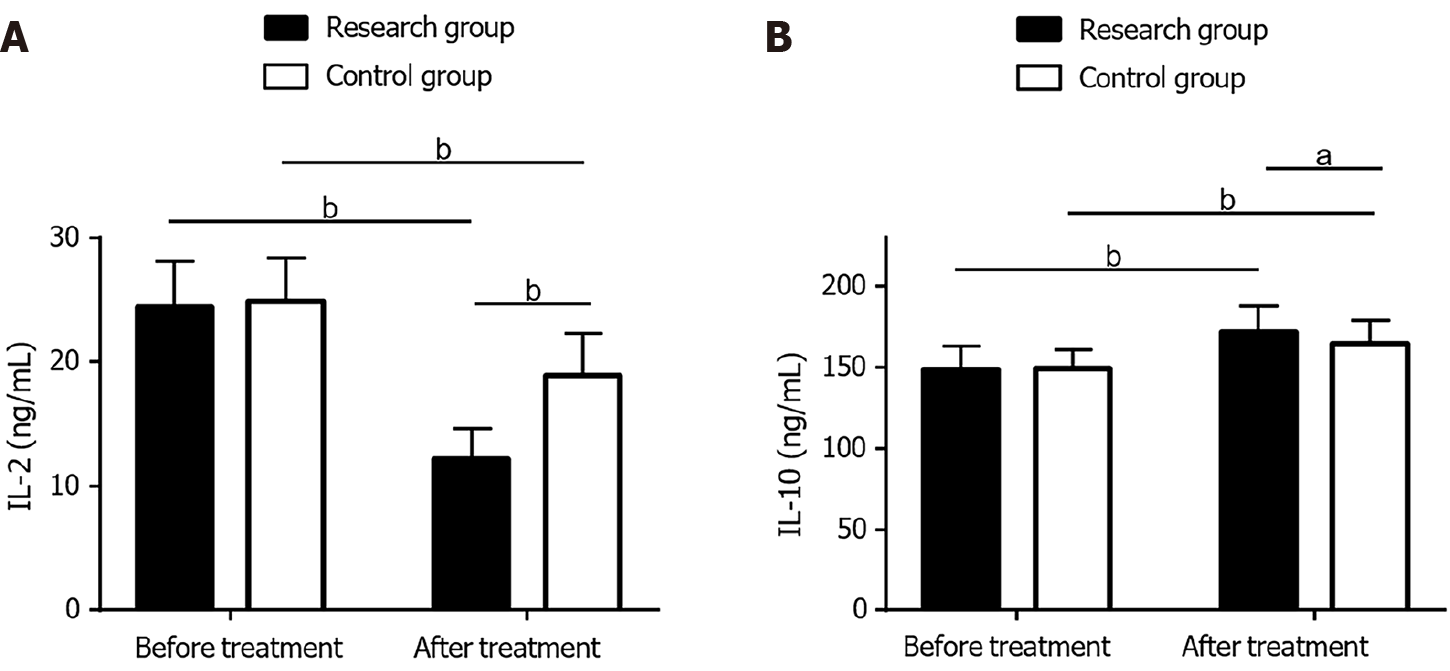
The RG and CG exhibited similar IL-2 and IL-10 Levels before treatment (P > 0.05). A marked reduction in IL-2 and a marked increase in IL-10 were observed in both groups following treatment (P < 0.05). The RG showed lower IL-2 and higher IL-10 Levels compared with the CG following treatment, with statistical significance (P < 0.05; Figure 2).
The QOL improvement in the two groups was assessed based on changes in KPS scores before and after treatment. The data indicated that a higher enhancement of QOL in the RG was observed compared with the CG (71.67% vs 52.63%, P < 0.05); however, the two groups were similar in terms of stability and decline in QOL (P > 0.05; Table 4).
| Classification | Research group (n = 60) | Control group (n = 57) | χ2 | P value |
| Enhancement | 43 (71.67) | 30 (52.63) | 4.514 | 0.034 |
| Stability | 15 (25.00) | 23 (40.35) | 3.141 | 0.076 |
| Decline | 2 (3.33) | 4 (7.02) | 0.816 | 0.367 |
As a fatal cancer, aGC carries the risks of tumor metastasis, high intratumoral heterogeneity, and chemotherapy resistance. It has an overall survival rate of only 25% worldwide and a median survival rate of usually less than one year[17-19]. To improve survival outcomes for aGC, it is necessary to optimize treatment regimens in these patients.
The RR and DCR were significantly higher in RG compared with CG, which indicates that CET + FOLFOX4 results in a superior treatment response and clinical efficacy for aGC patients who received EBC. CET exerts its anti-tumor effect by inducing G1 cell cycle arrest in tumor cells and inhibiting vascular endothelial growth factor production, tumor-related angiogenesis, and cancer cell invasion[20,21]. As reported by Lordick et al[22], the CET + FOLFOX4 regimen also has a high remission rate in the treatment of metastatic GC, suggesting the ability of CET to enhance the efficacy of the FOLFOX4 regimen, which is consistent with our findings. Chen et al[23] proposed that CET in GC has a positive effect on improving curative efficacy, the inhibition of tumor metastasis, and the improvement of immune function, which may be related to the activation of P38. With respect to safety, we primarily evaluated the occurrence of liver and kidney dysfunction, leukopenia, thrombocytopenia, rash, and diarrhea, and found no notable difference in the overall incidence between the RG and CG (10.00% vs 14.04%). This suggests that the CET + FOLFOX4 regimen is well-tolerated in aGC patients receiving EBC. Shi et al[24] reported that the CET plus FOLFOX4 regimen was clinically effective and well-tolerated in aGC patients, which supports our results.
Serum tumor markers (STMs) are special substances which may be proteins, sugars, or enzymes, and are released into the serum by tumor cells during differentiation. They are used to evaluate tumor status by assessing their abnormal expression or changes, thus guiding tumor diagnosis, survival prediction, and postoperative recurrence prediction[25,26]. In the present study, the STMs, CA19-9, CA72-4, and CEA, were analyzed. These indices in RG were significantly reduced after treatment, lower than pre-treatment levels, and those in the CG. This indicates that the abnormal expression of STMs in aGC patients may be significantly inhibited by the combined regimen, similar to the results of Yun et al[27]. Jia et al[28] reported that CA19-9 and CEA may also be used as predictors of efficacy and progression-free survival for the CET + FOLFOX4 regimen in patients with advanced colorectal cancer. Moreover, changes in the inflammatory microenvironment are strongly linked to tumorigenesis and progression, of which pro-inflammatory IL-2 may be related to GC progression and poor prognosis, whereas the deficiency of anti-inflammatory factor IL-10 may be related to Helicobacter pylori infection + chronic alcohol-induced GC[29,30]. This study measured inflammation markers, IL-2 and IL-10. The IL-2 Levels in RG following treatment were significantly lower compared with that of the pre-treatment levels and the CG, whereas IL-10 was significantly increased, suggesting that the combined scheme effectively regulates the inflammatory microenvironment of aGC patients receiving EBC, which is conducive to inhibiting excessive inflammatory reactions. We also found that the QOL improvement rate in RG vs CG was 71.67% and 52.63%, respectively, indicating that CET + FOLFOX4 is superior to FOLFOX4 alone in improving the QOL of aGC patients receiving EBC.
Several limitations of this study should be considered. First, the risk factors affecting the improvement in the QOL of aGC patients have not been analyzed. These should be addressed in the future to help further optimize the management of aGC patients. Second, there is no discussion regarding the risk factors affecting therapeutic safety in aGC patients. Further studies will be useful to minimize the risk of adverse events in aGC patients after treatment. Finally, the sample size included in this study is relatively small with only 117 cases, which may affect the accuracy of the results to a certain extent. In the future, more samples should be included to enhance the reliability of the findings.
Taken together, the first-line treatment of CET combined with the FOLFOX4 regimen has a good efficacy and safety profile in aGC patients receiving EBC.
| 1. | Bessède E, Mégraud F. Microbiota and gastric cancer. Semin Cancer Biol. 2022;86:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Johnston FM, Beckman M. Updates on Management of Gastric Cancer. Curr Oncol Rep. 2019;21:67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 316] [Article Influence: 52.7] [Reference Citation Analysis (1)] |
| 3. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 4. | Jung K, Park MI, Kim SE, Park SJ. Borrmann Type 4 Advanced Gastric Cancer: Focus on the Development of Scirrhous Gastric Cancer. Clin Endosc. 2016;49:336-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | de Nucci G, Gabbani T, Impellizzeri G, Deiana S, Biancheri P, Ottaviani L, Frazzoni L, Mandelli ED, Soriani P, Vecchi M, Manes G, Manno M. Linear EUS Accuracy in Preoperative Staging of Gastric Cancer: A Retrospective Multicenter Study. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, Yeoh KG, Hsu PI, Goh KL, Mahachai V, Gotoda T, Chang WL, Chen MJ, Chiang TH, Chen CC, Wu CY, Leow AH, Wu JY, Wu DC, Hong TC, Lu H, Yamaoka Y, Megraud F, Chan FKL, Sung JJ, Lin JT, Graham DY, Wu MS, El-Omar EM; Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM). Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 305] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 7. | Jiang Z, Cai Z, Ma Q, Shen C, Yin Y, Yin X, Liu C, Chang C, Zhao Z, Mu M, Zhang B. Comparative efficacy and safety of anti-HGF/MET pathway agents plus chemotherapy versus chemotherapy alone as first-line treatment in advanced gastric cancer: a protocol for a systematic review and meta-analysis. BMJ Open. 2021;11:e049575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Tan B, Li Y, Di Y, Fan L, Zhao Q, Liu Q, Wang D, Jia N. Clinical value of peripheral blood microRNA detection in evaluation of SOX regimen as neoadjuvant chemotherapy for gastric cancer. J Clin Lab Anal. 2018;32:e22363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Huang L, Shi Y. Editorial: The use of chemotherapy in treating gastric cancers. Front Oncol. 2022;12:974023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Xie H, Lu Q, Wang H, Zhu X, Guan Z. Two postoperative chemotherapies for gastric cancer: FOLFOX4 vs. TPF. Oncol Lett. 2019;17:933-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ouzon-Shubeita H, Baker M, Koag MC, Lee S. Structural basis for the bypass of the major oxaliplatin-DNA adducts by human DNA polymerase η. Biochem J. 2019;476:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Wang X, Wang Y, Qiu M, Li Q, Li ZP, He B, Xu F, Shen YL, Gou HF, Yang Y, Cao D, Yi C, Liu JY, Luo DY, Liao ZY, Bi F. Postoperative chemoradiotherapy in gastric cancer: a phase I study of radiotherapy with dose escalation of oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX regimen). Med Oncol. 2011;28 Suppl 1:S274-S279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Watanabe M, Katsumata K, Sumi T, Ishizaki T, Enomoto M, Shigoka M, Wada T, Kuwabara H, Mazaki J, Kasahara K, Tago T, Udo R, Nagakawa Y, Kawachi S, Tsuchida A. Nucleic Acid Metabolizing Enzyme Levels Predict Chemotherapy Effects in Advanced and Recurrent Colorectal Cancer. Asian Pac J Cancer Prev. 2022;23:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Chen Z, Zhou Z, Hu Z, Xu Q, Wang J. Effect of FOLFOX4 combined with Brucea javanica emulsion on VEGF in patients with gastric cancer. Oncol Lett. 2018;15:1079-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Götte H, Melezínková H, Moehler M; Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 680] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 16. | Zhang ZD, Kong Y, Yang W, Zhang B, Zhang YL, Ma EM, Liu HX, Chen XB, Hua YW. Clinical evaluation of cetuximab combined with an S-1 and oxaliplatin regimen for Chinese patients with advanced gastric cancer. World J Surg Oncol. 2014;12:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 460] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 18. | Stahl P, Seeschaaf C, Lebok P, Kutup A, Bockhorn M, Izbicki JR, Bokemeyer C, Simon R, Sauter G, Marx AH. Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol. 2015;15:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2856] [Article Influence: 571.2] [Reference Citation Analysis (5)] |
| 20. | Roy S, Curry SD, Bibbey MG, Chapnick DA, Liu X, Goodwin AP, Cha JN. Effect of covalent photoconjugation of affibodies to epidermal growth factor receptor (EGFR) on cellular quiescence. Biotechnol Bioeng. 2022;119:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Moehler M, Frings C, Mueller A, Gockel I, Schimanski CC, Biesterfeld S, Galle PR, Holtmann MH. VEGF-D expression correlates with colorectal cancer aggressiveness and is downregulated by cetuximab. World J Gastroenterol. 2008;14:4156-4167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Lordick F, Luber B, Lorenzen S, Hegewisch-Becker S, Folprecht G, Wöll E, Decker T, Endlicher E, Röthling N, Schuster T, Keller G, Fend F, Peschel C. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer. 2010;102:500-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Chen Q, Shen L, Chen C, He H, Fu Y, Xu L, Wang Y. Cetuximab combined with cisplatin improves the prognosis of gastric cancer patients and its effect on P38 MAPK expression. J BUON. 2019;24:2490-2498. [PubMed] |
| 24. | Shi M, Ji J, Wu J, Ma T, Liu Y, Zhou CF, Su Y, Ye ZB, Zhang J, Zhu ZG. Cetuximab combined with FOLFOX4 as the first-line treatment for advanced gastric cancer: report of 25 cases from a single institution. Hepatogastroenterology. 2012;59:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Wang H, Jin W, Wan C, Zhu C. Diagnostic value of combined detection of CA72-4, CA19-9, and carcinoembryonic antigen comparing to CA72-4 alone in gastric cancer: a systematic review and meta-analysis. Transl Cancer Res. 2022;11:848-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | Amiri FS. Serum tumor markers in chronic kidney disease: as clinical tool in diagnosis, treatment and prognosis of cancers. Ren Fail. 2016;38:530-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Yun X, Meng H, Zhou A, Jia J, Qian W. Efficacy of transcatheter arterial chemoembolization combined with capecitabine and cetuximab in the treatment of colorectal cancer with liver metastasis. J BUON. 2021;26:1002-1008. [PubMed] |
| 28. | Jia J, Zhang P, Gou M, Yang F, Qian N, Dai G. The Role of Serum CEA and CA19-9 in Efficacy Evaluations and Progression-Free Survival Predictions for Patients Treated with Cetuximab Combined with FOLFOX4 or FOLFIRI as a First-Line Treatment for Advanced Colorectal Cancer. Dis Markers. 2019;2019:6812045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Wang DP, Zhao R, Qi YH, Shen J, Hou JY, Wang MY, Bi XG, Guo XQ, Cao JM. High Expression of Interleukin-2 Receptor Subunit Gamma Reveals Poor Prognosis in Human Gastric Cancer. J Oncol. 2021;2021:6670834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Aziz F, Chakraborty A, Liu K, Zhang T, Li X, Du R, Monts J, Xu G, Li Y, Bai R, Dong Z. Gastric tumorigenesis induced by combining Helicobacter pylori infection and chronic alcohol through IL-10 inhibition. Carcinogenesis. 2022;43:126-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |