Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3351
Revised: April 12, 2024
Accepted: May 9, 2024
Published online: June 26, 2024
Processing time: 168 Days and 13.4 Hours
In rhinoplasty, calcification around silicone implants is frequently observed in the tip dorsum (TD) area. Additionally, based on a review of various literature, it is presumed that calcification in silicone implants occurs due to both inflammatory chemical reactions and physical friction against the tissue. The calcification of nasal silicone implants not only results in the functional loss of the implants, but also leads to material deformation. However, there is a lack of research on cal
To elucidate various clinical characteristics of calcification around nasal silicone implants, using histological and radiological analysis.
This study analyzed data from 16 patients of calcified nasal implants, who un
The most common location of calcification, as visually analyzed, was in the TD area, accounting for 56%. Additionally, the analysis of CT scans revealed a trend of increasing Hounsfield Unit values for calcification with the duration of implantation, although this trend was not statistically significant (P = 0.139).
Our study shows that reducing the frequency of calcification may be achievable by using softer silicone implants and by minimizing the damage to perioperative tissues.
Core Tip: Due to its relatively safe biocompatibility and stability, silicone is widely used as a material for nasal implants in rhinoplasty. However, calcification of silicone implants in the nose results in both aesthetic and functional loss of the implants. This study, through clinical analysis of calcification in silicone nasal implants, suggests that the highest frequency occurs in the tip dorsum area. It hypothesizes that the cause of calcification is attributed to both inflammatory reactions and continuous friction against the tissue. Therefore, using softer forms of silicone and minimizing tissue damage during surgery, could reduce the complications associated with calcification.
- Citation: Hwang YS, Kim TK, Yang DJ, Jang SH, Lee DW. Complicated calcified alloplastic implants in the nasal dorsum: A clinical analysis. World J Clin Cases 2024; 12(18): 3351-3359
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3351.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3351
While there is a significant popularity of rhinoplasty in Asia, in contrast to Western countries, augmentation rhinoplasty is predominantly performed using alloplastic implants rather than using autologous grafts[1]. Silicone, a medical-grade polymer with silicon-oxygen chains, stands out for its commendable mechanical properties and chemical stability[2]. The physical properties and morphology of medical silicone depend on the number of cross-links between chains. Higher number of cross-links results in solid properties, while fewer cross-links lead to gel-like properties[3].
Silicone implants, therefore, exhibit superior biocompatibility compared to that of other materials, as they do not dissolve in body fluids, are non-toxic, and carve easily desired shapes[2]. Their nonporous nature deters bacterial infiltration and facilitates straightforward sterilization with antiseptic solutions, making them a preferred choice in plastic surgeries like augmentation rhinoplasty, chin augmentation, and breast augmentation[4], leading to their widespread use. Particularly, silicone implants are popular in augmentation rhinoplasty due to their inherent stability, mimicking the natural nasal skeleton[4]. Newly designed silicone implants can be used to refine and reshape the nasal tip, enhance the projection, definition, or symmetry of the nasal tip[5]. Furthermore, it may be used to smooth out the profile of the nose in the correction of hump nose[6].
Despite its excellent biocompatibility, silicone implants may rarely lead to adverse reactions, such as foreign body reactions. Prolonged implantation may lead to complications like chronic inflammation, delayed hypersensitivity reactions, infections, capsular contracture, and calcification[7]. The nature of complications varies with the physical form of silicone: Gel-type induces chronic inflammation and delayed hypersensitivity reactions, while solid silicone associates with issues like capsule formation, infection, extrusion, and calcification[3].
Calcification, among these complications, occurs due to the chemical rejection response of the body to the implant, causing loss of flexibility and potentially deforming the material[8]. This not only leads to the functional loss of the implant, but also results in material deformation and damage. In cases of severe calcification of silicone implants used for aesthetic purposes, reports have described instances of palpable nodules resembling skin protrusions externally[1]. Severe calcification in nasal implants has even been misinterpreted as hypertrophic bone lesions in the nasal bone[9].
At present, extensive research has been conducted on calcification associated with various medical polymer materials. Cases of complications owing to calcification have been reported in silicone breast implants and silicone sheet implants used in ophthalmology[10,11]. The qualitative, quantitative, and three-dimensional structure of calcium deposits in silicone implants are somewhat understood. Efforts to identify the causes of calcification continue through the analysis of the histological characteristics of tissues in areas where calcification occurs; however, the exact causes have not been conclusively determined. Despite the widespread use of silicone nasal implants in Asia, research on the calcification of silicone nasal implants is limited, especially studies utilizing computed tomography (CT) scans[12,13].
This study aims to conduct an in-depth analysis of the calcification phenomenon in silicone nasal implants. The study focuses on investigating the prevalence of calcification around the implants in patients, who underwent revision rhinoplasty to remove existing silicone implants for various reasons after undergoing augmentation rhinoplasty. Additionally, the study aims to analyze the radiographic and histological characteristics of calcification on CT scans, and the clinical manifestations of patients. Through this, the study seeks to contribute new information to existing research on the calcification of silicone nasal implants, and suggests new clinical insights by understanding the mechanisms of calcification.
We focused on a cohort of 16 patients, who underwent revisional rhinoplasty to remove silicone nasal implants for va
A retrospective study was conducted using medical records, and information such as the duration of implantation, types of implants, location of calcification, and the presence of inflammatory reactions were investigated. CT scans prior to revision surgery were available for seven patients, allowing examination of Hounsfield Units (HU) of calcification and the existence of bony erosion. The study adhered to ethical guidelines, receiving approval from the Institutional Review Board (number: 2023-11-062).
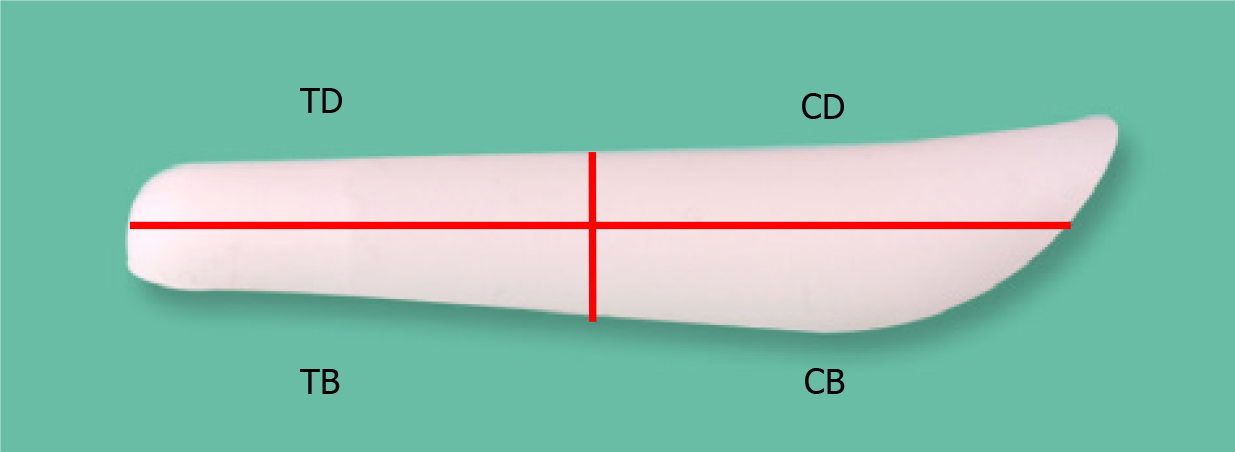
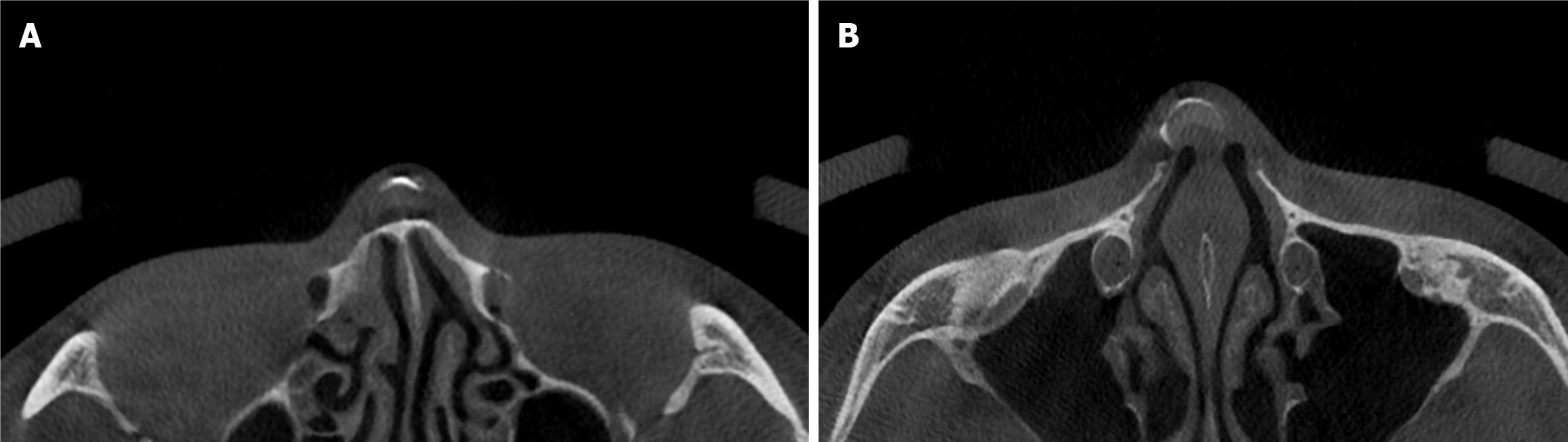
To assess calcification prevalence, the implant was segmented into four compartments: Tip dorsum (TD), tip base (TB), cephalic dorsum (CD), and cephalic base (CB) (Figure 1). The midpoint of the implant's transverse axis served as the reference, defining regions below and above it as caudal tip and cephalic portions, respectively. The areas in contact with nasal bone/cartilage and the skin envelope were designated as base and dorsum portions, respectively. In cases where the range of calcification was extensive and invaded multiple areas, if more than 80% of the calcification was skewed towards one specific compartment, that compartment was defined as the primary affected one. The results showed that calcification was localized at a single area in all cases.
The HU of calcified lesions observed in the CT scan, performed before the revisional surgery, was measured. The amount of X-ray energy absorbed into the tissues in CT is proportional to the density of the tissue. The higher the tissue density, the weaker the signal returned to the detector, resulting in a brighter image. HU is a numerical representation of this phenomenon. In other words, the higher the density of the tissue, the brighter it appears in CT, and consequently, the higher the HU value. For example, air, which is devoid of tissue, appears the darkest, showing HU values below -1000, while bone, being the densest tissue in the human body, appears the brightest, showing HU values above +1000. Calcification typically exhibits HU values of +300 or higher. As higher HU values indicate higher density of calcified deposits, it is indicative of more severe calcification[14]. In this study, the HU value was measured at the visually brightest spot of calcified lesions in the CT scan. Additionally, the median value of all HU measurements taken at 1 mm intervals in each case was investigated.
Statistical analyses were conducted using SPSS (version 27.0, IBM Corp., Armonk, NY, United States). Spearman's correlation analysis was used to explore variable relationships, with significance set at P < 0.05.
The study focused on 16 patients who exhibited calcification in silicone nasal implants, removed during revisional rhinoplasty. Patients' ages ranged from 21 to 64 years (mean age: 41.6 years), and the duration of implantation spanned 4 to 20 years (mean duration: 11 years). Investigation of the reasons for revisional rhinoplasty based on medical records revealed that the primary concern was deformity in nasal shape, with implant deviation being the most common reason in eight patients (50%). All 16 patients had consistent implant types, utilizing hard-type straight "I" shape silicone implants. Inflammation around calcification in the skin soft tissue envelope was observed in six patients (37.5%), while no bony erosion was detected in the seven patients who underwent CT imaging (Table 1).
| Number | Age | Sex | Chief complaint | Implant duration | Implant shape | Location of calcification | Bony erosion | Inflammation |
| 1 | 21 | Female | Implant deviation | 4 | Straight Silicone | TD | No | No |
| 2 | 64 | Female | Tip depression | 19 | Straight Silicone | TD | No | Yes |
| 3 | 49 | Female | Implant deviation | 15 | Straight Silicone | CD | No | Yes |
| 4 | 42 | Female | Implant deviation | 12 | Straight Silicone | TD | No | Yes |
| 5 | 42 | Female | Pinched tip | 12 | Straight Silicone | TD | No | No |
| 6 | 38 | Female | Implant deviation | 8 | Straight Silicone | TB | No | Yes |
| 7 | 57 | Female | Dorsum depression | 14 | Straight Silicone | TD | No | No |
| 8 | 48 | Female | Dorsum depression | 13 | Straight Silicone | CD | No | No |
| 9 | 51 | Female | Implant deviation | 15 | Straight Silicone | TD | No | No |
| 10 | 32 | Female | Hump, high nose | 7 | Straight Silicone | CB | No | No |
| 11 | 39 | Female | Pinched tip | 6 | Straight Silicone | CB | No | No |
| 12 | 34 | Female | Implant deviation | 15 | Straight Silicone | TD | No | Yes |
| 13 | 61 | Female | Implant deviation | 20 | Straight Silicone | TD | No | Yes |
| 14 | 31 | Female | Supratip break | 7 | Straight Silicone | TB | No | No |
| 15 | 30 | Female | Visibility | 4 | Straight Silicone | CD | No | No |
| 16 | 27 | Female | Implant deviation | 6 | Straight Silicone | TD | No | No |
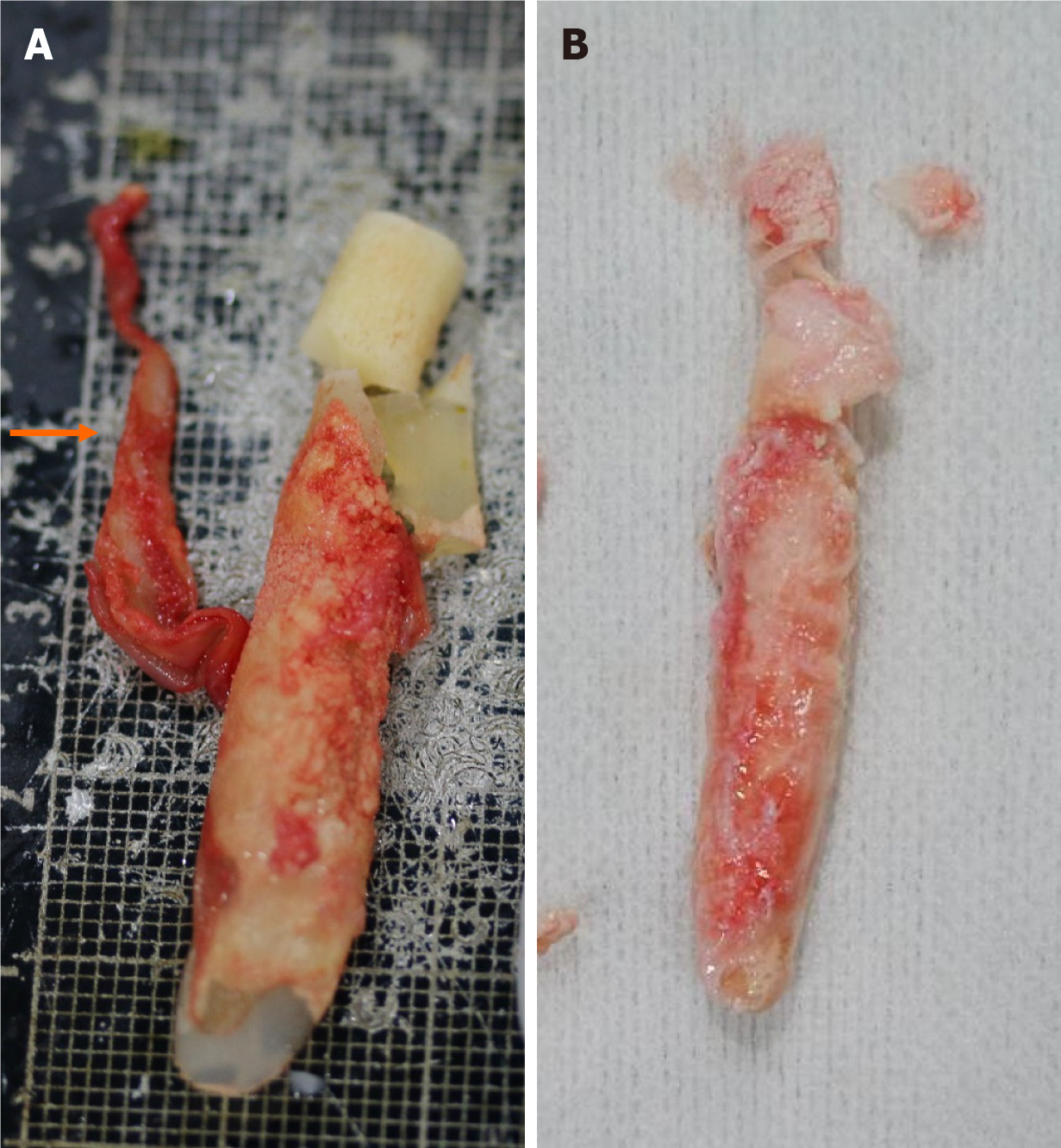
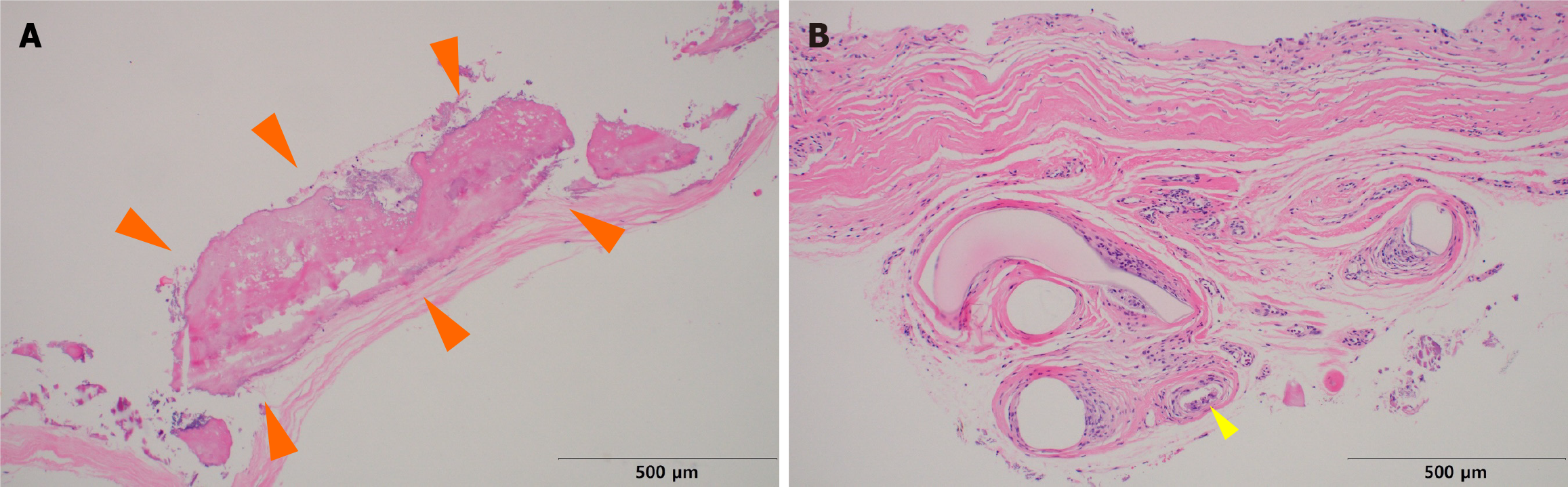
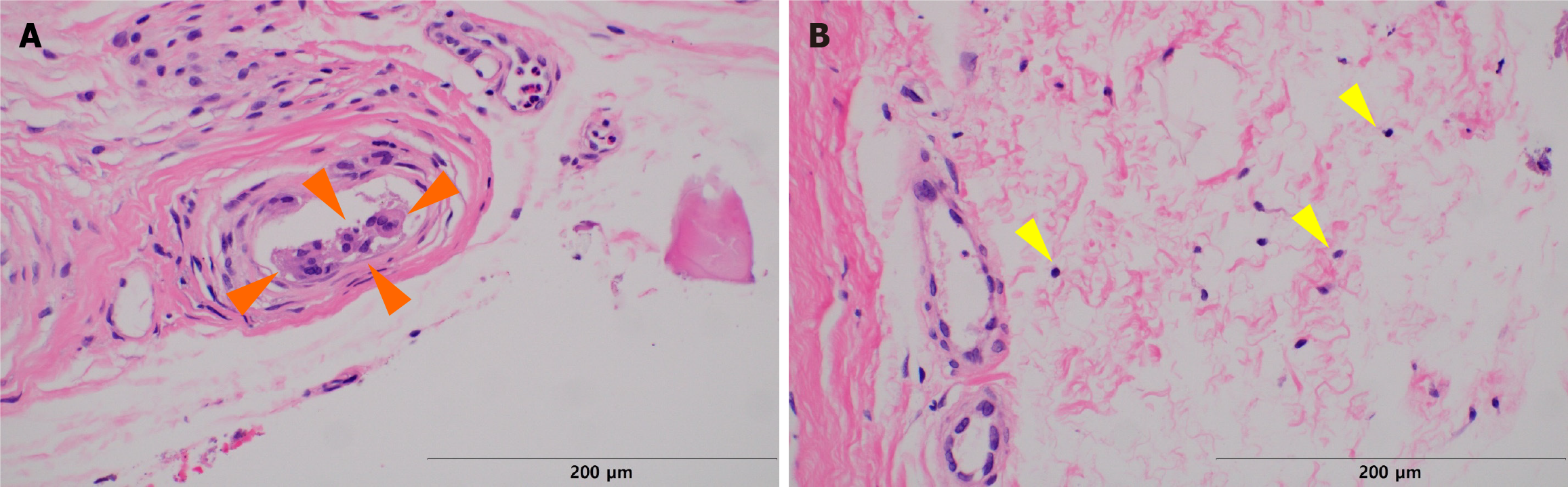
We collected samples of calcification present in the capsule around the silicone implant removed during revisional rhinoplasty. This sample was processed as formalin-fixed paraffin-embedded specimens. Grossly, calcified capsules were observed enveloping the silicone implant for nasal reshaping (Figure 2). Histological analysis was performed on an implant retrieved from a patient with severe inflammatory reactions using Hematoxylin and Eosin staining (500 μm), revealing inflammation characterized by lymphocyte infiltration (Figures 3 and 4). The primary affected area for calcification formation was investigated, and TD was the most prevalent (56%) among the previously mentioned four compartments. Following that, CD was more common (19%), while CB and TB each had two cases (12% each) (Table 2).
| Location of calcification | n | % |
| TD | 9 | 56.3 |
| CD | 3 | 18.8 |
| CB | 2 | 12.5 |
| TB | 2 | 12.5 |
| Total | 16 |
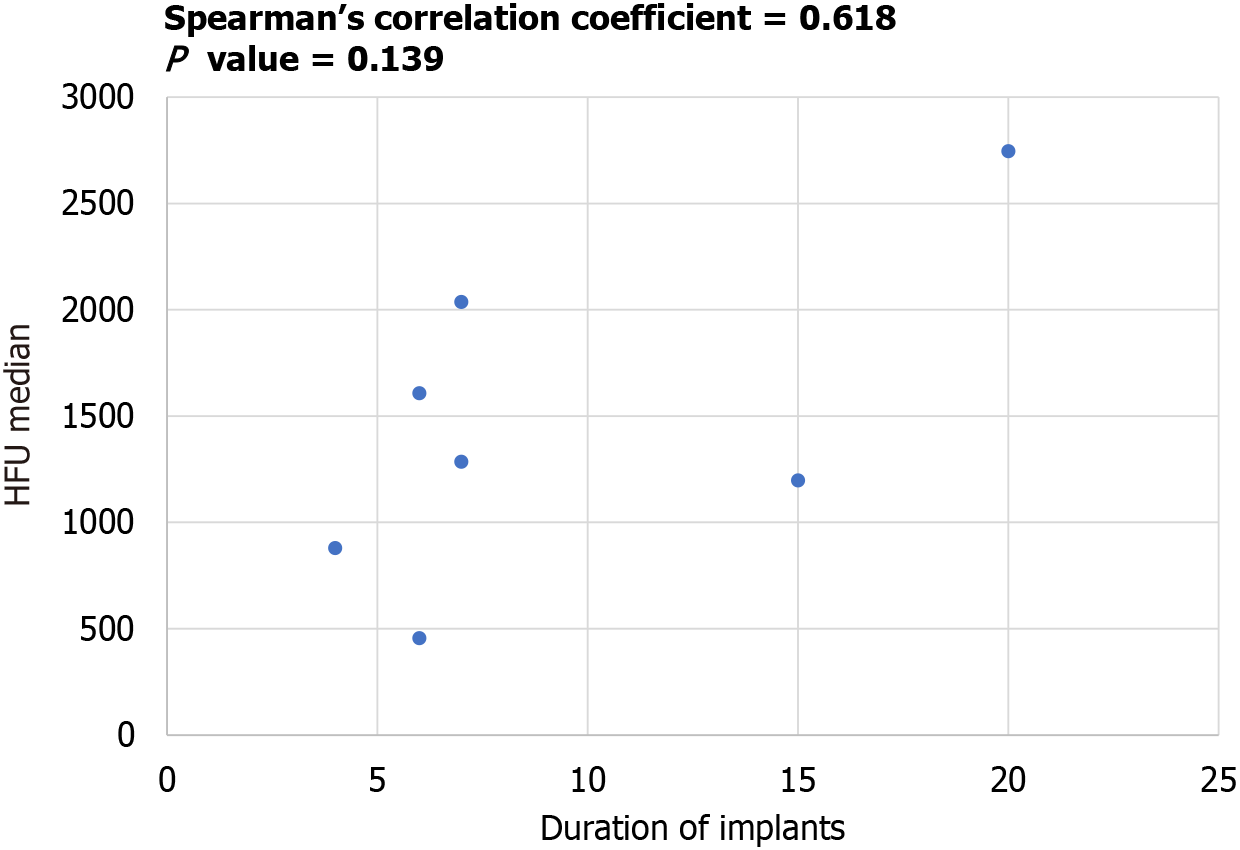
Given that CT scans were not performed on all patients, data was available for seven out of the 16 patients (Figure 5). The median HU values, calculated, ranged from 456 to 2746 (mean: 1459). The graph depicted the trend of increasing median HU values with longer durations of implantation (Figure 6). The Spearman correlation coefficient between the median HU values and duration of implants was 0.618, indicating a positive correlation. However, the P value was 0.139, suggesting that this correlation was not statistically significant.
Silicone, a medical-grade polymer, has been utilized in plastic surgery since the 1960s owing to its remarkable biocompatibility and stability. Particularly, it has gained prominence in breast augmentation procedures, being considered relatively safe from side effects such as breast cancer and autoimmune diseases[15]. Consequently, it is widely employed as an intra-body implant for tissue replacement in various body regions. Despite its benefits, complications such as infection, capsular contracture, and calcification have been also observed. Among these issues, calcification has become a recent focus of interest due to its delayed onset, potentially resulting in material deformation over time[8]. Once calcification occurs, the patient inevitably has high chance of silicone implant removal. This underscores the importance of heightened awareness among plastic surgeons using silicone implants.
Calcification tends to become more frequent and severe with prolonged periods of implantation. It is reported that calcification occurs frequently in silicone implants that are over 15 years old. Previous research by Jung et al[1] investigated calcification and capsular tissue around silicone nasal implants using optical microscopy. The results indicated an increase in size of calcification with prolonged in-body duration, accompanied by heightened inflammatory and foreign body reactions. Surprisingly, there had been no analysis of calcification around nasal implants using CT scans until this study. In our research, leveraging HU as a measuring tool for density of tissue on CT scans, we identified that higher-density calcification presented with longer implantation period. This confirms previous findings that calcification tends to be more severe over time with HU using CT scan.
However, it is crucial to note that calcification does not occur universally in all patients, and there are cases without inflammation. The degree of inflammation changes varying among individuals and whether these inflammation and foreign body reactions bring about aesthetic problem in nasal shape remain unclear.
The exact etiology of calcification around silicone implants are not well-established, although various hypotheses have been proposed. One hypothesis suggests that calcium cations present on the silicone surface promote calcification through chemical reactions with the plasma. The reason is that anions on the surface of high molecular weight substances such as silicone act as surfactants[16]. Another theory posits that defects on the implant surface induce membrane formation by fibroblasts or vascular endothelial cells, triggering calcification[17]. Mechanical stimulation between the implant and contacting muscle layers as well as osteogenic transformation of the bone cells due to mechanical friction force are also considered contributors to calcification[18]. As evidence to support this, experimental studies showing that continuous friction of the aortic valve is directly related to calcification of the valve, have shown that friction causes changes in the cells and the extracellular matrix of the valve and activates molecular signals that promote calcification[19]. There is another hypothesis which states that when calcium phosphate is deposited in tissues, calcified nodules are formed. Calcium phosphate is not easily deposited naturally in vivo, but can be easily deposited owing to decreased pH within tissue, when lactic acid increases due to increased bacterial metabolism following tissue damage or inflammatory lesions[20]. In experimental studies, apoptosis of smooth muscle cells caused by necrotic tissue or inflammatory tissue increases calcific nodules, and calcium aggregation is reportedly observed around apoptotic bodies[21].
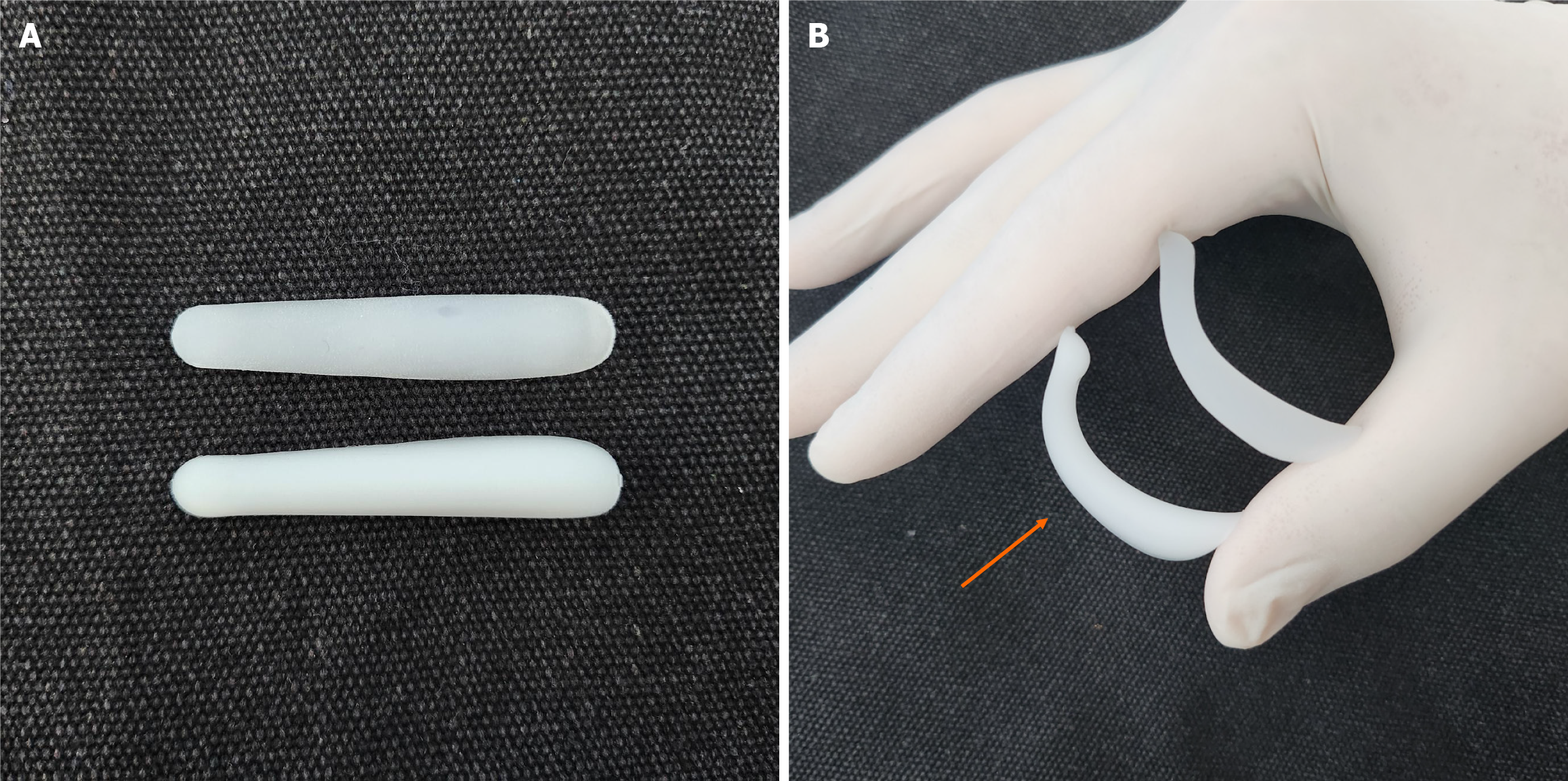
Considering these hypotheses, the cause of calcification in augmentation rhinoplasty using silicone implants may be assumed to be excessive mechanical or chemical stress against the tissue, such as friction force and chronic inflammatory response. These factors can be attributed to technical issues, for example implants not being securely fixed, leading to continuous movement under the nasal envelope. In addition, silicone materials with higher hardness can cause continuous stimulation on the nasal envelope, especially at the mobile nasal tip area, which is the most common compartment of calcification in this study. In other words, the softer is the implant, the lower is the risk of calcification. In the bending test of soft implants, the soft type implant exhibits higher bending strength compared to the hard type implant (Figure 7).
Regarding the location of calcification, a previous study observed that calcification areas were most extensive in the CD region. Jin et al[12] hypothesized that placing implants between the nasal bone and periosteum stimulates continuous bone formation of the periosteum, thereby causing calcification. Jin et al[12] included a large number of “L” shaped silicone implants, widely used in the past but infrequently used recently. This article also assumed that continuous mechanical stimulation causes calcification. This contrasts with our study, where all cases had straight implants. Our study suggested that calcification was the most frequently found at the TD, likely due to more frequent friction from the anatomical characteristics of the nose, where the lower cartilage structure allows more mobility than do the upper static structures including the nasal bone and upper lateral cartilage. In addition, postoperative inflammation and fibrosis due to excessive tip graft also triggers an overall inflammatory reaction contributing to increased calcification of TD. Among the tip areas, TD directly contacts with the skin and soft tissue envelope and generates more friction than does the TB, which matches TD as the most common calcification site.
The anatomical layers of nasal implant insertion in rhinoplasty can be broadly categorized into two regions: The bony vault towards the cephalic aspect and the cartilaginous vault towards the caudal aspect. In the bony vault, the implant is positioned between the nasal bone and periosteum. In contrast, in the cartilaginous vault, the implant is situated between the cartilage layer and the musculo-aponeurotic layer[22]. The increased physical friction, thought to contribute to TD, is attributed to movement of the musculo-aponeurotic layer. This phenomenon is believed to be influenced by the dynamic interaction between the implant and the surrounding anatomical structures during nasal tip movement[12]. Similar hypotheses have been proposed for breast implants, where calcification is primarily observed on the front surface when the implant is inserted below the breast parenchyma. This is attributed to greater movement of breast parenchymal tissue during physical activities such as running, compared to the relatively static pectoralis major muscle[23]. The study’s findings indicating a frequent occurrence of calcification on the anterior surface of breast implants, align with the findings of our study, where calcification was observed to be prevalent in TD.
There are some limitations in this study. First, the study variation results highlight the need for future studies comparing L-shaped and straight implants to elucidate the causes of calcification with silicone implants. Second, it is not easy to collect a large number of patients since not all cases are operated with revisional rhinoplasty. Finally, the possibility of technical error exists during the manual measuring of HUs from CT scans.
Our study revealed a high frequency of calcification around nasal silicone implants in the TD region with augmentation rhinoplasty. In addition, based on several literature reviews, it can be inferred that calcification caused by silicone implants is caused by both inflammatory reactions (chemical cause) and continuous friction against the tissue (physical cause). The reduction in calcification may be achievable by using softer implants and minimizing damage to perioperative tissues during rhinoplasty.
| 1. | Jung DH, Kim BR, Choi JY, Rho YS, Park HJ, Han WW. Gross and pathologic analysis of long-term silicone implants inserted into the human body for augmentation rhinoplasty: 221 revision cases. Plast Reconstr Surg. 2007;120:1997-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Thorne CH, Chung KC, Gosain AK, Gurtner GC, Mehrara BJ, Rubin PJ, Spear SL. Grabb and Smith's plastic surgery. 7th ed. Philadelphia: Lippincott Williams and Wilkins, 2014: 64-69. |
| 3. | Sclafani AP, Romo T 3rd. Biology and chemistry of facial implants. Facial Plast Surg. 2000;16:3-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Genther DJ, Papel ID. Surgical Nasal Implants: Indications and Risks. Facial Plast Surg. 2016;32:488-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Zhang J. Design and Application of a Novel Silicone Nasal Implant. Aesthet Surg J Open Forum. 2023;5:ojad040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Lee HI, Jung HW, Joo JB, Cho JE, Kim JY. Correction of Hump Nose Using Radix Augmentation with Silicone. Korean J Otorhinolaryngol-Head Neck Surg. 2010;53:486. [DOI] [Full Text] |
| 7. | Pak MW, Chan ES, van Hasselt CA. Late complications of nasal augmentation using silicone implants. J Laryngol Otol. 1998;112:1074-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Schoen FJ, Harasaki H, Kim KM, Anderson HC, Levy RJ. Biomaterial-associated calcification: pathology, mechanisms, and strategies for prevention. J Biomed Mater Res. 1988;22:11-36. [PubMed] |
| 9. | Lee SB, Min HJ. Circumferential Calcification of Silicone Implant Misunderstood as a Bony Substitute. J Craniofac Surg. 2016;27:e81-e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Peters W, Pritzker K, Smith D, Fornasier V, Holmyard D, Lugowski S, Kamel M, Visram F. Capsular calcification associated with silicone breast implants: incidence, determinants, and characterization. Ann Plast Surg. 1998;41:348-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Brockhurst RJ, Ward RC, Lou P, Ormerod D, Albert D. Dystrophic calcification of silicone scleral buckling implant materials. Am J Ophthalmol. 1993;115:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Jin HS, Kim HJ, Han KH. Calcification deposits in nasal silicone implants: regional distribution in relation to surrounding soft tissues. J Korean Soc Plast Reconstr Surg. 2004;31:315-323. |
| 13. | Lee MJ, Kim HJ, Han KH, Kim JH, Son DG. Calcification on the surface of silicone nasal implants: regional properties of calcification deposits. J Korean Soc Plast Reconstr Surg. 2006;33:324-329. Available from: https://koreascience.kr/article/JAKO200610103461118.view. |
| 14. | Kamalian S, Lev MH, Gupta R. Computed tomography imaging and angiography - principles. Handb Clin Neurol. 2016;135:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Brody GS. On the safety of breast implants. Plast Reconstr Surg. 1997;100:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Owen DR, Zone RM. Analysis of a possible mechanism of surface calcification on a biomedical elastomer. Trans Am Soc Artif Intern Organs. 1981;27:528-531. [PubMed] |
| 17. | Coleman DL, Lim D, Kessler T, Andrade JD. Calcification of nontextured implantable blood pumps. Trans Am Soc Artif Intern Organs. 1981;27:97-104. [PubMed] |
| 18. | Morris SF, Taylor GI. Predicting the survival of experimental skin flaps with a knowledge of the vascular architecture. Plast Reconstr Surg. 1993;92:1352-1361. [PubMed] |
| 19. | Dellimore K, Kemp I, Scheffer C, Weich H, Doubell A. The influence of leaflet skin friction and stiffness on the performance of bioprosthetic aortic valves. Australas Phys Eng Sci Med. 2013;36:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69:522-530. [PubMed] |
| 21. | Han KO. The Classification and Mechanism of Vascular Calcification. J Korean Soc Endocrinol. 2005;20:556. [DOI] [Full Text] |
| 22. | Daniel RK, Letourneau A. Rhinoplasty: nasal anatomy. Ann Plast Surg. 1988;20:5-13. [PubMed] |
| 23. | Peters W, Smith D, Lugowski S, Pritzker K, Holmyard D. Calcification properties of saline-filled breast implants. Plast Reconstr Surg. 2001;107:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |