Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3340
Revised: April 17, 2024
Accepted: April 23, 2024
Published online: June 26, 2024
Processing time: 106 Days and 1.8 Hours
Enhanced magnetic resonance imaging (MRI) is widely used in the diagnosis, treatment and prognosis of hepatocellular carcinoma (HCC), but it can not effectively reflect the heterogeneity within the tumor and evaluate the effect after treatment. Preoperative imaging analysis of voxel changes can effectively reflect the internal heterogeneity of the tumor and evaluate the progression-free survival (PFS).
To predict the PFS of patients with HCC before operation by building a model with enhanced MRI images.
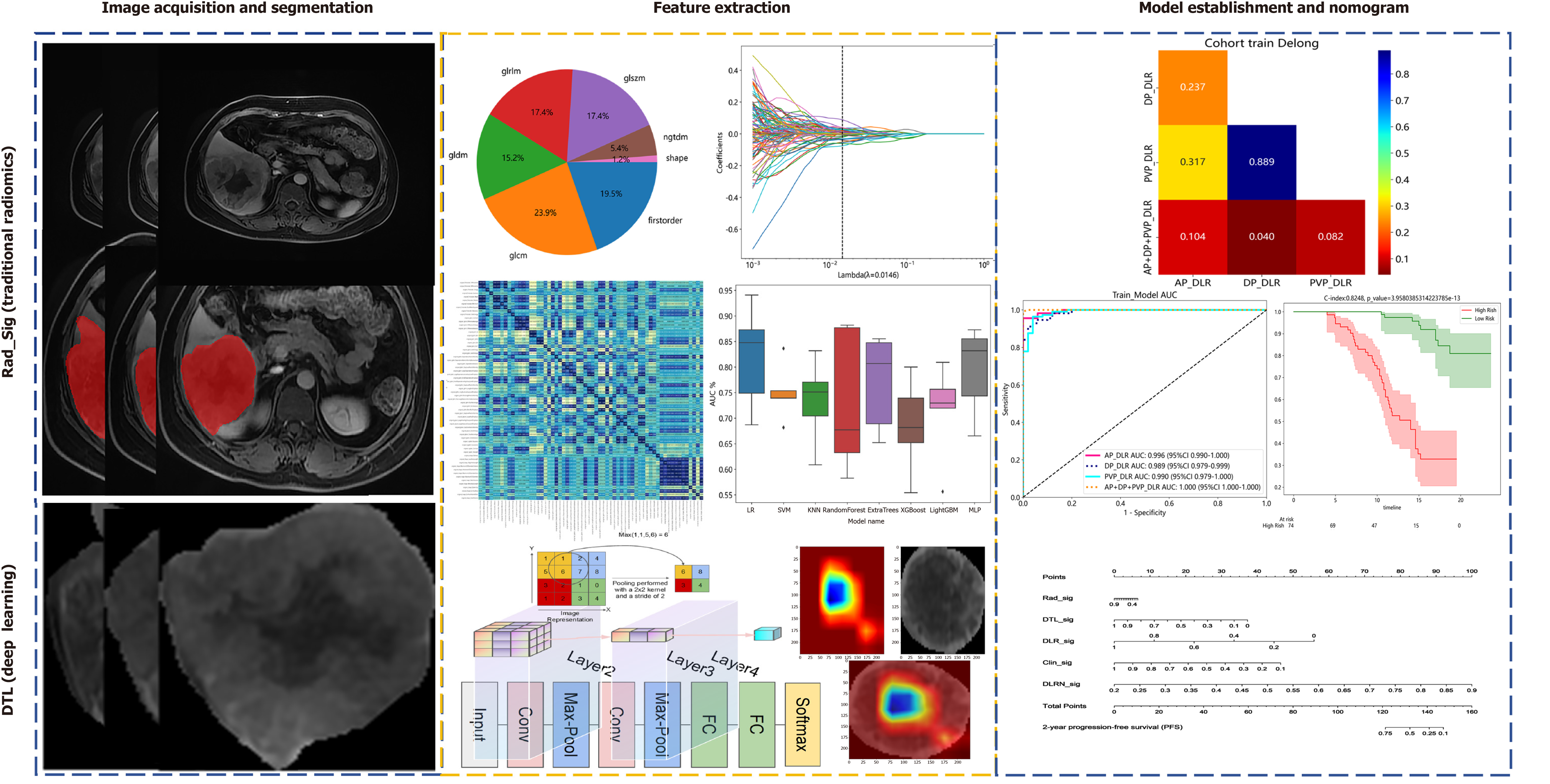
Delineate the regions of interest (ROI) in arterial phase, portal venous phase and delayed phase of enhanced MRI. After extracting the combinatorial features of ROI, the features are fused to obtain deep learning radiomics (DLR)_Sig. DeLong's test was used to evaluate the diagnostic performance of different typological features. K-M analysis was applied to assess PFS in different risk groups, and the discriminative ability of the model was evaluated using the C-index.
Tumor diameter and diolame were independent factors influencing the prognosis of PFS. Delong's test revealed multi-phase combined radiomic features had significantly greater area under the curve values than did those of the individual phases (P < 0.05).In deep transfer learning (DTL) and DLR, significant differences were observed between the multi-phase and individual phases feature sets (P < 0.05). K-M survival analysis revealed a median survival time of high risk group and low risk group was 12.8 and 14.2 months, respectively, and the predicted probabilities of 6 months, 1 year and 2 years were 92%, 60%, 40% and 98%, 90%,73%, respectively. The C-index was 0.764, indicating relatively good consistency between the predicted and observed results. DTL and DLR have higher predictive value for 2-year PFS in nomogram.
Based on the multi-temporal characteristics of enhanced MRI and the constructed Nomograph, it provides a new strategy for predicting the PFS of transarterial chemoembolization treatment of HCC.
Core Tip: In this study, traditional radiomics and deep learning (DL) were used to model hepatocellular carcinoma in order to reflect the heterogeneity of tumors through voxel changes in images. The main purpose of this method is to extract the regions of interest features of the three-phase images of enhanced magnetic resonance imaging obtained before transarterial chemoembolization (TACE), construct the prediction model and nomogram through feature screening and least absolute shrinkage and selection operator regression, to construct a prediction model and nomogram to evaluate the clinical value of DL radiomics omics feature model and to predict progression-free survival after TACE.
- Citation: Guan QL, Zhang HX, Gu JP, Cao GF, Ren WX. Omics-imaging signature-based nomogram to predict the progression-free survival of patients with hepatocellular carcinoma after transcatheter arterial chemoembolization. World J Clin Cases 2024; 12(18): 3340-3350
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3340.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3340
Hepatocellular carcinoma (HCC) accounts for 90% of primary liver tumors, and its incidence has been steadily increasing in recent years[1]. The five-year survival rate for unresectable HCC patients is approximately 12%, with a postoperative recurrence rate as high as 70%[2]. As recommended by the American Association for the Study of Liver Diseases and the Chinese Society of Clinical Oncology guidelines[3], transarterial chemoembolization (TACE) has become a diversified and comprehensive intervention for early, intermediate, and advanced-stage HCC. Therefore, in order to avoid liver failure after TACE, we should grasp the staging of liver cancer, and preoperative imaging evaluation is very important[4]. The mechanism of TACE involves the embolization of tumor-feeding arteries and the infusion of cytotoxic drugs directly into tumor-feeding arteries[5], with reported objective response rates reaching 70%[6]. Due to the broad indications of TACE, timely assessment of which HCC patients can benefit from TACE treatment is crucial for improving patient prognosis.
In comparison to traditional imaging features, radiomic features reveal the phenotype and biological heterogeneity of tumors. By using radiomics to convert preoperative conventional imaging data into high-throughput quantitative imaging features, scholars endeavor to assess patient survival[7,8]. Using radiomic models based on radiological data, clinicians are currently able to successfully predict the survival of cancer patients[9,10]. Radiomics via ultrasound or magnetic resonance imaging (MRI) has shown promise as an effective indicator for predicting short-term prognosis after TACE for HCC patients[11-13]. The application of deep learning (DL) techniques to computed tomography images can also enhance the prediction of TACE treatment response[14]. Therefore, radiomics is considered capable of predicting clinical endpoints, including disease progression, survival time, and treatment response. Recently, there have been an increasing number of studies on various radiomic patterns in different phases, tumor regions, and surrounding areas of enhanced MRI in HCC[15-17]. This is due to the close correlation between the radiomic expression of enhancement levels in different phases of HCC MRI and microvascular invasion density, making the study of enhancement levels in different phases crucial for predicting HCC prognosis.
In recent years, convolutional neural networks (CNNs) with various network structures have been widely applied in radiological research. Numerous useful DL features can be extracted for tumor grading prediction, survival analysis, and prognosis prediction[18]. However, constructing CNNs often requires a large training dataset. Transfer learning involves using a pretrained neural network on other images, typically from ImageNet, allowing the application of existing training models to unresolved problems and significantly reducing the need for a large amount of training data.
This study aimed to use radiomic features from enhanced MR images acquired during different phases of HCC progression to develop and validate a new radiomic model by combining clinical features. The goal of this study was to assess whether radiomics can provide relevant information on the efficacy of TACE and predict the progression-free survival (PFS) of patients after TACE treatment.
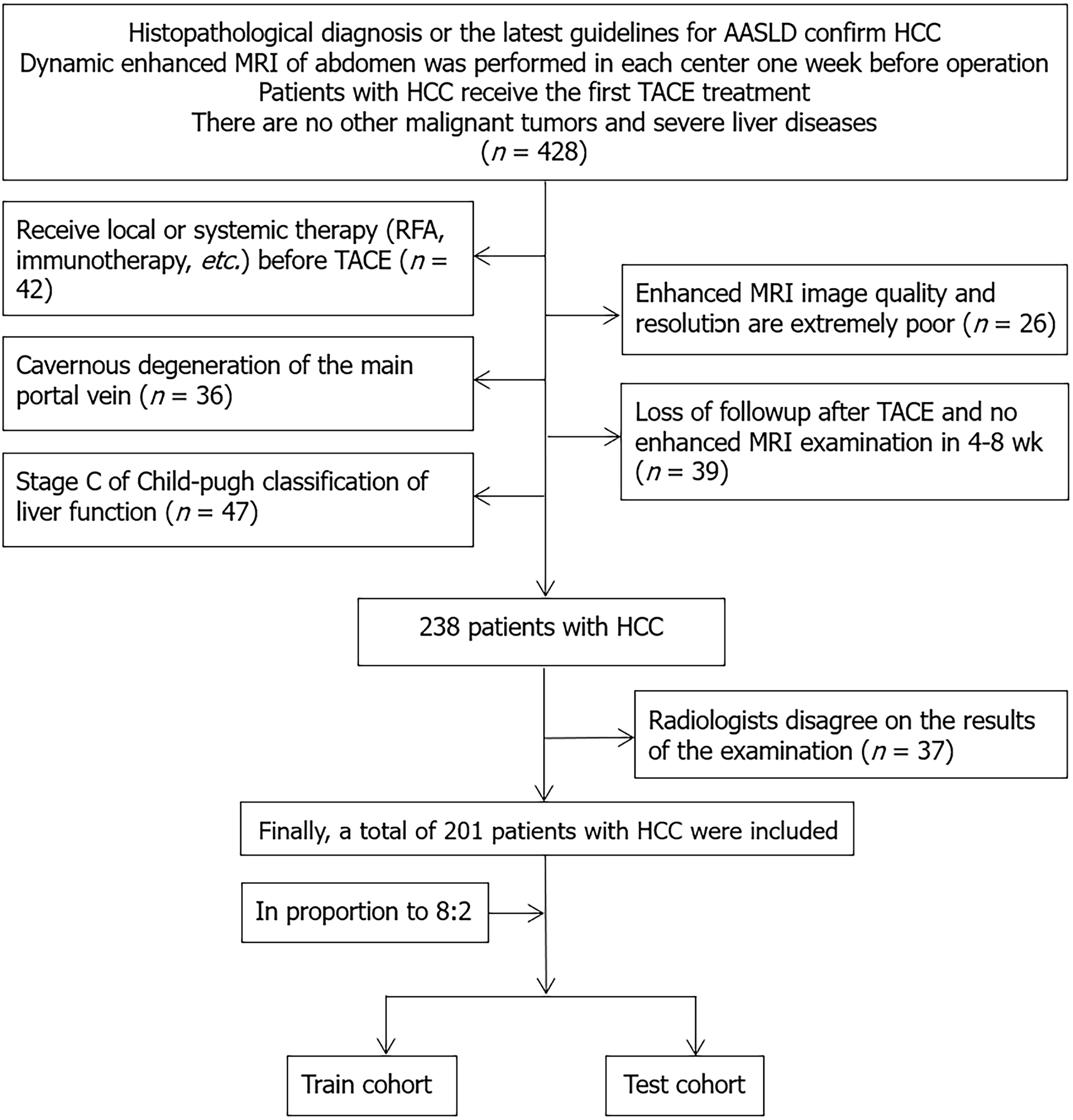
We retrospectively reviewed and collected data from 329 patients who underwent TACE at different medical centers from January 2021 to April 2023. The inclusion criteria for this study were as follows: (1) Had HCC classified as Child-Pugh grade A or B; (2) lacked indications for surgery or refused surgical intervention; (3) received TACE treatment before surgical procedures; and (4) underwent MRI scans within 1 wk before TACE and 4-8 wk after TACE. The exclusion criteria for patients were as follows: (1) Had Child-Pugh grade C disease; (2) received TACE, surgery, targeted drug therapy, or radiochemotherapy before admission; (3) failed to undergo timely follow-up examinations before and after TACE as needed; and (4) had concomitant other malignant tumors. A total of 201 patients met the inclusion criteria and were randomly allocated to a training cohort (n = 163) and a validation cohort (n = 38) at an 8:2 ratio as illustrated in the flowchart in Figure 1. Among these patients, 140 were from the Second Affiliated Hospital of Shandong First Medical University, 42 from Shandong Cancer Hospital, and 19 from the First Affiliated Hospital of Xinjiang Medical University. Clinical data and imaging features were collected from these patients within 1 wk before surgery (Figure 1).
Digital subtraction angiography was employed to guide the TACE procedure. Initially, the hepatic duct was catheterized to perform angiography of the abdominal aorta and superior mesenteric artery, enabling the precise identification of the tumor location, vascular supply, and presence of collateral circulation. After a microcatheter was selectively inserted into the tumor-feeding artery through the hepatic artery, the following chemical agents and embolic agents were chosen: iodized oil (5-20 mL), platinum-based agents (10-40 mg; C-TACE), or drug-eluting microspheres (CalliSpheres, 100-300 μm) with adsorbed doxorubicin (40-80 mg; DEB-TACE). Postoperatively, abnormal staining of the tumor and the disappearance of abnormal feeding arteries were evaluated. The TACE procedure was performed by two interventional surgeons with more than 10 years of experience each.
MRI scans were conducted using Siemens (Prisma, MAOMOM) and GE Healthcare (750w Discovery, HDi Signa) 3.0T magnetic resonance scanners before and after TACE treatment. Dynamic contrast-enhanced MRI was achieved by injecting gadopentetate dimeglumine (Guangzhou Kangchen Pharmaceutical Co., Ltd.) at a dose of 0.1 mmol/kg via the median cubital vein at a rate of 2.5 mL/s. The scanning parameters included the following: (1) Spectral presaturation inversion recovery T2-weighted sequence (3000/200 ms repetition time/echo time, 7 mm slice thickness, 1 mm interslice gap, 200 × 195 matrix size); (2) breath-hold axial scan and contrast-enhanced mdIXON-T1WI (water) sequence (3.6/1.31/2.2 ms repetition time/TE1/TE2; 400-314 mm field of view, 5 mm slice thickness, -2.5 mm interslice gap, 224 × 166 matrix size), divided into dynamic phases: Hepatic arterial phase (AP, 15 s), portal venous phase (PVP, 50 s), parenchymal phase (90 s), and delayed phase (DP, 180 s); and (3) breath-hold diffusion-weighted axial echo-planar sequence (2500/64 ms repetition time/TE; 40-343 mm field of view, 7 mm slice thickness). MRI images were exported in DICOM format for the AP, PVP, and DP.
After obtainment of the original images for the AP, PVP, and DP, image preprocessing, including normalization and uniform voxel sizing, was performed. The open-source software ITK-SNAP was used to manually outline regions of interest (ROI) along the tumor contours, and Python 3.7-based open-source software was used for ROI feature extraction. Radiomic features were extracted from the AP, PVP, and DP images.
Tumor feature selection involved inter- and intraobserver assessments. Features with intraclass correlation coefficient values consistently exceeding 0.80, indicating satisfactory repeatability and high stability of radiomic features, were retained for further dimensionality reduction. Redundant features with Spearman rank correlation coefficients greater than 0.90 were eliminated. The least absolute shrinkage and selection operator (LASSO) algorithm with 10-fold cross-validation was applied for penalty parameter tuning to identify the most valuable features. Radiomics scores were computed as a linear combination of the selected features multiplied by their corresponding λ coefficients. The final features were input into machine learning models, such as logistic regression and support vector machine models, for model building.
ResNet series models were selected as pretrained CNN models on the Python 3.7 platform to extract deep transfer learning (DTL)[19]. The images representing the maximum tumor area ROI for each patient were extracted, and principal component analysis was applied to compress the DL features. Z score normalization was performed on the compressed features. LASSO was used to filter out features with nonzero coefficients, selecting and reducing the fused features to obtain the optimal subset of fused features.
The data extraction features were obtained based on radiomic features (Rad_Sig, Rad Signature) and DTL. A pre-fusion of DL (DTL) + radiomics (Rad_Sig) features was performed to obtain DLR features. Thus, the feature data followed the same path and consistency for radiomics and DL features. After LASSO feature selection, the final features were input into machine learning models for predictive modeling, resulting in the final DLR features.
Cox analysis was also conducted to predict the independent risk factors affecting patient PFS. Clinical-radiomics-deep (transfer) learning models were established for AP, PVP, DP, and their combined three-phase data, constructing nomograms containing features from different phases. Receiver operating characteristic (ROC) curves were plotted to evaluate the diagnostic performance of the nomograms, and the Delong test was used to assess the ROC curve values. K-M analysis was applied to analyze PFS after TACE for 2 years according to different risk stratifications. The detailed workflow is illustrated in Figure 2.
All the statistical tests were performed using SPSS 26.0 (IBM, New York, United States) to analyze the baseline data of the patients. For normally distributed data, t tests were used to compare the differences in efficacy after TACE between the training and testing cohorts. Feature extraction and selection were conducted using the LASSO regression model with 10-fold cross-validation in Python 3.7. Multivariate analysis was initially performed using the Cox proportional hazards regression model, followed by univariate analysis. The C-index was used to evaluate the specificity and value of the predictive model.
A total of 201 HCC patients treated with TACE were included in this study (150 males, 51 females; age range 32-86 years, median age 58 years). Basic clinical and radiological feature data were collected preoperatively. Apart from tumor size and the presence of encapsulation (P value < 0.05). Univariate and multivariate Cox regression analyses for survival time TACE revealed that tumor diameter and encapsulation were independent prognostic factors (P value < 0.05, Table 1).
| Characteristics | Train cohort (n = 163) | P value | Test cohort (n = 38) | P value | Univariate analysis | Multivariate analysis | ||
| OR | P value | OR | P value | |||||
| Age | 58.82 ± 10.36 | 0.178 | 57.76 ± 10.56 | 0.489 | 1.004 | 0.205 | ||
| PIVKA | 152.98 ± 182.31 | 0.191 | 112.56 ± 129.18 | 0.128 | 1 | 0.577 | ||
| AST | 105.43 ± 120.89 | 0.842 | 74.66 ± 65.83 | 0.572 | 0.999 | 0.027 | 1 | 0.684 |
| ALT | 45.54 ± 59.10 | 0.211 | 50.20 ± 38.58 | 0.093 | 0.999 | 0.287 | ||
| GGT | 82.91 ± 84.65 | 0.166 | 99.26 ± 71.53 | 0.087 | 1 | 0.333 | ||
| AFP | 68.05 ± 100.64 | 0.239 | 105.27 ± 102.15 | 0.014 | 0.999 | 0.005 | 1 | 0.187 |
| Platelet | 143.44 ± 80.10 | 0.554 | 153.53 ± 112.02 | 0.153 | 1 | 0.907 | ||
| Tumor diameter | 7.25 ± 4.08 | < 0.001 | 7.44 ± 3.84 | 0.001 | 0.947 | 0.001 | 0.94 | 0.001 |
| Gender | 0.386 | 0.772 | 0.956 | 0.546 | ||||
| Female | 38 (23.31) | 13 (34.21) | ||||||
| Male | 125 (76.69) | 25 (65.79) | ||||||
| Hepatitis | 0.866 | 0.542 | 0.954 | 0.468 | ||||
| No | 88 (53.99) | 17 (44.74) | ||||||
| Yes | 75 (46.01) | 21 (55.26) | ||||||
| Diolame | 0.012 | 0.049 | 1.267 | 0.001 | 1.233 | 0.001 | ||
| No | 51 (31.29) | 18 (47.37) | ||||||
| Yes | 112 (68.71) | 20 (52.63) | ||||||
| Metastasis | 0.461 | 0.078 | 0.889 | 0.082 | ||||
| No | 103 (63.19) | 25 (65.79) | ||||||
| Yes | 60 (36.81) | 13 (34.21) | ||||||
| Necrosis | 0.081 | 0.542 | 0.869 | 0.032 | 1.101 | 0.14 | ||
| No | 90 (55.21) | 17 (44.74) | ||||||
| Yes | 73 (44.79) | 21 (55.26) | ||||||
| PVTT | 0.283 | 0.333 | 0.895 | 0.095 | ||||
| No | 106 (65.03) | 12 (31.58) | ||||||
| Yes | 57 (34.97) | 26 (68.42) | ||||||
| Ascites | 0.068 | 0.175 | 0.816 | 0.014 | 0.954 | 0.538 | ||
| No | 127 (77.91) | 36 (94.74) | ||||||
| Yes | 36 (22.09) | 2 (5.26) | ||||||
| Regular | 0.299 | 0.127 | 1.134 | 0.056 | ||||
| No | 70 (42.94) | 20 (52.63) | ||||||
| Yes | 93 (57.06) | 18 (47.37) | ||||||
| BCLC Stage | 0.088 | 0.081 | 0.842 | 0.009 | 1.095 | 0.181 | ||
| A stage | 96 (58.90) | 19 (50.00) | ||||||
| B stage | 67 (41.10) | 19 (50.00) | ||||||
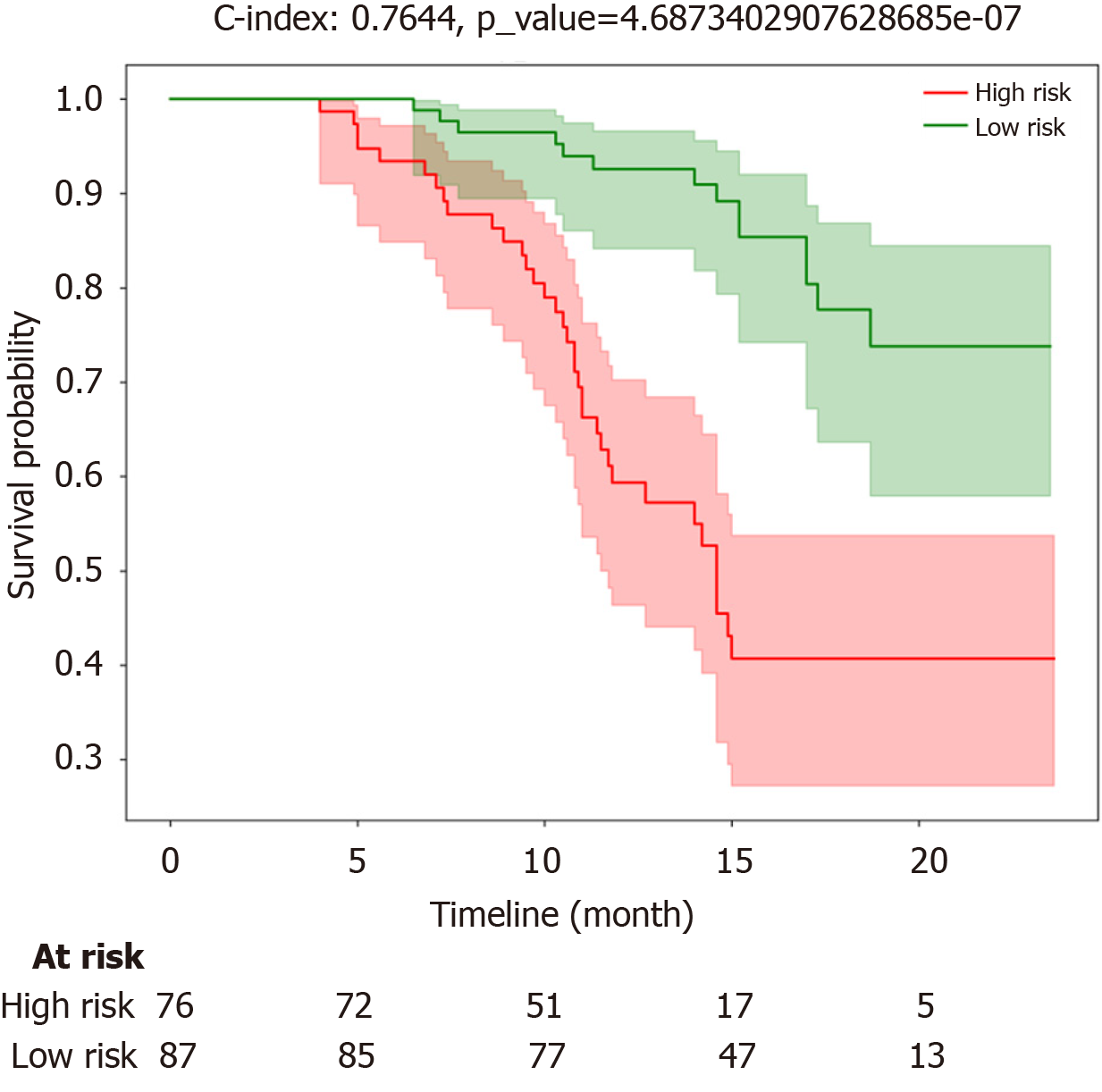
The tumor diameter and capsule of the tumor were classified according to the above multivariate analysis. K-M survival analysis revealed significantly shorter PFS in the high-risk group (red curve) than in the low-risk group (green curve). The median survival time of patients in the high-risk group was 12.8 months, with predicted probabilities at 6 months, 1 year, and 2 years being 92%, 60%, and 40%, respectively, and a survival time of 14.3 months. The low-risk group exhibited a median survival time of 14.2 months, with predicted probabilities at 6 months, 1 year, and 2 years being 98%, 90%, and 73%, respectively. In the training cohort, the C-index was 0.764, indicating a relatively good probability of consistency between the predicted and observed results (log-rank P < 0.001; Figure 3).
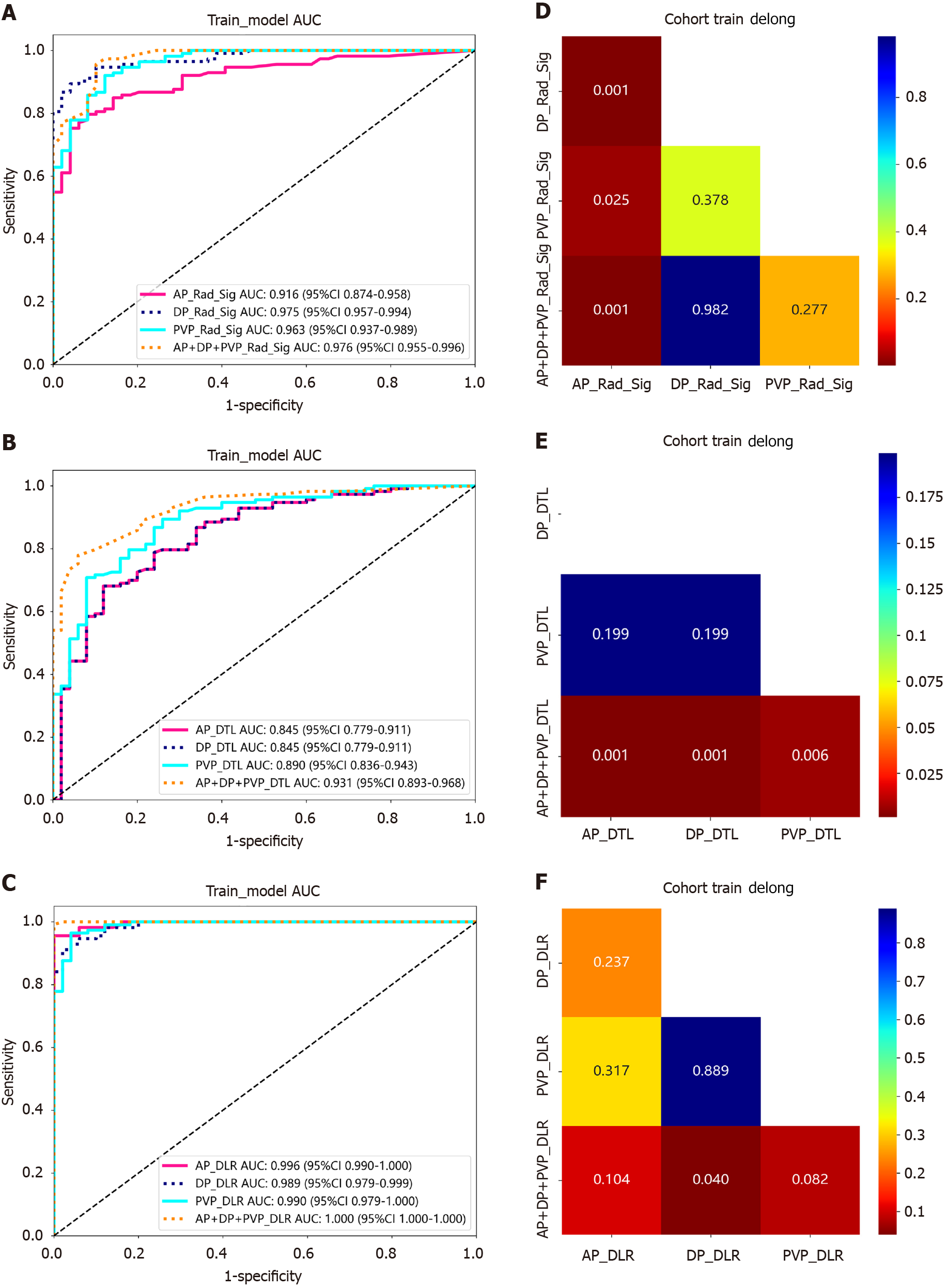
With respect to the training cohort, models constructed using different phases and combined phases demonstrated that the area under the curve (AUC) of the AP + PVP + DP + radiomics features model was significantly greater than that of the single-phase radiomic-feature models. The AUC values of the radiomic features for constructing DLRs were markedly greater than those for Rad_Sig and DTL (Figure 4A-C). DeLong tests were also conducted to assess the value of the different models in the training cohort. When comparing the AP_Rad_Sig radiomics features with the AP + PVP + DP_Rad_Sig, PVP_Rad_Sig, and DP_Rad_Sig radiomic-feature models, we detected statistically significant differences (P < 0.05, Figure 4D). Similarly, when comparing the AP + PVP + DP_DTL features with the AP_DTL, DP_DTL, and PVP_DTL features, significant differences were observed (P < 0.05, Figure 4E). However, there were significant differences in the AP + PVP + DP_DLR features compared to the DP_DLR features (P < 0.05, Figure 4F).
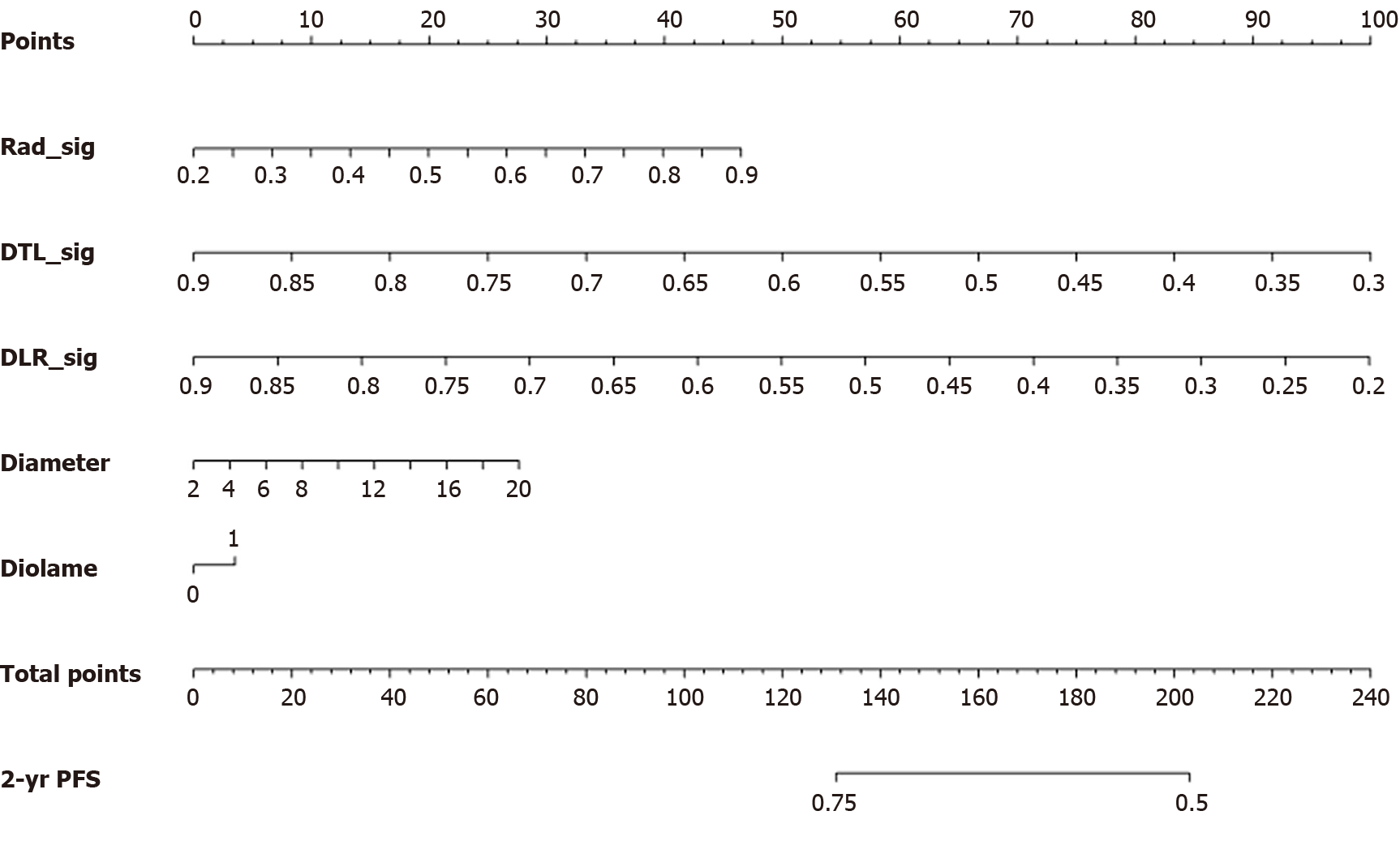
Using multiphase enhanced MRIs, a model combining clinical and radiomic features was constructed, and relevant radiomic features and prognostic risk factors were incorporated to predict 2-year PFS after TACE. Both radiomic features and risk factors were scored, with a total score ranging from 0 to 240. In this nomogram, only DTL_Sig and DLR_Sig can predict the probability of PFS. The chart indicates that the higher the total score, the lower the predicted 2-year PFS (Figure 5).
TACE post-treatment radiomics, a noninvasive computer-aided technique, explores high-throughput features to quantify tumor heterogeneity and invasiveness[20,21]. It describes patterns or spatial distributions of voxel intensities within the tumor's ROI from different perspectives to obtain comprehensive information about the tumor's internal aspects that are not observable to the naked eye. The degree of ischemia and necrosis after TACE treatment is usually associated with individual differences and spatial heterogeneity of the tumor[20].Therefore, in this study, based on enhanced MRI data, a model was developed for predicting PFS using a combination of single- and multiphase radiomic features and survival curve analysis.
In general, there was a statistically significant difference in tumor size and tumor encapsulation between the training and testing cohorts. Single-factor and multifactor Cox regression analyses revealed that tumor size and tumor encapsulation were independent risk factors affecting patient prognosis, indicating that as tumor volume increased, the integrity of the tumor's "pseudocapsule" worsened after expansive and invasive growth, leading to a greater likelihood of distant metastasis and ultimately a poorer prognosis. For operable HCC, recurrence and metastasis are the main causes of poor prognosis. Among patients undergoing curative resection, approximately 70% experience recurrence within 5 years. According to the relevant risk factors in this study, such as tumor size and encapsulation, patients were assigned to high-risk and low-risk groups. The median survival times in the high-risk and low-risk groups were 12.8 months and 14.2 months, respectively, with predicted probabilities at 6 months, 1 year, and 2 years being 92%, 60%, and 40%, respectively, in the high-risk group and 98%, 90%, and 73%, respectively, in the low-risk group. In the training cohort, the C-index was 0.764, indicating relatively good consistency between the predicted and observed results and emphasizing the urgent need for a detection method that can assess survival time earlier preoperatively.
There are many ways to predict the survival of HCC patients[22,23]. With respect to multiphase images in radiomics research, a study compared the predictive efficacy of radiomic models that included arterial, portal venous, and hepatic venous data and various enhancement phases of MRI scans for preoperatively predicting the pathological differentiation degree of a single HCC lesion. The results showed that the predictive performance of the enhanced three-phase radiomic model was the highest[24]. In this study, after obtaining radiomic features for AP, PVP, DP, and AP + PVP + DP, Rad_Sig, DTL, and DLR models were constructed. The optimal model was confirmed to be the DLR model with joint radiomic features of AP + PVP + DP, indicating superior performance in predicting TACE efficacy. The ROC curve demonstrated that the diagnostic performance of the radiomics features in the DLR model was significantly greater than that of the other radiomic features. Delong's test comparing the diagnostic performance of different ROC curves showed no apparent overfitting. In conclusion, multiphase enhanced MRIs can reflect tumor heterogeneity and vascularization patterns. Thus, combining different imaging phases and joint imaging can reveal information related to tumor invasiveness and prognosis. The nomogram can provide individualized predictions post-TACE, with higher total scores indicating more accurate PFS predictions. Previous studies have shown that radiomic feature models can predict post-TACE treatment response and recurrence in HCC patients.
While this was a multicenter retrospective study, it still faced limitations, such as a small sample size and limited follow-up information, leading to inevitable selection bias. Surgical resection and combined targeted and immune therapies were chosen for some patients after successful TACE, greatly influencing patient prognosis. Therefore, PFS was not an indicator of the overall biological behavior of these patients. Future research should focus on more accurate assessments and further investigations. In the future, based on traditional histology and DL, we can further derive the habitat analysis to construct the HCC prognostic risk model to understand the protein and characteristic gene expression of the tumor after treatment, and evaluate its prognosis[25].
In the era of big data, a variety of artificial intelligence applications began to be used in clinic to serve clinical doctors in different fields. At present, traditional radiological studies based on enhanced MRI have made significant progress in prognostic factors and prognostic prediction of hepatic malignant tumors after TACE. In this study, based on the multi-phase features of enhanced MRI extracted images, DLR_Sig omics features were obtained based on traditional radiology and DL to truly reflect the internal heterogeneity of the tumor and predict the prognosis of liver malignant tumors after TACE treatment. Therefore, it is safe and effective to construct a combined model to predict the PFS map of patients with liver cancer treated with TACE. In the future, the joint feature model of multitemporal imaging can be used as a new strategy to predict PFS in patients with liver cancer undergoing TACE.
We would like to express our great appreciation to the editor and anonymous reviewers for their comments, which helped us to improve the quality of our paper. And for advice regarding the code used in this revised manuscript, we thank OnekeyAI platform and its developers.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Sonntag R, Germany S-Editor: Lin C L-Editor: A P-Editor: Li X
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3170] [Article Influence: 528.3] [Reference Citation Analysis (37)] |
| 2. | Akce M, El-Rayes BF, Wajapeyee N. Combinatorial targeting of immune checkpoints and epigenetic regulators for hepatocellular carcinoma therapy. Oncogene. 2023;42:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3026] [Article Influence: 432.3] [Reference Citation Analysis (3)] |
| 4. | Yuan M, Chen TY, Chen XR, Lu YF, Shi J, Zhang WS, Ye C, Tang BZ, Yang ZG. Identification of predictive factors for post-transarterial chemoembolization liver failure in hepatocellular carcinoma patients: A retrospective study. World J Clin Cases. 2022;10:8535-8546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Reference Citation Analysis (0)] |
| 5. | Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 6. | Li J, Kong M, Yu G, Wang S, Shi Z, Han H, Lin Y, Shi J, Song J. Safety and efficacy of transarterial chemoembolization combined with tyrosine kinase inhibitors and camrelizumab in the treatment of patients with advanced unresectable hepatocellular carcinoma. Front Immunol. 2023;14:1188308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Lee G, Bak SH, Lee HY. CT Radiomics in Thoracic Oncology: Technique and Clinical Applications. Nucl Med Mol Imaging. 2018;52:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Möller K, Safai Zadeh E, Görg C, Dong Y, Cui X, Lim A, de Molo C, Serra C, Martín Algíbez A, Berzigotti A, Piscaglia F, Faiss S, Dietrich CF. Focal Liver Lesions other than Hepatocellular Carcinoma in Cirrhosis: Diagnostic Challenges. J Transl Int Med. 2022;10:308-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Reference Citation Analysis (0)] |
| 9. | Ji GW, Zhang YD, Zhang H, Zhu FP, Wang K, Xia YX, Jiang WJ, Li XC, Wang XH. Biliary Tract Cancer at CT: A Radiomics-based Model to Predict Lymph Node Metastasis and Survival Outcomes. Radiology. 2019;290:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 10. | Park H, Lim Y, Ko ES, Cho HH, Lee JE, Han BK, Ko EY, Choi JS, Park KW. Radiomics Signature on Magnetic Resonance Imaging: Association with Disease-Free Survival in Patients with Invasive Breast Cancer. Clin Cancer Res. 2018;24:4705-4714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 11. | Liu D, Liu F, Xie X, Su L, Liu M, Kuang M, Huang G, Wang Y, Zhou H, Wang K, Lin M, Tian J. Accurate prediction of responses to transarterial chemoembolization for patients with hepatocellular carcinoma by using artificial intelligence in contrast-enhanced ultrasound. Eur Radiol. 2020;30:2365-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 12. | Sun Y, Bai H, Xia W, Wang D, Zhou B, Zhao X, Yang G, Xu L, Zhang W, Liu P, Xu J, Meng S, Liu R, Gao X. Predicting the Outcome of Transcatheter Arterial Embolization Therapy for Unresectable Hepatocellular Carcinoma Based on Radiomics of Preoperative Multiparameter MRI. J Magn Reson Imaging. 2020;52:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Weng W, Lü XL, Zhang QQ, Zhao XM, Chen CM, Kong CL, Lu CY, Chen MJ, Ji JS. [Prediction of short-term prognosis of hepatocellular carcinoma after TACE surgery based on MRI texture analysis technology]. Zhonghua Yi Xue Za Zhi. 2020;100:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Peng J, Kang S, Ning Z, Deng H, Shen J, Xu Y, Zhang J, Zhao W, Li X, Gong W, Huang J, Liu L. Residual convolutional neural network for predicting response of transarterial chemoembolization in hepatocellular carcinoma from CT imaging. Eur Radiol. 2020;30:413-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 15. | Tian Y, Hua H, Peng Q, Zhang Z, Wang X, Han J, Ma W, Chen J. Preoperative Evaluation of Gd-EOB-DTPA-Enhanced MRI Radiomics-Based Nomogram in Small Solitary Hepatocellular Carcinoma (≤3 cm) With Microvascular Invasion: A Two-Center Study. J Magn Reson Imaging. 2022;56:1459-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Song W, Yu X, Guo D, Liu H, Tang Z, Liu X, Zhou J, Zhang H, Liu Y. MRI-Based Radiomics: Associations With the Recurrence-Free Survival of Patients With Hepatocellular Carcinoma Treated With Conventional Transcatheter Arterial Chemoembolization. J Magn Reson Imaging. 2020;52:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Wang F, Cheng M, Du B, Li LM, Huang WP, Gao JB. Use of radiomics containing an effective peritumoral area to predict early recurrence of solitary hepatocellular carcinoma ≤5 cm in diameter. Front Oncol. 2022;12:1032115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Liu S, Sun W, Yang S, Duan L, Huang C, Xu J, Hou F, Hao D, Yu T, Wang H. Deep learning radiomic nomogram to predict recurrence in soft tissue sarcoma: a multi-institutional study. Eur Radiol. 2022;32:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Romero M, Interian Y, Solberg T, Valdes G. Targeted transfer learning to improve performance in small medical physics datasets. Med Phys. 2020;47:6246-6256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Yang G, Yan H, Tang Y, Yuan F, Cao M, Ren Y, Li Y, He Z, Su X, Yao Z, Deng M. Advancements in understanding mechanisms of hepatocellular carcinoma radiosensitivity: A comprehensive review. Chin J Cancer Res. 2023;35:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 21. | Lévi-Strauss T, Tortorici B, Lopez O, Viau P, Ouizeman DJ, Schall B, Adhoute X, Humbert O, Chevallier P, Gual P, Fillatre L, Anty R. Radiomics, a Promising New Discipline: Example of Hepatocellular Carcinoma. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Li H, Lin Z, Zhuo J, Yang M, Shen W, Hu Z, Ding Y, Chen H, He C, Yang X, Dong S, Wei X, Sun B, Zheng S, Lang R, Lu D, Xu X. TNFR2 is a potent prognostic biomarker for post-transplant lung metastasis in patients with hepatocellular carcinoma. Chin J Cancer Res. 2023;35:66-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Liu Y, Liu Y, Ye S, Feng H, Ma L. A new ferroptosis-related signature model including messenger RNAs and long non-coding RNAs predicts the prognosis of gastric cancer patients. J Transl Int Med. 2023;11:145-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Kim S, Shin J, Kim DY, Choi GH, Kim MJ, Choi JY. Radiomics on Gadoxetic Acid-Enhanced Magnetic Resonance Imaging for Prediction of Postoperative Early and Late Recurrence of Single Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:3847-3855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 25. | Zhang GX, Ding XS, Wang YL. Prognostic model of hepatocellular carcinoma based on cancer grade. World J Clin Cases. 2023;11:6383-6397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |