Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.3188
Revised: April 9, 2024
Accepted: May 6, 2024
Published online: June 16, 2024
Processing time: 123 Days and 2.4 Hours
Low grade serous carcinoma of the ovary (LGSOC) is a rare type of epithelial ova
We report a 54 years old postmenopausal woman who was hospitalized with a lower abdominal mass and underwent surgical treatment. The final pathological confirmation was low-grade serous carcinoma of the right ovary and low-grade serous carcinoma of the left fallopian tube. No special treatment was performed after the surgery, and the patient was instructed to undergo regular follow-up without any signs of disease progression.
The prognosis of LGSOC is relatively good, over 80% of patients still experience disease recurrence.
Core Tip: Although the prognosis of low grade serous carcinoma of the ovary (LGSOC) is relatively good, over 80% of patients still experience disease recurrence. In most cases, these patients are relatively young and have the potential to receive active treatment and achieve a longer survival period. The treatment methods for LGSOC recurrence include re-tumor reduction surgery, chemotherapy, targeted therapy, hormone therapy and clinical trials. Currently, a large amount of research focuses on gene testing and targeted therapy. I hope that the progress of genome research and targeted therapy can change the therapeutic prospects of LGSOC and bring better therapeutic effects to patients with this disease.
- Citation: Bai SN, Wu Q, Song LY. Primary ovarian cancer combined with primary fallopian tube cancer: A case report. World J Clin Cases 2024; 12(17): 3188-3193
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/3188.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.3188
Low grade serous carcinoma of the ovary (LGSOC) is a rare type of epithelial ovarian cancer. Primary fallopian tube cancer is the lowest incidence rate among female reproductive system malignancies. The origin of ovarian cancer has always been a hot topic in gynecological oncology research, and some scholars believe that the origin of ovarian malignant tumors is the fallopian tubes.
We present a case of ovarian cancer combined with fallopian tube cancer in this manuscript.
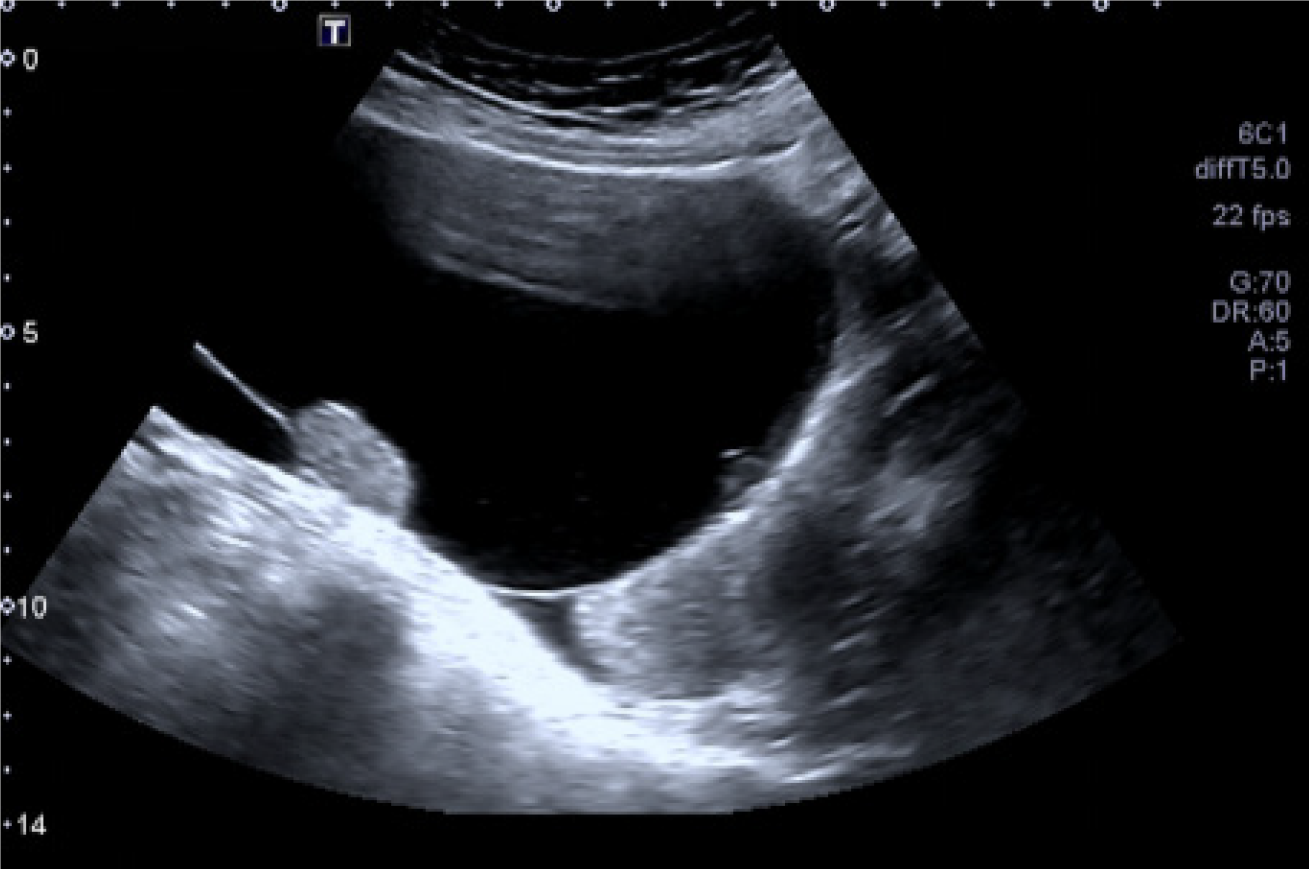
A 54 years old postmenopausal woman visited our hospital on July 30, 2023 due to the discovery of a lower abdominal mass for half a month. She has no abnormal vaginal bleeding or discharge, and no abdominal pain. Her gynecological ultrasound showed a pelvic cyst (A liquid dark area of approximately 117 mm × 97 mm × 89 mm in size can be seen in front of the uterus, with good internal sound transmission. Several hyperechoic masses can be seen attached to the wall, with the larger area being approximately 28 mm × 31 mm × 19 mm in size) (Figure 1).
The patient has a history of good physical health and no history of chronic diseases.
Her menstrual cycle is regular, with a menarche age of 16 years old, a menstrual period of 5 d, and a menstrual cycle of 28-30 d. At the age of 52, she experienced menopause and had no history of dysmenorrhea or irregular vaginal bleeding. She had two children and had a history of induced abortion. Her body mass index (BMI) is 22.0. She has not undergone regular gynecological examinations before.
There is no history of malignant tumors in her family.
After the patient checked into our department, we conducted a detailed physical examination on her, and no abnor
After the patient was admitted, all preoperative examinations were completed, and her female tumor markers showed no abnormalities, while the gastroscopy examination showed no abnormalities. The postoperative pathological report of this patient is as follows: (1) Low grade serous carcinoma of the right ovary, with no intravascular tumor thrombus or nerve infiltration. No cancer was found in the right fallopian tube. Immunohistochemical staining: P53 (wild-type), ER (partial+), PR (rarely+), CA125 (+), CK7 (+), CK20 (-), Ki-67 (about 20%+), P16 (-), WT-1 (+), Villin (-), HNF1-Beta (small focal weak+); (2) Low grade serous carcinoma of the left fallopian tube. No cancer was found in the left ovary; (3) No cancer was found in the greater omentum. No cancer was found in the peritoneum of the left and right pelvic walls, the peritoneum of the left and right colonic sulcus, the peritoneum of the rectal fossa, and the retroperitoneum of the bladder; (4) No cancer metastasis found in lymph nodes; and (5) No cancer cells were found in the peritoneal lavage solution.
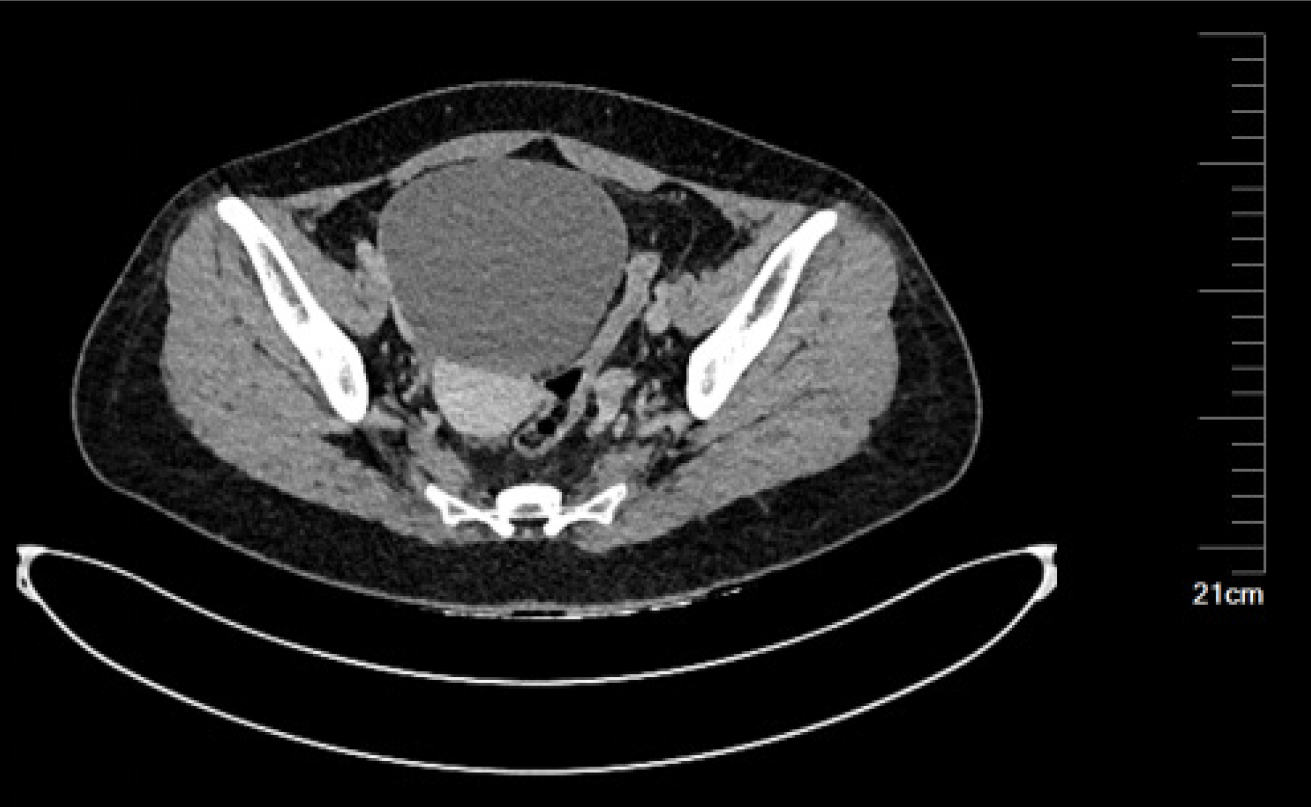
Her pelvic computed tomography (CT) showed a circular cystic solid mass shadow on the right adnexal area of the pelvic cavity, with clear boundaries and a size of approximately 105 mm × 113 mm × 126 mm, no obvious enhancement was observed in the cystic part of the enhanced scan, and the local thickening of the cyst wall was not uniformly enhanced. Linear enhancement septa were visible inside the cyst, and the solid components inside the cyst showed obvious progressive enhancement (Figure 2).
The final diagnosis of this patient is: (1) Primary ovarian low-grade serous carcinoma stage IA; and (2) Primary fallopian tube low-grade serous carcinoma stage IA.
On August 3, 2023, we performed surgical treatment on the patient. During the operation, we found no ascites in the abdominal cavity, the uterus was smaller than normal, and a tumor with a diameter of about 10 cm was visible in the right ovary, mainly cystic, with a smooth surface. There were no obvious abnormalities in the appearance of the right fallopian tube and left appendix. We removed the right accessory and opened it. After opening, we found solid cauliflower like tissue inside the cyst, which was sent for intraoperative frozen pathological examination. The frozen pathological result is a cystic adenoid tumor (right attachment), locally infiltrating carcinoma, to be further confirmed by paraffin section. During the surgery, we explored the omentum, colon, small intestine, appendix, bladder, liver, stomach, and spleen, but no cancer nodules were found on the surface. The diaphragm and peritoneum were smooth, and no lesions were found. During the surgery, we explained the patient's condition to the authorized person and obtained informed consent. After obtaining informed consent, we performed ovarian cancer staging surgery on the patient, including transabdominal total hysterectomy, bilateral ovarian and fallopian tube resection, greater omentum resection, pelvic and paraaortic lymph node resection, and peritoneal multipoint biopsy.
We didn't provide her with additional treatment after the surgery, we just suggested that she needs regular follow-up.
LGSOC is a rare type of epithelial ovarian cancer. Its incidence rate is low, accounting for 2% of all epithelial ovarian cancer and 4.7% of serous ovarian cancer[1]. It is reported that primary fallopian tube cancer is the lowest incidence rate among female reproductive system malignancies. There are only a few reports in the literature, but the mortality rate is very high. However, in reality, fallopian tube cancer is very common in clinical work, but in most cases, it is considered a type of ovarian cancer, resulting in limited reports on primary fallopian tube cancer. Because the incidence rate of LGSOC is low, there is no clear guidance and consensus at present. This article describes the LGSOC by providing a case of primary LGSOC with primary low-grade serous carcinoma of the fallopian tube.
LGSOC generally grows slowly and has a longer course. Its staging distribution is similar to that of high grade serous ovarian carcinoma (HGSOC), with 80% of patients diagnosed as advanced[2]. Compared with HGSOC, LGSOC has unique molecular biological characteristics and clinical manifestations, therefore, its treatment method is also different from HGSOC. With the establishment of the binary classification theory of ovarian serous cancer, many scholars have been prompted to study LGSOC as an independent rare disease. Due to its low incidence rate and limited number of cases reported at present, its etiology and prognostic factors are not completely clear. The onset age of LGSOC is relatively young, with a median onset age between 43 and 47 years old[3,4]. Currently, there are no clear genetic or environmental susceptibility factors for LGSOC. Some scholars believe that LGSOC may originate from benign ovarian serous tumors, then transform into borderline serous tumors, and ultimately develop into low-grade serous cancer[5], or it can itself be low-grade serous cancer. A comprehensive population study conducted by Vang et al[6] included 1042 cases of ovarian borderline tumors diagnosed in Denmark between 1978 and 2002, of which 4% developed ovarian cancer, 93% had low-grade serous cancer, and 7% had high-grade serous cancer. At present, several studies have found that age of first onset, BMI, and smoking status may be associated with the prognosis of LGSOC patients[3,4,7]. Among patients in stage II-IV, patients diagnosed after 35 years old have longer progression free survival and overall survival compared to patients diagnosed before 35 years old, and the likelihood of tumor progression and recurrence is also lower. Higher BMI (≥ 35 kg/m2) and smoking both increase the likelihood of disease progression and recurrence in LGSOC patients, shortening their survival time. The characteristic of LGSOC is the abnormal mitogen activated protein kinase (MAPK) pathway, which is a major intracellular signaling pathway responsible for regulating important cellular activities such as cell survival, proliferation, migration, and angiogenesis. MAPK mediates the transmission of growth signals to the nucleus. Mutations in upstream regulatory factors BRAF or KRAS of the MAPK pathway lead to constitutive ac
In the early stages of LGSOC, there may be no symptoms. As the disease progresses, there may be abdominal dis
Surgical treatment is the cornerstone of LGSOC treatment, and the most basic surgical methods for early patients without fertility requirements are total hysterectomy, bilateral fallopian tube oophorectomy, pelvic and paraaortic lymph node resection, omentectomy, and peritoneal multipoint biopsy. For advanced patients, tumor cell reduction surgery is necessary, and every effort must be made to remove all visible metastatic lesions. Many scholars have discussed the impact of residual diseases on the prognosis of LGSOC. In the auxiliary analysis of GOG182, Fader et al[15] published a study on the survival rate of 189 LGSOC patients, and only residual disease status was significantly correlated with survival rate. The median progression free survival (PFS) of residues smaller than 1 cm and larger than 1 cm under the microscope were 32.2 months, 14.65 months, and 14.1 months, respectively. Similarly, the median overall survival of microscopic residues smaller than 1 cm and larger than 1 cm were 96.6 months, 45 months, and 42 months, respectively. After controlling for other variables, the disease progression adjusted hazard ratio (HR) for LGSOC and measurable residual disease patients after initial cell reduction surgery was 2.28, and the death HR was 2.12, which is comparable to the results of HGSOC for measurable diseases[16,17]. In addition, Gershenson et al[3] concluded that compared to the absence of visible lesions at the end of the surgery, the presence of measurable lesions at the end of the initial cytoreductive surgery increases the risk of disease progression or recurrence, as well as the risk of death. Vatansever et al[18] also reached the same conclusion. For early patients with fertility requirements, surgery to preserve fertility function can preserve the patient's hope of pregnancy while treating the disease, and is suitable for patients of childbearing age from IA to IC1. Many scholars have conducted retrospective studies on the safety of fertility preserving surgery, and have concluded that it is safe and does not increase mortality for early patients[19,20]. Angiogenesis has been proven to play an important role in the occurrence and development of tumors. Bevacizumab is a monoclonal antibody that can inhibit vascular endothelial growth factor, but its application in the treatment of LGSOC has not yet been supported by high-level evidence, and further research on its therapeutic effect requires large-scale clinical trials. Hormone maintenance therapy after adjuvant chemotherapy can improve prognosis. Gershenson et al[21] retrospectively studied the results of hormone maintenance therapy in 203 patients with stage II-IV LGSOC. The study found that patients who added hormone maintenance therapy after surgery and chemotherapy had significantly longer PFS and lower risk of disease progression compared to those who chose to observe after surgery and chemotherapy[21].
Although the prognosis of LGSOC is relatively good, over 80% of patients still experience disease recurrence. In most cases, these patients are relatively young and have the potential to receive active treatment and achieve a longer survival period. The treatment options for LGSOC recurrence include re-tumor reduction surgery, chemotherapy, targeted therapy, hormone therapy and clinical trials. Currently, a large amount of research focuses on gene testing and targeted therapy. I hope that the progress of genome research and targeted therapy can change the therapeutic prospects of LGSOC and bring better therapeutic effects to patients with this disease.
| 1. | Matsuo K, Machida H, Grubbs BH, Sood AK, Gershenson DM. Trends of low-grade serous ovarian carcinoma in the United States. J Gynecol Oncol. 2018;29:e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 2. | Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, Silva EG. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 479] [Article Influence: 22.8] [Reference Citation Analysis (33)] |
| 3. | Gershenson DM, Bodurka DC, Lu KH, Nathan LC, Milojevic L, Wong KK, Malpica A, Sun CC. Impact of Age and Primary Disease Site on Outcome in Women With Low-Grade Serous Carcinoma of the Ovary or Peritoneum: Results of a Large Single-Institution Registry of a Rare Tumor. J Clin Oncol. 2015;33:2675-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (33)] |
| 4. | Gershenson DM. Low-grade serous carcinoma of the ovary or peritoneum. Ann Oncol. 2016;27 Suppl 1:i45-i49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (33)] |
| 5. | Kurman RJ, Shih IeM. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol. 2016;186:733-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 715] [Article Influence: 79.4] [Reference Citation Analysis (33)] |
| 6. | Vang R, Hannibal CG, Junge J, Frederiksen K, Kjaer SK, Kurman RJ. Long-term Behavior of Serous Borderline Tumors Subdivided Into Atypical Proliferative Tumors and Noninvasive Low-grade Carcinomas: A Population-based Clinicopathologic Study of 942 Cases. Am J Surg Pathol. 2017;41:725-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (33)] |
| 7. | Schlumbrecht MP, Sun CC, Wong KN, Broaddus RR, Gershenson DM, Bodurka DC. Clinicodemographic factors influencing outcomes in patients with low-grade serous ovarian carcinoma. Cancer. 2011;117:3741-3749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (34)] |
| 8. | Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3924] [Cited by in RCA: 4008] [Article Influence: 167.0] [Reference Citation Analysis (33)] |
| 9. | Wong KK, Lu KH, Malpica A, Bodurka DC, Shvartsman HS, Schmandt RE, Thornton AD, Deavers MT, Silva EG, Gershenson DM. Significantly greater expression of ER, PR, and ECAD in advanced-stage low-grade ovarian serous carcinoma as revealed by immunohistochemical analysis. Int J Gynecol Pathol. 2007;26:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 10. | Vineyard MA, Daniels MS, Urbauer DL, Deavers MT, Sun CC, Boerwinkle E, Bodurka DC, Gershenson DM, Crawford J, Lu KH. Is low-grade serous ovarian cancer part of the tumor spectrum of hereditary breast and ovarian cancer? Gynecol Oncol. 2011;120:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 11. | O'Neill CJ, Deavers MT, Malpica A, Foster H, McCluggage WG. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol. 2005;29:1034-1041. [PubMed] |
| 12. | Singer G, Stöhr R, Cope L, Dehari R, Hartmann A, Cao DF, Wang TL, Kurman RJ, Shih IeM. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 318] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 13. | Piek JM, van Diest PJ, Zweemer RP, Kenemans P, Verheijen RH. Tubal ligation and risk of ovarian cancer. Lancet. 2001;358:844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 14. | Piek JM, Verheijen RH, Kenemans P, Massuger LF, Bulten H, van Diest PJ. BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol Oncol. 2003;90:491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 15. | Fader AN, Java J, Krivak TC, Bristow RE, Tergas AI, Bookman MA, Armstrong DK, Tanner EJ, Gershenson DM. The prognostic significance of pre- and post-treatment CA-125 in grade 1 serous ovarian carcinoma: a gynecologic Oncology Group study. Gynecol Oncol. 2014;132:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 16. | Babaier A, Mal H, Alselwi W, Ghatage P. Low-Grade Serous Carcinoma of the Ovary: The Current Status. Diagnostics (Basel). 2022;12:458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (2)] |
| 17. | Nickles Fader A, Java J, Ueda S, Bristow RE, Armstrong DK, Bookman MA, Gershenson DM; Gynecologic Oncology Group (GOG)*. Survival in women with grade 1 serous ovarian carcinoma. Obstet Gynecol. 2013;122:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 18. | Vatansever D, Taskiran C, Mutlu Meydanli M, Gungorduk K, Akbayir O, Yalcin I, Demirkiran F, Sozen H, Ozgul N, Celik H, Onan MA, Taskin S, Oge T, Simsek T, Abboud S, Yuksel IT, Ayhan A. Impact of cytoreductive surgery on survival of patients with low-grade serous ovarian carcinoma: A multicentric study of Turkish Society of Gynecologic Oncology (TRSGO-OvCa-001). J Surg Oncol. 2021;123:1801-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 19. | Jiang X, Yang J, Yu M, Xie W, Cao D, Wu M, Pan L, Huang H, You Y, Shen K. Oncofertility in patients with stage I epithelial ovarian cancer: fertility-sparing surgery in young women of reproductive age. World J Surg Oncol. 2017;15:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 20. | Fruscio R, Ceppi L, Corso S, Galli F, Dell'Anna T, Dell'Orto F, Giuliani D, Garbi A, Chiari S, Mangioni C, Milani R, Floriani I, Colombo N, Bonazzi CM. Long-term results of fertility-sparing treatment compared with standard radical surgery for early-stage epithelial ovarian cancer. Br J Cancer. 2016;115:641-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 21. | Gershenson DM, Bodurka DC, Coleman RL, Lu KH, Malpica A, Sun CC. Hormonal Maintenance Therapy for Women With Low-Grade Serous Cancer of the Ovary or Peritoneum. J Clin Oncol. 2017;35:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (1)] |