Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.3177
Revised: April 13, 2024
Accepted: April 25, 2024
Published online: June 16, 2024
Processing time: 127 Days and 9.2 Hours
Supernumerary phantom limb (SPL) sensation is the experience of additional limbs, either single or a pair of limbs. Unique to traumatic spinal cord injuries, we report effect of transcranial direct current stimulation (tDCS) on SPL pain in a patient with cervical cord injury.
The subject was a 57-year-old man who was diagnosed with complete spinal cord injury (C6/C5, motor level; C5/C5, sensory level; AIS-A) approximately three months ago. After a period of 2 wk, we administered anodal tDCS over the motor cortex for 15 minutes at an intensity of 1.5 mA. Following that treatment, the patient experienced a decrease of SPL pain intensity and frequency, which lasted for 1 week after the end of treatment.
Targeting the motor cortex through neuromodulation appears to be a promising option for the management of SPL pain.
Core tip: Supernumerary phantom limb (SPL) sensation is the experience of additional limbs, either a single or a pair of limbs. SPL after a traumatic spinal cord injury is extremely rare and SPL pain is unique. Regarding SPL pain, there is no established standard treatment yet. We administered anodal transcranial direct current stimulation over the motor cortex for 15 min at an intensity of 1.5 mA. After the management, the patient experienced a decrease in SPL pain intensity and frequency. Targeting the motor cortex through neuromodulation appears to be a promising option for the management of supernumerary phantom limb pain.
- Citation: Park HS, Kim JH. Effect of transcranial direct current stimulation on supernumerary phantom limb pain in spinal cord injured patient: A case report. World J Clin Cases 2024; 12(17): 3177-3182
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/3177.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.3177
Phantom sensations include all non-painful sensations (volume, position, and movement of the phantom limb) perceived in the amputated limb. Beyond phantom sensation, supernumerary phantom limb (SPL) sensation is the experience of additional limbs, either as single limbs (upper or lower limb) or even as a pair of limbs[1]. SPL after a traumatic spinal cord injury (SCI) is extremely rare, with few reported cases in the literature. While the underlying pathophysiology of SPL after an SCI is unclear, pain is frequently associated with phantom limb after amputation, However, SPL pain is very rare[2]. We report a case of a two-week treatment of motor cortex stimulation with transcranial direct current stimulation to induce stable relief from SPL pain.
The patient was a 57-year-old man reported the emergence of SPL and SPL pain at 30 d after the trauma.
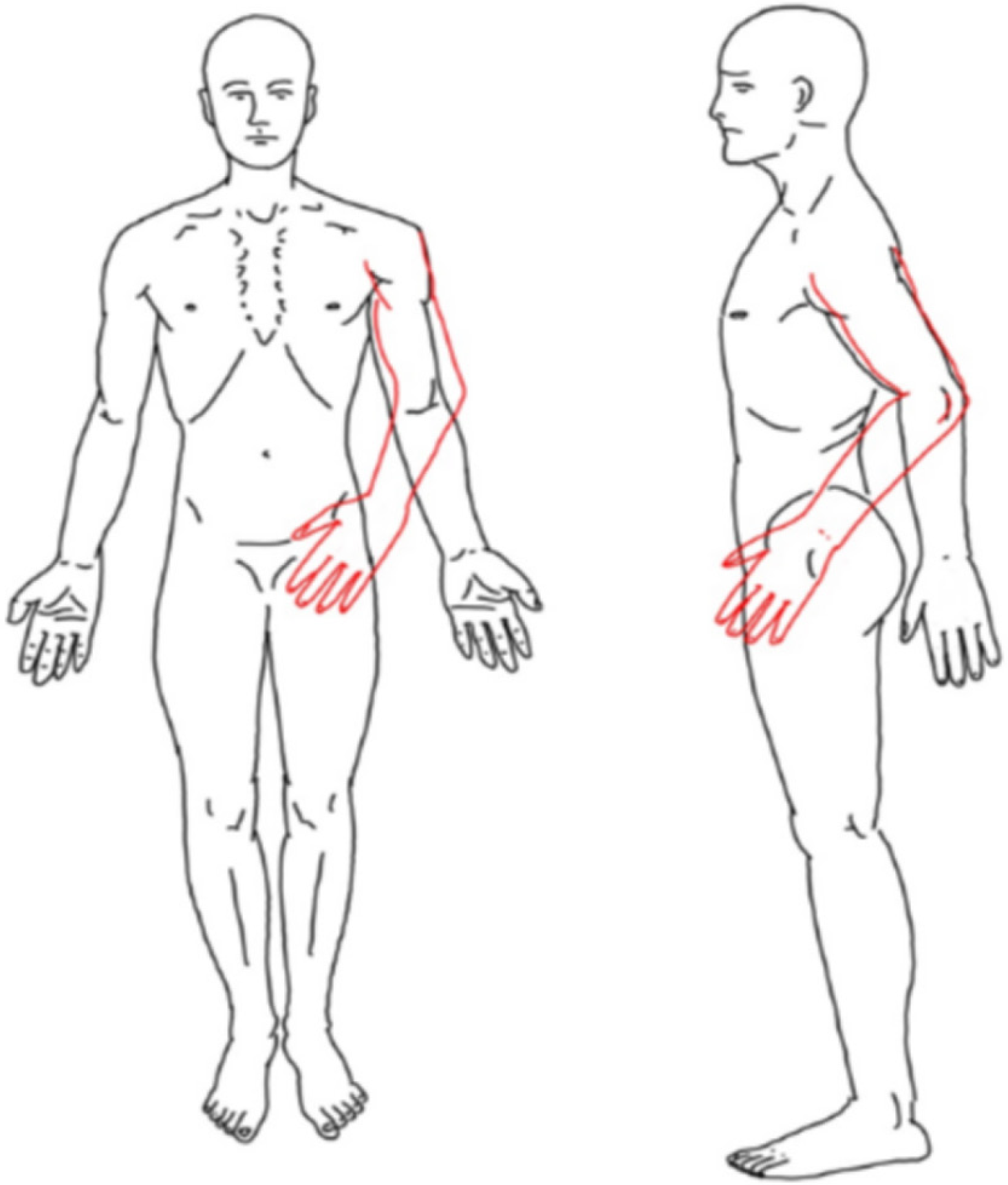
Symptoms started 30 d after the traumatic cervical spinal cord injury, experiencing a sensation of an extra left shoulder and the arm protruding forward with accompanying pain (Figure 1).
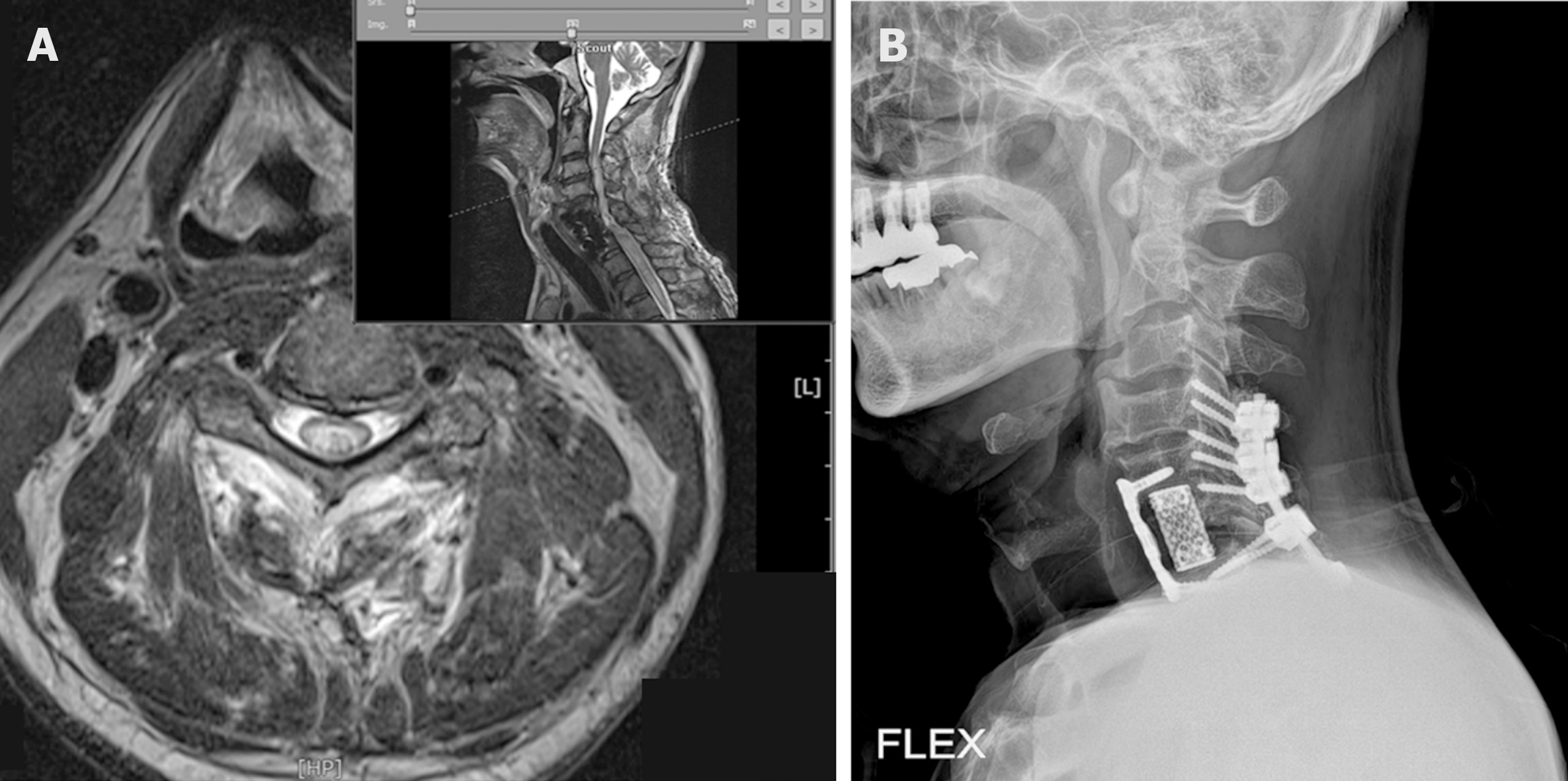
The patient was diagnosed with a complete cervical spinal cord injury approximately three months ago. The patient was a tetraplegia after suffering C6/7 fracture dislocation (Figure 2A and B) due to a fall. Following an unsuccessful reduction of the C6 fracture with a C4-T2 posterior fusion, the patient underwent a C5-7 anterior mass fusion and mesh cage insertion with C6 corpectomy. Postoperatively, the patient remained a C5 tetraplegia (C6/C5, motor level; C5/C5, sensory level; AIS-A).
There was no history of drug or alcohol abuse.
The patient's pain state was assessed using a numeric rating scale (NRS), which included evaluating the maximal intensity and background intensity of SPL pain (i.e., pain experienced in the phantom limb persistently or most of the day; 0 = absent, 10 = the worst possible SPL pain) and the frequency of SPL pain paroxysms (i.e., when SPL pain significantly intensifies above the background level; 0 = never during the day, 10 = very frequent). The patient also reported experiencing depression related to pain, and the Beck depression inventory (BDI) was used to assess whether there was an improvement in depression following pain relief. It was conducted on the initial day of treatment, before the first transcranial direct current stimulation (tDCS) session, at the end of the second week of treatment, and following the final tDCS session.
No abnormality was found in routine blood and urine analyses.
There was no concomitant brain injury on brain computed tomography scans.
The modified Bathel Index score was 2/100 points, indicating total dependence for activities of daily living. Cognitive tests including scores of Global Deterioration Scale and Mini-Mental Status Examination showed intact cognition. Psychiatric evaluation revealed no evidence of delusion, hallucination, or any other specific psychiatric abnormality. The patient showed good insight regarding various clinical conditions related to SCI.
Combined with the patient’s symptom, medical history, the final diagnosis was SPL and SPL pain.
Prior to tDCS therapy, the patient received basic rehabilitation therapies including physical therapy (functional electrical stimulation, passive range of motion exercise, and spastic muscle stretching exercise). For pain management, the patient was prescribed 150 mg oral pregabalin, twice a day, and no other treatments were implanted aside from pharmacological therapy. However, the oral medication was not effective enough to relieve SPL pain symptoms. To alleviate pain while maintaining pharmacological management, we administered adjunctive tDCS therapy. The patient underwent tDCS therapy five days a week for two weeks, with anodal tDCS applied over the motor cortex for 15 minutes at an intensity of 1.5 mA. The tDCS was delivered with a transcranial direct current stimulator (MINDD STIM, YDS-301N, Ybrain INC., Seongnam-si, Republic of Korea; http://www.ybrain.com). A set of surface saline-soaked sponge electrodes were positioned on the patient’s scalp. The anodal electrode was positioned above C3 or C4 (according to the 10-20 electroencephalograph system for electrode placement) to stimulate M1 opposite to the affected area, while the cathode electrode over the contralateral supraorbital area. We carefully monitored potential side effects during tDCS treatment period. At the end of tDCS treatment, participants completed an 8-item questionnaire to check potential side effects of tDCS. Items included headache, neck discomfort, scalp discomfort, scalp irritation, prickle, flushed skin, drowsiness, and difficulty concentrating.
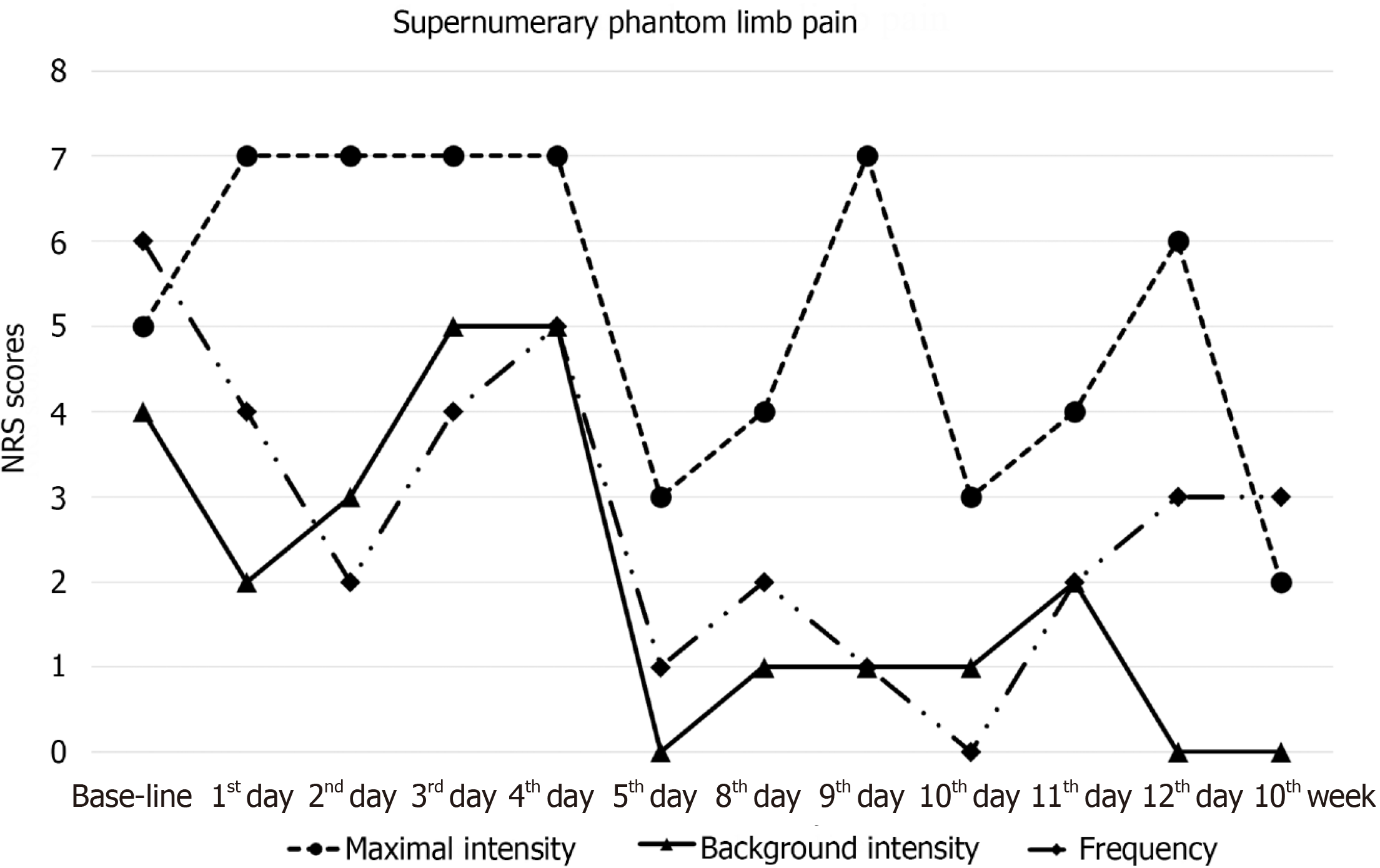
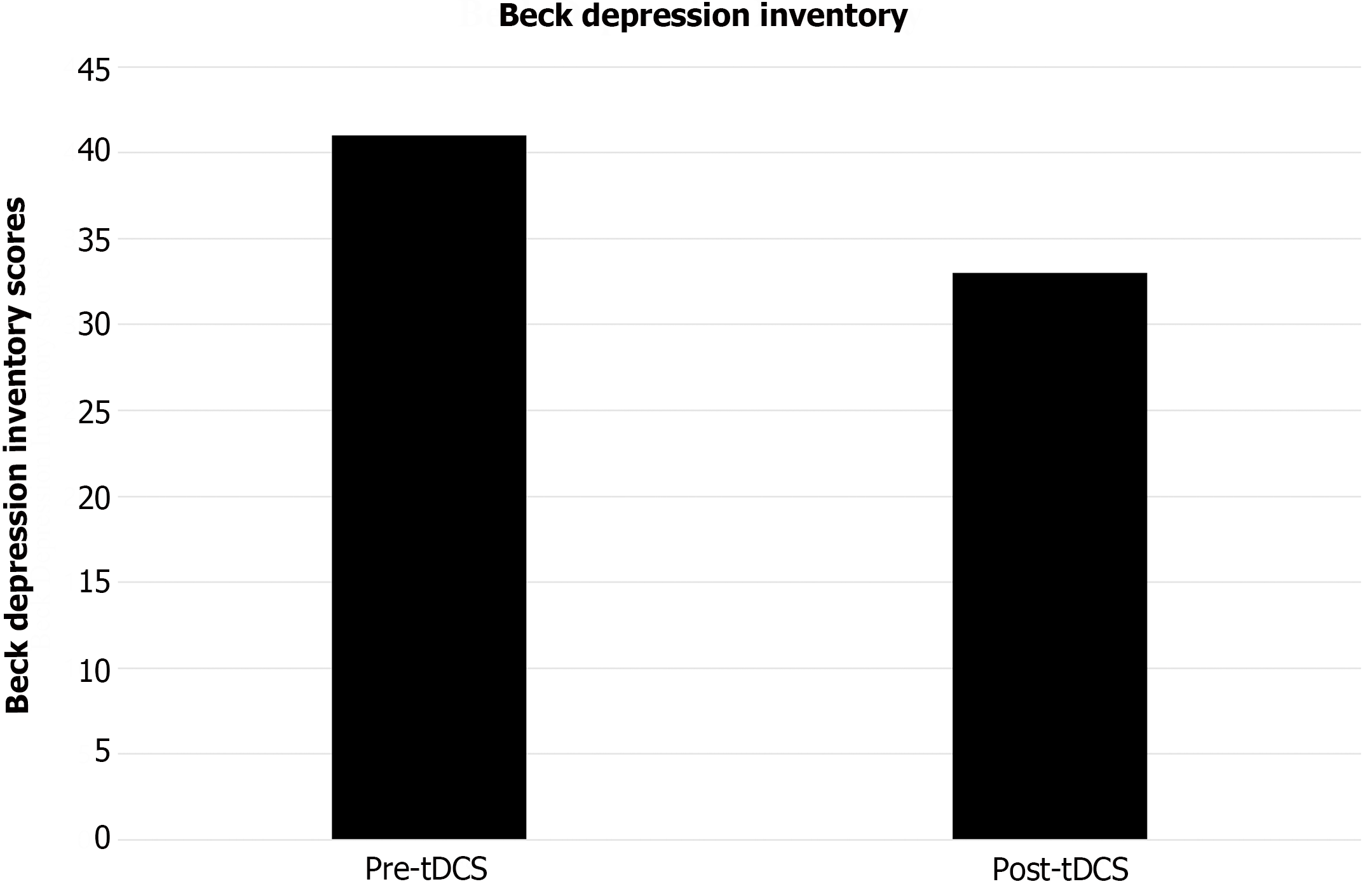
Throughout the tDCS treatment period, the patient experienced persistent SPL pain, although its intensity fluctuated with exacerbation in the afternoon and evening. The supernumerary arm was also associated with lancinating and piercing pain around the left hand and wrist. The SPL pain was rated between 2 to 7 points on the NRS during the treatment period. Following the 2-wk tDCS treatment, there was a sustained decrease in the maximal value of SPL pain score along with improvements in background intensity and frequency of SPL pain paroxysms (Figure 3). However, there was no change in SPL sensation. The BDI score also showed improvement after tDCS treatment (Figure 4). Side effects related to tDCS were not reported.
Phantom sensations include all non-painful sensations (volume, position, and movement of the phantom limb) perceived in the amputated limb. Beyond phantom sensation, supernumerary phantom limb sensation is the experience of additional limbs, either as single limbs (upper or lower limb) or even as a pair of limbs[1]. SPL after a traumatic SCI is extremely rare, with few reported cases in the literature. While the underlying pathophysiology of SPL after an SCI is unclear, pain is frequently associated with phantom limb after amputation, However, SPL pain is very rare[2].
Visual-tactile feedback (VTF), repetitive transcranial magnetic stimulation (rTMS), extensive reassurance and education about SPL, and neuropathic pain medication have been used for SPL sensation treatment[2,3]. In SCI patients, standardized and internationally recognized treatment protocols are not yet available to appropriately follow SPL sensation. Studies about effects of tDCS on SPL pain and sensation have not been reported yet. Therefore, we tried to determine effects of tDCS on SPL pain and sensation. To date, mechanisms underlying SPL caused by SCI remain unclear. After reviewing relevant literature, multiple theories have been proposed. Among these theories, the most frequently mentioned hypothesis suggests a mismatch between central command and sensory feedback from the periphery, which arises due to impaired sensory roots resulting from SCI[3]. This mismatch can lead to an ineffective integration of motor information, ultimately resulting in abnormal physical experiences.
The tDCS is a non-invasive modulation method where different brain regions are stimulated below the threshold of neuronal damage. Low intensity current modulates brain regions, and this leads to the excitation or inhibition of different functions[4]. In the present case, tDCS demonstrated effectiveness in alleviating pain associated with SPL. Previous studies have suggested that SPL pain shares similarities with phantom pain experienced by amputees[2,3]. In prior studies investigating the treatment of phantom limb pain utilizing tDCS, there were investigations employing tDCS for a duration of 2 wk with 10 sessions, as well as studies administering tDCS for 20 min each session over 2 wk. Consequently, in our study, we also implemented tDCS for 2 weeks across 10 sessions[5,6]. While the exact mechanisms underlying the pain-alleviating effects of anodal tDCS over the motor cortex in phantom pain are still unknown, it has been observed that depolarization of the affected sensorimotor cortex through anodal tDCS can reduce phantom limb pain[7]. This hypothesis regarding the effectiveness of tDCS in treating phantom pain in amputees can also be extended to SPL pain. Therefore, it is believed that tDCS has a pain-reducing effect.
A previous study has found that combining rTMS and VTF can improve SPL sensation[8]. In our case, we only used tDCS as a standalone therapy for SPL pain and sensation because we exclusively investigated effects of tDCS on SPL pain and sensation without considering any other treatments. Therefore, the fact that we did not simultaneously administer VTF could be one possible reason why it improved SPL pain without improving SPL sensation. Additionally, results of this study showed that tDCS not only reduced pain for the patient with SPL, but also improved depressive symptoms.
The occurrence of SPL sensation has been observed symmetrically in the bilateral upper limbs and/or lower limb in previously published research studies[3]. However, in the present case, SPL sensation was asymmetrically reported in only the left upper limb. Previous studies either did not provide data on the motor level associated with SPL sensation or reported that all key muscles in the upper limbs had scores of zero[3]. In contrast, the motor level of our case was determined as C6 on the right side and C5 on the left side. It was hypothesized that the manifestation of SPL sensation and pain occurred unilaterally due to different motor levels involved. Differences in motor levels can lead to a mismatch between central command and sensory feedback from the periphery. This mismatch can result in a different integration of motor information, leading to unilateral SPL sensation.
Limitations of this study include the inability to assess long-term effects of tDCS treatment on SPL pain. The exact mechanisms underlying SPL pain treatment are not yet clearly understood. Another limitation was that the patient's symptoms had a subacute onset, which did not completely exclude the possibility of spontaneous neural recovery. Based on these factors, a standardized protocol of tDCS for SPL pain is needed. Further research is necessary to fully understand the underlying mechanisms and develop more effective treatments for this condition.
In conclusion, this is a novel case report describing the successful use of tDCS to treat SPL pain in a high cervical cord injured patient. Results of this case suggest that a two-week treatment of motor cortex stimulation with tDCS could provide some relief for SPL pain.
This study complied with the Declaration of Helsinki regarding studies on human subjects. Informed consent was obtained from the patient and patient’s family.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rehabilitation
Country/Territory of origin: South Korea
Peer-review report’s classification
Scientific Quality: Grade C, Grade D
Novelty: Grade B, Grade C
Creativity or Innovation: Grade C, Grade C
Scientific Significance: Grade C, Grade C
P-Reviewer: Han J, China S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Finnoff J. Differentiation and treatment of phantom sensation, phantom pain, and residual-limb pain. J Am Podiatr Med Assoc. 2001;91:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Choi JY, Kim HI, Lee KC, Han ZA. Atypical supernumerary phantom limb and phantom limb pain in a patient with spinal cord injury: case report. Ann Rehabil Med. 2013;37:901-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Diaz-Segarra N, McKay O, Kirshblum S, Yonclas P. Management of nonpainful supernumerary phantom limbs after incomplete spinal cord injury with visual-tactile feedback therapy: a case report. Spinal Cord Ser Cases. 2020;6:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Kinakool AN, Abualait TS. Polarity-dependent cerebellar transcranial direct current stimulation modulates online functions of the alerting attention network. Neurosciences (Riyadh). 2021;26:323-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Segal N, Pud D, Amir H, Ratmansky M, Kuperman P, Honigman L, Treister R. Additive Analgesic Effect of Transcranial Direct Current Stimulation Together with Mirror Therapy for the Treatment of Phantom Pain. Pain Med. 2021;22:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Gunduz ME, Pacheco-Barrios K, Bonin Pinto C, Duarte D, Vélez FGS, Gianlorenco ACL, Teixeira PEP, Giannoni-Luza S, Crandell D, Battistella LR, Simis M, Fregni F. Effects of Combined and Alone Transcranial Motor Cortex Stimulation and Mirror Therapy in Phantom Limb Pain: A Randomized Factorial Trial. Neurorehabil Neural Repair. 2021;35:704-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 7. | Damercheli S, Ramne M, Ortiz-Catalan M. transcranial Direct Current Stimulation (tDCS) for the treatment and investigation of Phantom Limb Pain (PLP). Psychoradiology. 2022;2:23-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Lu YS, Tong P, Guo TC, Ding XH, Zhang S, Zhang XJ. Effects of combined rTMS and visual feedback on the rehabilitation of supernumerary phantom limbs in a patient with spinal cord injury: A case report. World J Clin Cases. 2019;7:3120-3125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |