Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.3168
Revised: March 4, 2024
Accepted: May 7, 2024
Published online: June 16, 2024
Processing time: 132 Days and 1.2 Hours
High-dose vitamin C treatment (HVCT) can reduce the adverse effect of chemo
Two patients with colorectal cancer developed hemolytic anemia after using 1 g/kg HVCT. In contrast to previous cases, the lowest hemoglobin level in the two cases was < 50 g/L, which was lower than previously reported. This may be because Case 1 had chronic hepatitis B for many years, which caused abnormal liver reserve function, and Case 2 had grade II bone marrow suppression. Both patients improved and were discharged after blood replacement therapy. Our cases had the most severe degree of hemolysis but the best prognosis, suggesting that our treatment may be helpful for rescue of drug-induced hemolysis. This is the first review of the literature on hemolysis caused by HVCT, and we found that all patients with G6PD deficiency developed hemolysis after HVCT.
G6PD deficiency should be considered as a contraindication to HVCT, and it is not recommended for patients with bone marrow suppression, moderate-to-severe anemia, hematopoietic abnormalities, or abnormal liver and kidney function. Early blood purification and steroid therapy may avoid acute kidney injury or death caused by HVCT-related hemolytic anemia.
Core Tip: Two patients suffered from extremely severe hemolysis after high dose vitamin C treatment (HVCT), and both patients had glucose-6-phosphatase dehydrogenase (G6PD) deficiency. By reviewing the literature and summarizing the findings of available case reports, we conclude for the first time that patients who develop hemolytic anemia after HVCT may have G6PD deficiency, thus G6PD deficiency is an absolute contraindication for HVCT. Our treatment protected the patients from acute kidney injury in the context of extremely severe hemolysis. Our experience may be helpful for the treatment of similar cases.
- Citation: Wang SW, Zhang XW, Qu JX, Rao YZ, Lu S, Wang B, He J, Zhao Y, Rao BQ. Hemolysis attributed to high dose vitamin C: Two case reports. World J Clin Cases 2024; 12(17): 3168-3176
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/3168.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.3168
We report two cases of hemolytic anemia induced by high dose vitamin C treatment (HVCT) in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. This is the first report of this condition in patients with combined myelosuppression and liver dysfunction.
We conducted a literature search in PubMed, CNKI and OVID, and found a total of 7 articles, and the data in them were compared with our patients’ data. Combined with our case, the 9 patients with hemolysis after HVCT reported to date were summarized, and G6PD deficiency was found in all cases. We analyzed the reasons for this occurrence. We recommend strict control of the contraindications of HVCT, and HVCT should be prohibited for G6PD deficiency patients[1,2].
Our patients had more severe hemolysis after HVCT, which may be related to their comorbidities, but they recovered best after treatment. Therefore, we also compared and summarized the treatment regimens of these 9 patients, and our treatment experience may be helpful for the treatment of drug-induced hemolysis.
Both Case 1 and Case 2 presented with jaundice after HVCT used for anti-tumor therapy.
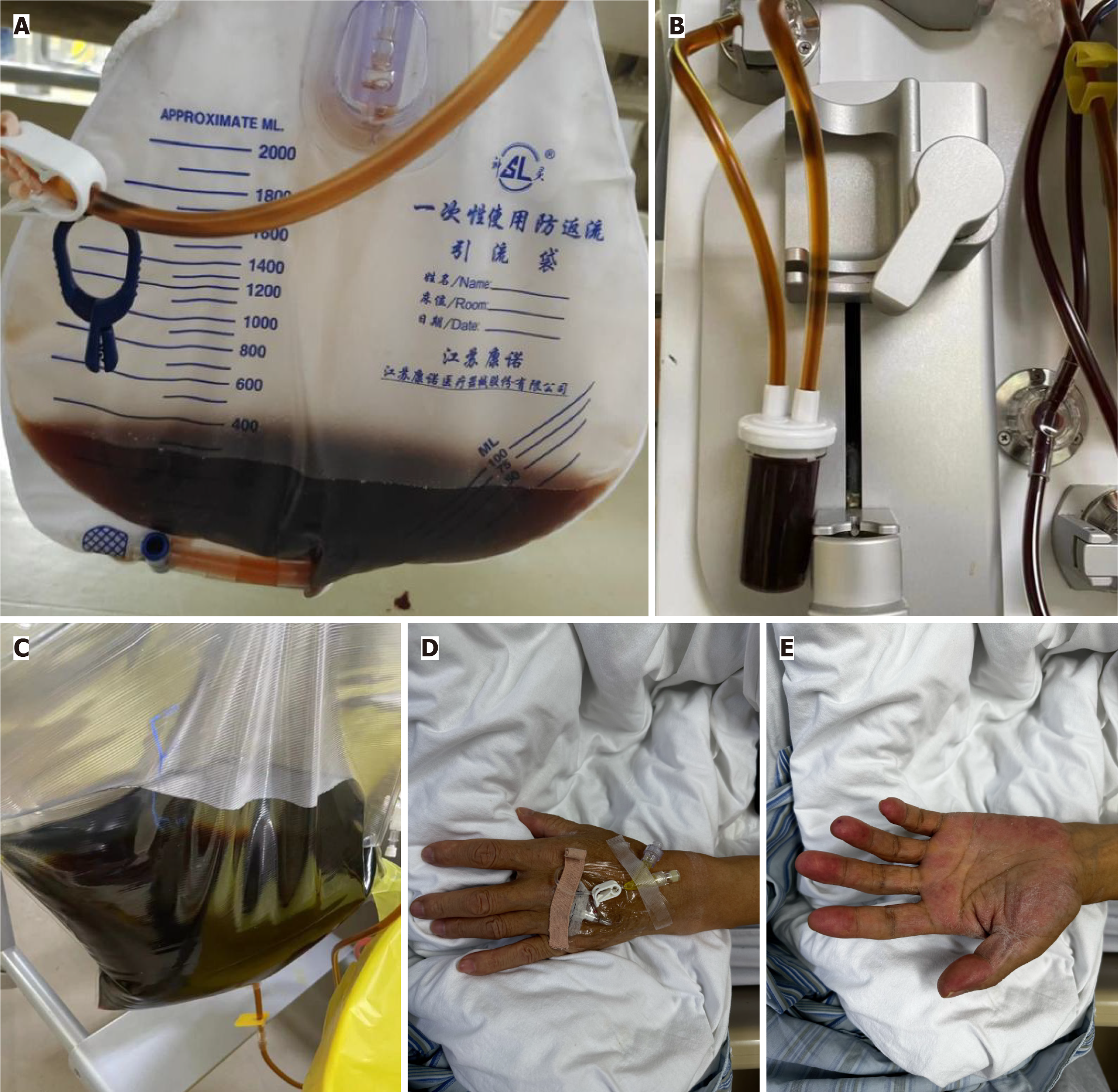
Case 1: A 48-year-old man from Yongfeng County, Jiangxi Province, China, received FOLFOX (oxaliplatin + calcium folinate +fluorouracil) chemotherapy + bevacizumab with concurrent HVCT (1 g/kg, 90 g, qd, ivgtt for 3 consecutive days) for colon cancer. After finishing treatment, the patient was admitted to the emergency department because of jaundice of the skin and sclera and fatigue for 1 d, which worsened for 3 h. On the day of presentation, the patient had severe fatigue, inability to walk, and nonexertional shortness of breath, with hematuria that rapidly progressed to soy-colored urine within 1 d (Figure 1A). He had no fever, chills, hematemesis, hematochezia, cough, chest pain or back pain.
Case 2: The patient was a 54-year-old woman from Yongfeng County, Jiangxi Province, China. She was given single HVCT (10 g, qd, ivgtt, days 1 and 2) to establish tolerance, and the dose increased to 1 g/kg, 60 g, qd, ivgtt, on days 3 and 4, after no discomfort was observed. On the morning of day 5 of infusion, the patient complained of fatigue, dark yellow urine, skin jaundice and icteric sclera. She had no other complaints and her general condition was fair.
Case 1: The patient reported no jaundice, but he had stopped anti-tumor treatment because of abnormal liver function, and was treated with intermittent oral antiviral drug entecavir previously. In addition, the patient developed COVID-19 with tachycardia as the main manifestation 3 months previously, without dyspnea and standard antiviral treatment.
Case 2: She received anti-tumor treatment with one cycle of Xelox (oxaliplatin and capecitabine); nine cycles of FOLFOX + cetuximab; eight cycles of FOLFOXIRI (Fluorouracil + leucovorin + oxaliplatin + irinotecan)+ cetuximab; and four cycles of TAS-102 (trifluridine tipiracil tablets) + bevacizumab for rectal cancer with liver and lung metastases for > 1 year. At the time of the last chemotherapy in April 2023, no signs of tumor remission were observed, and grade II bone marrow suppression occurred.
Case 1: His son had jaundice but the cause was not established. He had not previously received any food or medication that might have induced hemolysis, except HVCT.
Case 2: The patient and her next of kin had no history of jaundice or hemolysis, and she had not previously received any food or medication that might have induced hemolysis, except HVCT.
Case 1: The patient had obvious skin and scleral jaundice and poor consciousness. After emergency admission, the patient underwent electrocardiographic monitoring, which showed a heart rate 110 beats/min, blood pressure 115/80 mmHg, oxygen inhalation 10 L/min, and peripheral oxygen saturation 62% (Figure 2).
Case 2: Her vital signs were stable, but the skin and the sclera were jaundiced, with soy sauce colored urine.
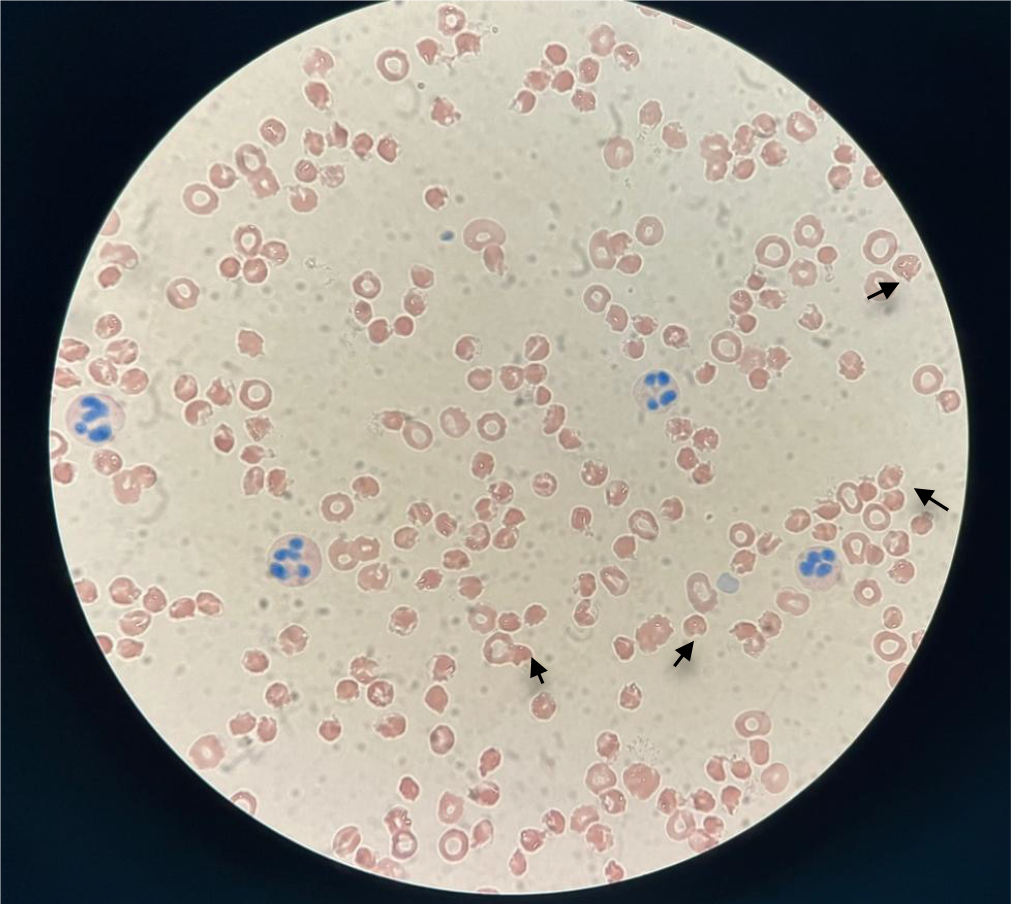
Case 1: Blood gas analysis showed partial pressure of oxygen 83 mmHg, partial pressure of carbon dioxide 42 mmHg, pH 7.43, and total hemoglobin 5.8 g/dL. Routine blood tests showed white blood cell count 23.65 × 109/L, red blood cell count 1.75 × 1012/L, hemoglobin 62 g/L, total bilirubin 179.9 μmol/L, direct bilirubin 31.2 μmol/L, indirect bilirubin 148.7 μmol/L, and serum iron 42.5 μmol/L (Table 1). The direct antiglobulin test was negative.
| Items | Case 1 | Case 2 | Reference ranges | Units | ||||||
| Before VC | After VC | Minimum hemoglobin | Post-treatment | Before VC | After VC | Minimum hemoglobin | Post-treatment | |||
| RBC | 4.81 | 1.75 | 1.06 | 3.54 | 2.69 | 1.26 | 1.16 | 2.69 | 4.3-5.8 | × 1012/L |
| HGB | 152 | 63 | 37 | 112 | 96 | 44 | 41 | 86 | 130-175 | g/L |
| HCT | 0.444 | 0.173 | 0.105 | 0.335 | 0.285 | 0.144 | 0.129 | 0.274 | 0.40-0.50 | L/L |
| MCV | 92.3 | 98.9 | 99.1 | 94.7 | 105.9 | 114.8 | 110.9 | 101.9 | 82-100 | fL |
| MCH | 31.6 | 36 | 34.9 | 31.6 | 35.7 | 35.4 | 35.2 | 32 | 27-34 | pg |
| MCHC | 342 | 364 | 352 | 334 | 2.69 | 1.26 | 1.16 | 2.69 | 316-354 | g/L |
Case 2: The blood examination showed red blood cell count 1.26 × 1012/L, hemoglobin concentration 44 g/L, total bilirubin 148.8 μmol/L, direct bilirubin 48.4 μmol/L, and indirect bilirubin 100.4 μmol/L (Table 1).
Case 1: The patient was diagnosed with intravascular hemolysis, hemolytic anemia, hemolytic jaundice, methemoglobinemia, and malignancy of the sigmoid colon.
Case 2: The patient was diagnosed with intravascular hemolysis, hemolytic anemia, hemolytic jaundice, rectal malignancy, secondary liver malignancy, and secondary lung malignancy.
Case 1: The patient was in critical condition and was transferred to intensive care unit for plasma exchange (Figure 1B–E). On the next day, the disease continued to progress and the patient became unconscious. He was treated with endotracheal intubation and mechanical ventilation, and then plasmapheresis, blood transfusion, hemofiltration and high-dose steroid pulse therapy. The endotracheal tube was extubated 33 h after intubation, and the patient was transferred back to the general ward 1 d later. He was discharged after a step-down treatment with high-dose steroids.
Case 2: HVCT was stopped immediately and the patient was transferred to intensive care unit. Electrocardiographic monitoring, oxygen inhalation, gammaglobulin 15 g, dexamethasone 10 g, and methylprednisolone 40 mg pulse therapy were administered for two consecutive days. A total of 7 U red blood cells were transfused and hemofiltration was performed once.
In both cases, hemoglobin levels gradually increased after treatment, their condition gradually stabilized, and they were discharged safely. No acute kidney injury occurred during the follow-up period.
After clarifying the two polarities of vitamin C dose effect, which is, reductive at low dose and oxidative high dose[3], HVCT has been shown to prolong survival and improve quality of life of patients with colorectal cancer harboring KRAS mutation[4], and has a good synergistic effect in some antitumor therapies[5]. This is closely related to the production of a large number of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) and superoxide anion by HVCT, which directly or indirectly kill tumor cells.
G6PD is a key enzyme in the pentose phosphate pathway (PPP), which is widely expressed in the body to assist glu
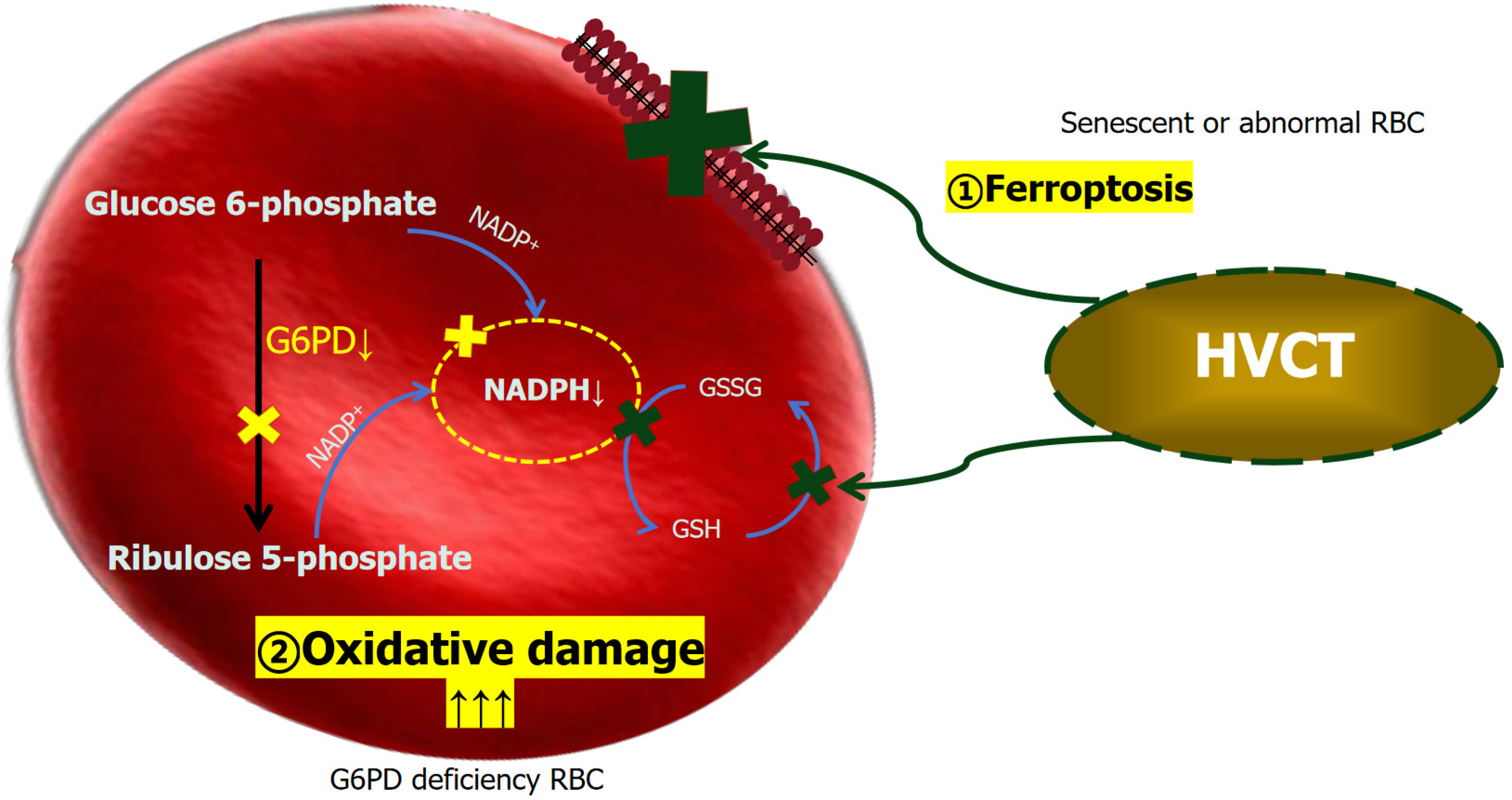
There are 400 million people around the world who carry mutations in the G6PD gene associated with enzyme deficiency. G1388A, G1376T and A95G are the most common mutations in the Chinese population. The degree of enzyme deficiency triggered by genetic defects varies. It is more common in male than female patients, with obvious racial and geographic differences, and it is common in Africa, Asia and the Middle East. It is often found in areas with high incidence of malaria, thalassemia and abnormal blood protein diseases[8-12]. The distribution in China shows a trend of high in the south and low in the north, especially in Guangdong, Hainan, Guangxi, Yunnan, Jiangxi, Guizhou, and Sichuan, with incidence rates of 4%–15% and up to 40% in some areas[13]. The occurrence of hemolytic anemia in patients with G6PD deficiency after intravenous HVCT may be related to the strong oxidation of HVCT, which produces a large amount of ROS, especially H2O2, inside and outside the cells, causing oxidative damage to the cells[5]. When G6PD is deficient, red blood cells fail to produce sufficient NADPH, a key molecule for restoring glutathione depletion due to vitamin C-induced oxidative stress, which leads to fragmentation of red blood cells and thus hemolysis[14] (Figure 3).
The key words "ascorbic acid", "vitamin C" and "hemolysis" were used for our literature search, and 7 cases of hemolysis caused by HVCT were screened out. Together with the 2 patients reported here, a total of 9 cases were recorded, all of whom had G6PD deficiency. Among such patients, the probability of acute kidney injury or methemoglobinemia at the time of hemolysis are 44% respectively, and the probability of death is 11% (Table 2).
| Year | Nationality | Sex | G6PD deficiency | Dose, g/d | The lowest level of hemoglobin, g/L | Treatment | Complications and prognosis | Ref. |
| 1975 | United States | M | Yes | 80 | 58 | Blood transfusion + glucocorticoids (day 3) + hemodialysis (after anuria) | AKI, DIC, death | [19] |
| 1993 | United Kingdom | M | Yes | 80 | 67 | Drink plenty of water + folic acid orally | AKI | [20] |
| 2014 | Taiwan, China | M | Yes | 75 | 54 | Supportive treatment | None | [21] |
| 2017 | United States | M | Yes | 60 | 59 | Intravenous fluids + glucocorticoids | AKI, methemoglobinemia, acute oxalate nephropathy | [22] |
| 2019 | United States | M | Yes | 65-200 | - | - | None | [23] |
| 2020 | Hong Kong, China | F | Yes | 30 | 63 | Blood transfusion | Methemoglobinemia | [6] |
| 2022 | Taiwan, China | M | Yes | 50 | 71 | - | AKI | [24] |
| 2023 | Mainland China | M | Yes | 90 | 37 | Plasmapheresis + glucocorticoids | Methemoglobinemia | This text |
| 2023 | Mainland China | F | Yes | 60 | 41 | Hemofiltration + glucocorticoids | Methemoglobinemia | This text |
In clinical application of HVCT, it is necessary to check the G6PD related indicators to detect G6PD enzyme activity for patients in advance, for example, methemoglobin reduction test, fluorescent spot test, nitrotetrazolium disk method. G6PD deficiency should also be considered as one of the exclusion criteria in the clinical experimental study of HVCT[5]. During the infusion, patients should be paid attention to whether they have adverse reactions and hemoglobin content should be monitored.
HVCT is now mostly used as adjuvant therapy for antitumor therapy, such as combined chemotherapy, radiotherapy, immune or targeted therapy. When combined, the killing effect on normal cells increases[15], which may be one of the risk factors for hemolysis induced by HVCT. Therefore, special attention should be paid to the hematopoietic and metabolic functions of patients when combined. If the patient has bone marrow suppression and reticulocytosis, indicating reduced hematopoietic function and tolerance to injury, HVCT may be more likely to induce hemolytic reaction (Table 3). Therefore, when HVCT is used in combination, especially with antitumor drugs that may lead to autoimmune hemolytic anemia, HVCT may be more likely to induce a hemolytic reaction. When used with anti-programmed cell death protein-1 (e.g., nivolumab, pembrolizumab), cytotoxic T lymphocyte-associated protein 4 (e.g., ipilimumab), the dosage should be reduced as appropriate[16]. Patients with impaired liver function have a reduced ability to deal with schistocytes, and the degree of hemolysis is more severe than that of patients with normal liver function, and the condition is more difficult to control. For patients with hepatitis, cirrhosis, liver cancer and other diseases that affect liver metabolism, the indocyanine green (ICG) test should be supplemented under the condition of normal liver function to determine whether the liver reserve capacity can cope with hemolysis induced by HVCT.
| Items | Results | Units | Reference ranges | |
| Case 1 | Case 2 | |||
| RET1 | 1.8 | 6.9 | % | 0.59-2.07 |
| RET2 | 32.2 | 207.8 | ´109/L | 22.4-93.5 |
| LFR | 92.4 | 77.3 | % | 87.8-99.5 |
| MFR | 7.4 | 15.7 | % | 1.8-14.4 |
| HFR | 0.2 | 7 | % | 0-2.4 |
| IRF | 7.6 | 22.7 | % | 2.1-17.5 |
Since HVCT also have a certain destructive effect on about 5% G6PD normal erythrocytes[17], may be related to the ferroptosis of lipid peroxidation on the surface of red blood cells caused by HVCT. The manifestation of this effect in patient is an extremely slight decrease in hemoglobin. Therefore, in addition to G6PD detection, routine blood and biochemical tests, reticulocyte count and liver function storage test (ICG test) are necessary before administration of HVCT. At the same time, the above blood indicators should be measured on the second day before administration of HVCT to determine whether the patient can tolerate the treatment, and the infusion cannot be continued until there is no significant change, otherwise the infusion should be stopped permanently.
In patients with acute hemolysis, all oxidative drugs including HVCT should be stopped immediately. During the hemolytic phase, adequate hydration, correction of electrolyte imbalance, oral or intravenous supplementation of sodium bicarbonate, and alkaline urine should be maintained to prevent the deposition of hemoglobin in the renal tubules. Renal function should be closely monitored to prevent acute kidney injury and acute oxalate nephropathy. Mild anemia caused by HVCT (hemoglobin 60-90 g/L) generally recovers within 1 wk after withdrawal in patients without serious cardiopulmonary comorbidities[18]. The patients with severe anemia (hemoglobin < 60 g/L) or decreased hemoglobin, rapid increase in bilirubin, fatigue, shortness of breath, tachycardia and other symptoms of anemia, especially those with coronary heart disease, pulmonary heart disease or cerebrovascular disease, can be transfused with red blood cells to correct anemia. Patients with severe hemolysis should be treated with blood purification as soon as possible to prevent the occurrence of bilirubin encephalopathy or acute kidney injury or renal failure. Early blood purification and hormone pulse therapy may be the methods to avoid acute kidney injury caused by HVCT-related hemolysis.
HVCT is a mild antitumor therapy for most patients, except for those with G6PD deficiency and those with a risk of adverse effects such as renal insufficiency, recurrent urolithiasis, or hematopoietic abnormalities. Our cases were unique due to their complications and severity of the hemolysis. With the early blood purification and hormone therapy that we administered, these patients were spared acute kidney injury, renal failure, or death that occurred in the other patients, suggesting that these treatments may be effective in patients with similar conditions. In addition, we suggest that patients should be screened for enzyme activity to exclude G6PD deficiency before HVCT, and changes in hemoglobin content and hemolysis-related indicators should be detected in all patients during administration, especially after the first day of treatment. A standardized administration regimen is the best solution to avoid HVCT-related hemolysis.
| 1. | Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis. 2009;42:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 462] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 2. | Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 497] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 3. | Padayatty SJ, Riordan HD, Hewitt SM, Katz A, Hoffer LJ, Levine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006;174:937-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Wang F, He MM, Xiao J, Zhang YQ, Yuan XL, Fang WJ, Zhang Y, Wang W, Hu XH, Ma ZG, Yao YC, Zhuang ZX, Zhou FX, Ying JE, Yuan Y, Zou QF, Guo ZQ, Wu XY, Jin Y, Mai ZJ, Wang ZQ, Qiu H, Guo Y, Shi SM, Chen SZ, Luo HY, Zhang DS, Wang FH, Li YH, Xu RH. A Randomized, Open-Label, Multicenter, Phase 3 Study of High-Dose Vitamin C Plus FOLFOX ± Bevacizumab versus FOLFOX ± Bevacizumab in Unresectable Untreated Metastatic Colorectal Cancer (VITALITY Study). Clin Cancer Res. 2022;28:4232-4239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 5. | Böttger F, Vallés-Martí A, Cahn L, Jimenez CR. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J Exp Clin Cancer Res. 2021;40:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 6. | Lo YH, Mok KL. High dose vitamin C induced methemoglobinemia and hemolytic anemia in glucose-6-phosphate dehydrogenase deficiency. Am J Emerg Med. 2020;38:2488.e3-2488.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Yanase F, Fujii T, Naorungroj T, Belletti A, Luethi N, Carr AC, Young PJ, Bellomo R. Harm of IV High-Dose Vitamin C Therapy in Adult Patients: A Scoping Review. Crit Care Med. 2020;48:e620-e628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Israel A, Schäffer AA, Berkovitch M, Ozeri DJ, Merzon E, Green I, Golan-Cohen A, Ruppin E, Vinker S, Magen E. Glucose-6-phosphate dehydrogenase deficiency and long-term risk of immune-related disorders. Front Immunol. 2023;14:1232560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Luzzatto L, Nannelli C, Notaro R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol Oncol Clin North Am. 2016;30:373-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 10. | Relling MV, McDonagh EM, Chang T, Caudle KE, McLeod HL, Haidar CE, Klein T, Luzzatto L; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clin Pharmacol Ther. 2014;96:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Pukrittayakamee S, Jittamala P, Watson JA, Hanboonkunupakarn B, Leungsinsiri P, Poovorawan K, Chotivanich K, Bancone G, Chu CS, Imwong M, Day NPJ, Taylor WRJ, White NJ. Primaquine in glucose-6-phosphate dehydrogenase deficiency: an adaptive pharmacometric assessment of ascending dose regimens in healthy volunteers. Elife. 2024;12:RP87318. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 959] [Cited by in RCA: 941] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 13. | Liang HF, Lin F, Lu J, Chen ZX, Yang LY. Glucose-6-phosphate dehydrogenase deficiency screening and gene analysis in blood donors of Guangdong province. Hematology. 2022;27:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Youngster I, Arcavi L, Schechmaster R, Akayzen Y, Popliski H, Shimonov J, Beig S, Berkovitch M. Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review. Drug Saf. 2010;33:713-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Mussa A, Mohd Idris RA, Ahmed N, Ahmad S, Murtadha AH, Tengku Din TADAA, Yean CY, Wan Abdul Rahman WF, Mat Lazim N, Uskoković V, Hajissa K, Mokhtar NF, Mohamud R, Hassan R. High-Dose Vitamin C for Cancer Therapy. Pharmaceuticals (Basel). 2022;15:711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | The Society of Chemotherapy. Chinese Anti-Cancer Association. A consensus on the clinical diagnosis, treatment, and prevention of cancer- and chemotherapy-related anemia in China. Chin J Clin Onco. 2019;46:869-875. [DOI] [Full Text] |
| 17. | Ibrahim IH, Sallam SM, Omar H, Rizk M. Oxidative hemolysis of erythrocytes induced by various vitamins. Int J Biomed Sci. 2006;2:295-298. [PubMed] |
| 18. | Juneja D, Jain R, Nasa P. Vitamin C-induced Hemolysis: Meta-summary and Review of Literature. Indian J Crit Care Med. 2022;26:224-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Campbell GD Jr, Steinberg MH, Bower JD. Letter: Ascorbic acid-induced hemolysis in G-6-PD deficiency. Ann Intern Med. 1975;82:810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 75] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Rees DC, Kelsey H, Richards JD. Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. BMJ. 1993;306:841-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Huang YC, Chang TK, Fu YC, Jan SL. C for colored urine: acute hemolysis induced by high-dose ascorbic acid. Clin Toxicol (Phila). 2014;52:984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Quinn J, Gerber B, Fouche R, Kenyon K, Blom Z, Muthukanagaraj P. Effect of High-Dose Vitamin C Infusion in a Glucose-6-Phosphate Dehydrogenase-Deficient Patient. Case Rep Med. 2017;2017:5202606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Lin H, Lin BW, Lin JD. Effects of different ascorbic acid doses on the mortality of critically ill patients: a meta-analysis. Ann Intensive Care. 2019;9:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Chen HJ, Lin KT, Chou CC, Lin YR. New-Onset Methemoglobinemia After Receiving Ozone Autohemotherapy and High-Dose Vitamin C Injection. Transfus Med Rev. 2022;36:68-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |