Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.3130
Revised: March 16, 2024
Accepted: April 22, 2024
Published online: June 16, 2024
Processing time: 121 Days and 6.9 Hours
Neurological complications are common in the management of venoarterial extracorporeal membrane oxygenation (VA-ECMO), with most patients requiring sedation and intubation, limiting the assessment of neurological function. There-fore, we must rely on advanced neuroimaging techniques, such as computed tomography angiography (CTA) and computed tomography perfusion (CTP). Because ECMO changes the normal blood flow pattern, it may interfere with the contrast medium in some special cases, leading to artifacts and ultimately mis-leading clinical decisions.
A 61-year-old man presented to a local hospital with chest tightness and pain 1 d prior to presentation. The patient was treated with VA-ECMO after sudden car-diac and respiratory arrest at a local hospital. For further treatment, the patient was transferred to our hospital. The initial consciousness assessment was not clear, and routine CTP was performed to understand the intracranial changes, which suggested a large area of cerebral infarction on the right side; however, the cerebral oxygen was not consistent with the CTP results, and the reexamination of CTA still suggested a right cerebral infarction. To identify this difference, bedside transcranial Doppler was performed, and the blood flow on both sides was different. By reducing the ECMO flow, CTP reexamination showed that the results were normal and consistent with the clinical results. On day 3, the patient was alert and showed good limb movements.
In patients with peripheral VA-ECMO, cerebral perfusion confirmed by CTP and CTA may lead to false cerebral infarction.
Core Tip: In venoarterial extracorporeal membrane oxygenation (VA-ECMO) patients, neurological complications are common and require sedation and tracheal intubation, limiting neurological assessment and necessitating reliance on advanced neuroimaging techniques, such as computed tomography angiography (CTA) and computed tomography perfusion (CTP). We report a rare case of VA-ECMO-induced abnormal flow patterns causing unique CTA and CTP artifacts mimicking cerebral infarction. Combined with the analysis of PubMed index cases, we found that peripheral VA-ECMO CTA and CTP may generate rare artifacts, which may mislead clinicians in diagnosing acute stroke, posing serious consequences for patients. Early detection is vital for prognosis, and noninvasive monitoring methods (transcranial Doppler, near-infrared spectroscopy, and continuous electroencephalography) can aid in identification.
- Citation: Xu M, Yan JY, Jin JJ, Li T. Cerebral pseudoinfarction due to venoarterial extracorporeal membrane oxygenation: A case report. World J Clin Cases 2024; 12(17): 3130-3137
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/3130.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.3130
Mortality from all causes remains high in patients with severe cardiogenic shock. The use of venoarterial extracorporeal membrane oxygenation (VA-ECMO), which improves outcomes, has increased more than 10-fold in the past decade and is listed in several guidelines as an important treatment for severe cardiogenic shock[1]. Neurological complications are common[2,3]. Most patients on ECMO require sedation and tracheal intubation, limiting the assessment of neurological function[4]; therefore, they have to rely on advanced neuroimaging techniques such as computed tomography angiography (CTA) and computed tomography perfusion (CTP). Both methods involve the administration of intravenous contrast material, which may give false results in peripherally catheterized ECMO because of a mixture of reversed ECMO flow in the femoral artery and native cardiac output[5]. Most of the junction planes are in the thoracoabdominal aorta, and fewer are mixed in the aortic arch and intracranial region. Therefore, if the contrast agent is administered through conventional systemic venous access, the appearance of the head and neck arterial supply on CTA and processed CTP images will change significantly. Thus, there are obvious limitations in using CTA and CTP to diagnose intracranial perfusion. However, this is not only an incidental imaging artifact; altered flow patterns on CTA and CTP may have significant hemodynamic and perfusion consequences on the brain, with specific pathophysiological consequences depending on the individual arterial anatomy and collateral flow[5,6].
In clinical practice, these artifacts may mislead diagnosis, treatment, and prognosis, resulting in the need for additional anticoagulation or interventional therapy, accompanied by serious consequences. Here, we present a case of cerebral pseudoinfarction due to VA-ECMO in a 61-year-old man and a literature review of previous cases, summarizing the imaging findings and possible methods of differentiation.
A 61-year-old man admitted to the hospital with chest tightness and chest pain 1 d earlier was treated with VA-ECMO after sudden cardiac and respiratory arrest in the hospital.
One day earlier, the patient presented to a local hospital with chest tightness and pain. During hospitalization, the patient experienced sudden cardiac and respiratory arrest and was immediately treated with cardiopulmonary resuscitation, which restored sinus rhythm; however, it was still difficult to maintain circulation after the use of large doses of vasopressor drugs. VA-ECMO (flow rate 2.95 L/min, speed 3205 r/min) support treatment was performed. Due to a lack of further management experience in the local hospital, he was transferred to our hospital for further treatment.
Diagnosed over 10 years ago with coronary heart disease [New York Heart Association functional classification (NYHA) III-IV], renal insufficiency 3 years prior, and type 2 diabetes mellitus for 5 years, the patient also had a history of alcohol consumption, having been abstinent for over 1 year.
The patient had no relevant personal or family histories.
The patient was 80 kg and had a body mass index of 26. The vital signs were as follows: Body temperature, 36.1 ℃; heart rate, 54 beats per minute; blood pressure, 11.3/10.1 kPa; respiration, 12 breaths per minute (mechanical ventilation); endotracheal intubation, mechanical ventilation, PC mode, inspiratory pressure 2.5 kPa, positive end-expiratory pressure 0.1 kPa; and bilateral pupils were equal and round, with a diameter of about 2 mm.
The mean partial thromboplastin time (APTT) was 46.8 s. No abnormalities were found on routine blood or urine analyses.
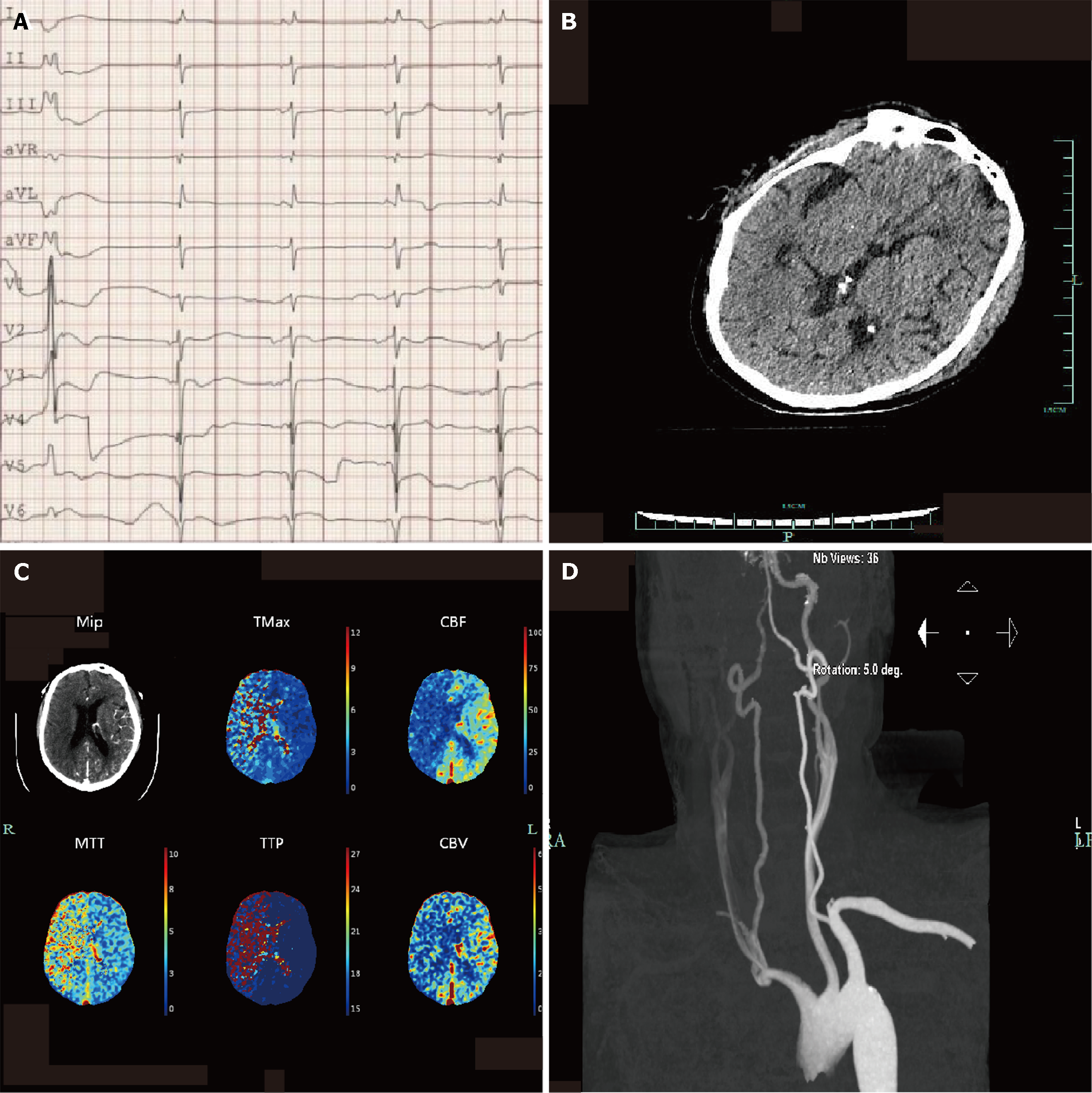
Electrocardiogram: On admission, the electrocardiogram revealed sinus rhythm, atrial arrhythmia, premature ventricular contraction, occasional junctional arrhythmia, acute anterior wall myocardial infarction, anterior lateral wall myocardial infarction, and atrioventricular block (Figure 1A).
Echocardiography was performed, and the results showed left heart enlargement and decreased left ventricular systolic function (estimated LVEF: 10%–15%), apical ventricular aneurysm formed mitral regurgitation (mild to moderate), decreased left ventricular diastolic function, main valve degeneration with mild regurgitation, and tricuspid valve mild regurgitation (bedside ultrasound images were not available).
Plain head computed tomography (CT): Images obtained on admission showed no obvious signs of stroke (Figure 1B).
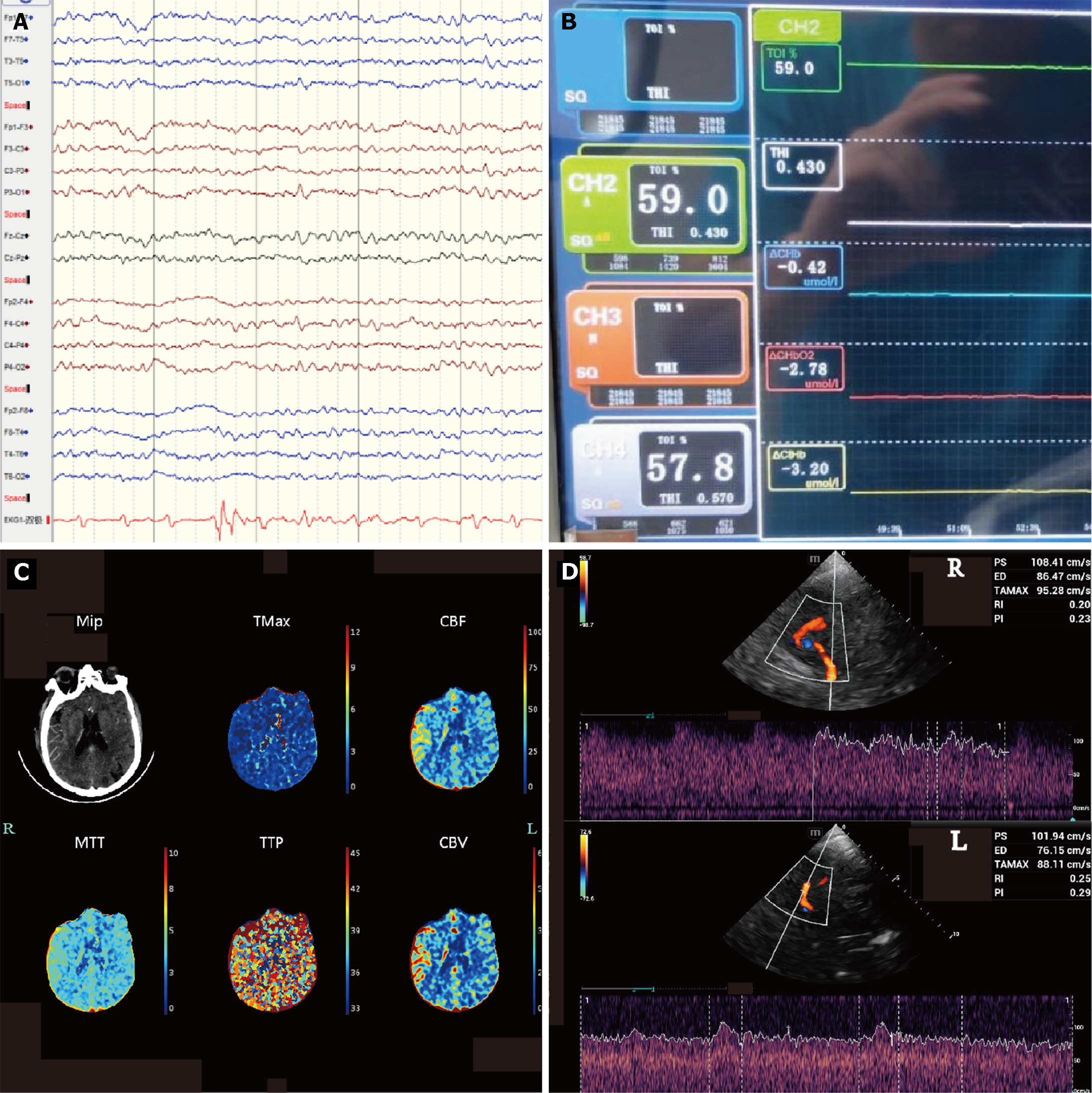
The initial neurological function evaluation on the first day after admission failed to confirm the patient's mental state. After the administration of naloxone and flumazenil, the Glasgow Coma Scale (GCS) scores were 2 + endotracheal tube + 3. Considering that the patient had experienced cardiac arrest and had a long history of cerebral ischemia, head CTP was performed to rule out stroke. The image showed a large ischemic hypoperfusion in the right frontotemporal lobe, suggesting cerebral infarction and poor compensatory blood flow (Figure 1C). Concurrent CTA of the neck performed to identify the vessel of infarction showed sparse blood flow in the right common carotid artery (CCA), M1 segment of the right middle cerebral artery (MCA), and vertebral artery (VA) (Figure 1D). However, the results of other continuous monitoring at this time were unusual, with no corresponding pathological signs on clinical examination, no significant changes in bilateral continuous electroencephalography (cEEG) monitoring (Figure 2A), and no differences in near-infrared spectroscopy (NIRS) (Figure 2B). Combined with the analysis of other cases, this "false" cerebral infarction may be caused by differential blood flow. We reduced the ECMO flow and repeated CTP, which showed symmetric perfusion in the cerebral hemispheres and cerebellum (Figure 2C), while pulsatile flow in the right MCA and advection in the left MCA were found in transcranial Doppler (TCD) images, with similar flow rates (Figure 2D).
The patient was diagnosed with cardiac arrest successfully managed with VA-ECMO maintenance, coronary atherogenic heart disease (NYHA grade IV), pneumonia and respiratory failure, renal insufficiency (continuous renal replacement therap status), and type 2 diabetes mellitus.
The patient underwent continuous VA-ECMO maintenance and received norepinephrine for circulation, aspirin for antiplatelet therapy, and heparin to maintain APTT (60–80 s) during anticoagulant therapy.
The patient discontinued treatments, such as heart transplantation, due to financial problems.
With the increasing use of VA-ECMO, there has been more focus on the acute neurological function of patients[7]. Many studies have found a high incidence of neurological complications[8], which may be related to various factors, including acute ischemic stroke or cerebral hemorrhage, seizures, toxicity, metabolic or electrolyte disorders, hypoxia, global or regional hypoperfusion, and hyperperfusion[5]. The The Extracorporeal Life Support Organization (ELSO) guidelines and latest literature suggest increased attention and higher requirements for the monitoring of cerebral perfusion[2], especially for patients with low heart rates after CPR and VA-ECMO, who exhibit an increased incidence of neurological complications[9]. However, these patients often require brain protection and tracheal intubation, making routine mental health assessment more difficult. Therefore, it is necessary to rely on imaging methods for evaluation. Head CTP and CTA are the most common monitoring methods[5]; their diagnostic specificity and sensitivity are high, and they are widely used in clinical practice.
The contrast agent must be injected during CTP and CTA, which may cause artifacts owing to the abnormal blood flow pattern of VA-ECMO once it circulates throughout the body. An increasing number of detailed studies have been conducted on this topic[4]. This phenomenon is due to the fact that peripheral VA-ECMO is usually performed by femoral artery and vein catheterization. ECMO perfusion blood flow is retrograde to the heart through the femoral artery and is hybridized with the antegrade stroke blood flow of the heart. The intersection of the two blood flows is called the watershed region, which produces blood flow-related artifacts on first-pass CTP/CTA images. The location of the watershed region depends on the native cardiac function and ECMO flow.
Theoretically, as cardiac output gradually decreases, the watershed level gradually increases. In smaller cases, the watershed level may appear in the central part of the aortic arch (between the brachiocetic trunk and the left CCA), resulting in the left side of the brain being perfused by advection ECMO blood flow and the right side of the brain being perfused by the own heart blood flow. On CTA, acute stroke appears. These cases are difficult to identify clinically. Aboul Nour et al[6] reported that this phenomenon occurs during CTA when the perfusion tube is entered through the axillary artery, resulting in a performance similar to that of an acute stroke. However, peripheral femoral VA-ECMO is more common in clinical practice, and related cases are rare. Acharya et al[5] reported that the intubation of patients with peripheral femoral-femoral VA-ECMO and an aortic arch watershed can lead to strange artifacts on head and neck CTA images. These erroneous imaging findings have significant clinical implications.
No large patient studies have described how various VA-ECMO configurations affect imaging quality and interpretation in CTP/CTA. However, it is important to recognize and understand potential CT imaging deficits in patients receiving VA-ECMO. In critically ill patients, it is necessary to evaluate the neurological function more accurately and quickly; however, this is very difficult. For example, traditional magnetic resonance imaging cannot be performed normally in patients undergoing ECMO. Therefore, the ELSO guidelines suggest bedside, noninvasive, and continuous neurological monitoring, including TCD, NIRS, and cEEG[2].
TCD may be a reasonable alternative or adjunctive option, as it can detect flow in the internal carotid artery and VA without contrast injection. Moreover, TCD detected intracranial occlusions (sensitivity, 50%; specificity, 100% for total MCA occlusion). In a systematic review of nine studies, TCD displays had an estimated sensitivity of 95% and specificity of 95% for the detection of intracranial large vessel occlusion[10]. Another major advantage of TCD is that it is portable and fast. Therefore, any ultrasound doctor and formally trained emergency intensive care unit doctor can quickly and skillfully perform a TCD examination at any time. This can serve as a differential means of CTA and CTP; even when CTP and CTA cannot be distinguished, it can provide supplementary evidence for auxiliary diagnosis. Additionally, TCD may have utility for patients undergoing ECMO. In particular, the display of the differential mean flow velocity and pulsatility index can play a "quantitative" role in continuous neurological function monitoring[11]. In this case, bedside TCD showed excellent function, not only indicating a difference in flow patterns between the left and left middle cerebral arteries but also indicating that there was no significant deterioration in perfusion on either side, allowing us to make a more accurate diagnosis.
Another technique for continuous neurological monitoring is NIRS, which is a noninvasive method for monitoring continuous trends in oxygen saturation in non-pulsatile areas (SrO2) that can be used to monitor cerebral oxygen saturation (usually on the left and right forehead) in sealed patients[12]. In recent years, it has emerged as a crucial option for noninvasive and continuous monitoring of brain function. It monitors the oxygen supply to the cerebral cortex through infrared technology and dynamically observes changes in neural function. In this patient, there was no significant change in cerebral oxygen levels, even in the presence of positive CTP. It plays a pivotal role in identifying cerebral infarctions.
cEEG, as the basis of the multimodal brain, is increasingly used in ECMO patients, which is helpful for the prognosis of patients, especially for the prognosis of those with cardiac arrest. Previous studies have shown that cEEG has unique advantages in the prognosis of neurological function and determination of the survival rate in patients with brain injury[13].
Common strategies to mitigate the effects of ECMO include temporary cessation or slowing of the ECMO flow, direct injection of contrast material into the ECMO outflow zone, and increased delay periods[14]. These strategies are used to manipulate the location of a watershed region, change the order of contrast enhancement, and use blood recirculation to deliver contrast to the area to be examined. Among them, reducing the ECMO flow to guide more contrast material through the natural circulation or to perform delayed-phase imaging has been mentioned in many studies. Unfortunately, most extracorporeal pulmonary resuscitation and cardiac arrest patients with low cardiac output have significant hemodynamic instability; thus, reducing ECMO flow to complete CTP/CTA is risky behavior, and transferring these critically ill patients to CT imaging or catheter angiography increases the risk of transport.
In our case, after the patient experienced cardiac arrest, he presented with a comatose state (GCS score, 6), and the conventional physical examination could not provide an accurate evaluation. Therefore, it is necessary to quickly determine whether a stroke has occurred. To confirm the diagnosis, we performed CTP after the circulation was relatively stable. To reduce artifacts, contrast material was injected through an ECMO perfusion tube. However, the results suggested large ischemic hypoperfusion in the right frontotemporal lobe (Figure 1C). Concurrent CTA of the neck was performed to identify the infarcted vessel, which revealed no perfusion in the left MCA (Figure 1D). Such results would almost certainly confirm the diagnosis of stroke. However, the findings of other continuous monitoring at this time were unusual, with no corresponding pathological signs on clinical examination, no differences in NIRS (Figure 2B), and no significant changes in bilateral cEEG monitoring (Figure 2A). Therefore, we believe that this "false" cerebral infarction may have been caused by differential blood flow. To confirm this hypothesis, we reduced the ECMO flow and repeated the CTP, which showed symmetric perfusion in the cerebral hemispheres and cerebellum (Figure 2C), with a remarkable difference of only a few hours between the two CTPs. On the following day, the patient's limb movements were symmetrical on both sides, which is consistent with the results. Unfortunately, due to financial reasons, the patient discontinued treatment on the 7th day of treatment.
Our findings, akin to those of previous results, revealed a very rare case of cerebral pseudoinfarction due to an ECMO watershed in the center of the aortic arch, despite utilizing as many monitoring methods as possible to ascertain neurological function and preclude a false clinical diagnosis. However, our study also presents some limitations. First, DSA is often considered the gold standard for diagnosing vascular diseases. Due to the patient's circulatory instability, DSA was deemed too risky by interventional experts and consequently not performed. Second, reducing the ECMO flow rate is a common method to avoid artifacts in many ECMO centers, but it also increases the risk to patients during the examination, especially for those without cardiac function. This method was not preferred to avoid artifacts in this patient. Finally, the patient discontinued the treatment due to financial reasons; therefore, no follow-up was conducted to confirm our hypothesis.
Due to the limited number of reports and lack of additional research data, there are no mature clinical recommendations. The clinical decisions for such patients are also very different in different ECMO centers. Whether routine CTP/CTA can detect acute stroke earlier is not supported by large-scale research data, and the risk of transport and contrast media-induced acute kindey injury must be considered.
Watersheds in the center of the aortic arch caused by VA-ECMO are rare, and recognizing this situation is necessary. We suggest that clinicians use noninvasive, continuous monitoring methods, such as TCD, NIRS, and cEEG, as a means of identification. Furthermore, TCD has been continuously monitored in recent studies, which may benefit clinical management.
CTP and CTA of peripheral VA-ECMO may lead to the appearance of pseudostroke. In such cases, TCD, NIRS, and cEEG can be used as early identification methods. Reducing ECMO flow can improve the quality of imaging; however, it is associated with some risks. Early identification of image artifacts can prevent the serious consequences of misdiagnosis. Currently, there is a lack of specific and sensitive indicators to assess the neurological function after VA-ECMO. Multiple indicators and continuous brain function evaluation can help to more accurately assess the neurological function of patients receiving VA-ECMO.
| 1. | Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, Naar J, Smalcova J, Hubatova M, Hromadka M, Volovar S, Seyfrydova M, Jarkovsky J, Svoboda M, Linhart A, Belohlavek J; ECMO-CS Investigators. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation. 2023;147:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 257] [Article Influence: 128.5] [Reference Citation Analysis (0)] |
| 2. | Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Meyns B, Haft J, Vercaemst L, Pappalardo F, Bermudez C, Belohlavek J, Hou X, Boeken U, Castillo R, Donker DW, Abrams D, Ranucci M, Hryniewicz K, Chavez I, Chen YS, Salazar L, Whitman G. ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients. ASAIO J. 2021;67:827-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 3. | Sutter R, Tisljar K, Marsch S. Acute Neurologic Complications During Extracorporeal Membrane Oxygenation: A Systematic Review. Crit Care Med. 2018;46:1506-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Shen J, Tse JR, Chan F, Fleischmann D. CT Angiography of Venoarterial Extracorporeal Membrane Oxygenation. Radiographics. 2022;42:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Acharya J, Rajamohan AG, Skalski MR, Law M, Kim P, Gibbs W. CT Angiography of the Head in Extracorporeal Membrane Oxygenation. AJNR Am J Neuroradiol. 2017;38:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Aboul Nour H, Poyiadji N, Mohamed G, Alsrouji OK, Ramadan AR, Griffith B, Marin H, Chebl AB. Challenges of acute phase neuroimaging in VA-ECMO, pitfalls and alternative imaging options. Interv Neuroradiol. 2021;27:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 7. | Le Guennec L, Cholet C, Huang F, Schmidt M, Bréchot N, Hékimian G, Besset S, Lebreton G, Nieszkowska A, Leprince P, Combes A, Luyt CE. Ischemic and hemorrhagic brain injury during venoarterial-extracorporeal membrane oxygenation. Ann Intensive Care. 2018;8:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Hunt MF, Clark KT, Whitman G, Choi CW, Geocadin RG, Cho SM. The Use of Cerebral NIRS Monitoring to Identify Acute Brain Injury in Patients With VA-ECMO. J Intensive Care Med. 2021;36:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Low CJW, Ramanathan K, Ling RR, Ho MJC, Chen Y, Lorusso R, MacLaren G, Shekar K, Brodie D. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with cardiac arrest: a comparative meta-analysis and trial sequential analysis. Lancet Respir Med. 2023;11:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 10. | Mattioni A, Cenciarelli S, Eusebi P, Brazzelli M, Mazzoli T, Del Sette M, Gandolfo C, Marinoni M, Finocchi C, Saia V, Ricci S. Transcranial Doppler sonography for detecting stenosis or occlusion of intracranial arteries in people with acute ischaemic stroke. Cochrane Database Syst Rev. 2020;2:CD010722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Salna M, Ikegami H, Willey JZ, Garan AR, Cevasco M, Chan C, Takayama H, Colombo PC, Naka Y, Takeda K. Transcranial Doppler is an effective method in assessing cerebral blood flow patterns during peripheral venoarterial extracorporeal membrane oxygenation. J Card Surg. 2019;34:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Douflé G, Ferguson ND. Monitoring during extracorporeal membrane oxygenation. Curr Opin Crit Care. 2016;22:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | deCampo D, Husari KS, Bembea MM, Habela CW, Ritzl EK. Continuous Electroencephalography (EEG) Protocol Improves Seizure Detection in Children on Extracorporeal Membrane Oxygenation. J Child Neurol. 2023;38:581-589. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Gullberg Lidegran M, Gordon Murkes L, Andersson Lindholm J, Frenckner B. Optimizing Contrast-Enhanced Thoracoabdominal CT in Patients During Extracorporeal Membrane Oxygenation. Acad Radiol. 2021;28:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |