Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.3004
Revised: April 12, 2024
Accepted: April 23, 2024
Published online: June 16, 2024
Processing time: 104 Days and 1.9 Hours
Coronary heart disease is associated with coronary atherosclerosis indicated by carotid intima-media thickness (CIMT) thickening and altered vascular elasticity. The epicardial adipose layer can secrete proinflammatory factors that promote the formation of coronary atherosclerosis. Thus, the epicardial fat layer thickness (EAT) may also predict coronary heart disease.
To determine the role of common carotid artery ultrasound parameters and EAT in the early diagnosis of coronary artery disease.
Based on coronary angiography, patients with newly suspected coronary heart disease were divided into case (n = 107) and control (n = 41) groups. The carotid ultrasound parameters, including vascular stiffness (β), elastic coefficient (EP), pulse wave conduction velocity (PWV-β), CIMT, and EAT were compared between the case and control groups and among patients with different lesion numbers in the case group. Pearson correlation was used to evaluate the early diagnostic value of EAT, common carotid artery elasticity, and CIMT for coronary heart disease.
EP, β, PWV-β, CIMT, and EAT were significantly higher in the case group compared with the levels in the control group (all P < 0.001). In the case group, lesions were detected in one vessel in 34 patients, two vessels in 38 patients, and three vessels in 35 patients. Within the case group, β, EP, PWV-β, CIMT, and EAT levels significantly increased with an increased number of lesions (all P < 0.001). EAT positively correlated with β, EP, PWV-β, and CIMT (all P < 0.01). The area under the curve for diagnosing coronary heart disease using EAT combined with CIMT and carotid elasticity was 0.893, and the sensitivity and specificity were 0.890 and 0.837.
EAT correlated well with changes in carotid artery elasticity and CIMT in patients with coronary heart disease. The combination of EAT, carotid artery elasticity, and CIMT facilitates the early diagnosis of coronary heart disease.
Core Tip: Carotid intima-media thickening (CIMT) is a precursor to atherosclerosis. The epicardial fat layer secretes proinflammatory factors that contribute to coronary artery disease caused by coronary atherosclerosis. Our study focused on determining the correlation between epicardial adipose layer thickness (EAT) and changes in common carotid artery elasticity and CIMT on ultrasound. The combined detection of EAT, carotid artery elasticity, and CIMT facilitates the early diagnosis of coronary heart disease.
- Citation: Xu M, Lu ZY. Early diagnostic value of carotid artery ultrasound parameters combined with epicardial adipose layer thickness in coronary heart disease. World J Clin Cases 2024; 12(17): 3004-3011
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/3004.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.3004
Coronary heart disease is a vascular disease caused by coronary atherosclerotic plaque formation, which causes vascular stenosis or obstruction, leading to myocardial ischemia, hypoxia, and even necrosis[1]. The formation of atherosclerotic plaques is a chronic process[2]. During progression of the disease, a vicious cycle develops; coronary atherosclerotic plaques increase and the coronary artery lumen gradually narrows exacerbating myocardial ischemia and hypoxia. The development of coronary heart disease can be assessed using coronary angiography (CAG), which can clearly and dyna
Arterial intima thickening is a precursor to atherosclerosis[4]. The carotid intima-media thickness (CIMT) directly affects vascular elasticity; thickening decreases carotid artery elasticity, leading to atherosclerosis[5]. Thus, the assessment of vascular elasticity by carotid artery ultrasound may predict atherosclerosis. The surface of the myocardium is covered with a layer of visceral adipose tissue, called the epicardial adipose layer, which developed from embryonic brown adipose tissue. The epicardial adipose layer can secrete various proinflammatory factors[6] to promote the formation of coronary atherosclerosis[7]. Therefore, epicardial fat layer thickness (EAT), which is measured by ultrasound, may predict coronary atherosclerosis. Coronary artery stenosis is closely related to lesions in the internal and external carotid arteries[8]. However, the correlation between EAT, CIMT, and changes in carotid artery elasticity is unclear. In this study, the correlation between EAT and changes in carotid artery elasticity and CIMT was determined in 148 patients with suspected coronary heart disease at the initial diagnosis. The use of EAT and changes in carotid artery elasticity and CIMT for the early diagnosis of coronary heart disease was investigated.
The data for 148 patients with suspected coronary heart disease at the time of initial diagnosis at Suzhou Ninth People’s Hospital from January 2022 to December 2023 were collected. Inclusion criteria were as follows: (1) No prior history of coronary heart disease; (2) admitted to the hospital due to symptoms, including chest tightness, shortness of breath, and fatigue, at the initial diagnosis, and the electrocardiogram examination was abnormal, leading to the suspicion of coronary heart disease; (3) age ≥ 20 years old; (4) complete clinical data; (5) no smoking history or smoking cessation for more than 5 years; and (6) carotid artery ultrasound examination and EAT results available. The exclusion criteria were as follows: (1) Severe valvular disease or congenital heart disease; (2) malignant tumor; (3) unclear ultrasound image so pericardial effusion or epicardial fat layer could not be identified; (4) severe liver or kidney dysfunction; (5) common carotid artery stenosis; and (6) thyroid disease. Patients were grouped into the case group (n = 107) if coronary heart disease was confirmed by CAG. Patients diagnosed with noncoronary heart disease based on the CAG were grouped into the control group (n = 41).
The following data were retrospectively analyzed: Clinical data, including age, gender, body mass index (BMI), heart rate, right atrial diameter, history of alcohol consumption (drinking ≥ 50 mL per day for more than six months), fasting blood glucose (FPG), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), and ultrasound data, including vascular stiffness (β), elastic coefficient (EP), pulse wave conduction velocity (PWV-β), CIMT, and EAT.
An MP20 monitor (Philips, Netherlands) was used for routine monitoring of SBP, DBP, and heart rate. Venous blood (3–5 mL) was collected from patients on an empty stomach (fasting for more than 8 h) in the morning and FPG, TG, TC, LDL-C, and HDL-C were detected using an automatic biochemical analyzer (Beckman Coulter AU5800). CAG was performed using an ALOKA a10 color Doppler ultrasound diagnostic instrument (Japan) connected to an electrocardiogram. The patient was placed in a supine position to expose the neck. The long axis section of the carotid artery was selected for CIMT detection, The elastic parameters (β, EP, and PWV-β) of the common carotid artery were calculated using an echo tracking technique. After examining the common carotid artery, the patient was placed in the left lateral position and an S5-1 probe (1–5 MHz) was used to explore the left ventricular long axis section. EAT was measured perpendicular to the anterior wall of the right ventricle at the end of systole (the thickest part of the epicardial adipose layer). The measurement was repeated three times and the average was used in the analysis.
After admission, all patients underwent CAG examinations. CAG is the gold standard for diagnosing coronary heart disease. CAG was performed using the standard radial or femoral Judkins method. Each blood vessel underwent at least 3 multiposition exposures. Coronary heart disease was defined as at least one epicardial coronary artery or the main branch of the coronary artery with a degree of stenosis ≥ 50%, and coronary heart disease patients were classified based on the number of narrowed vessels detected by CAG.
SPSS 27.0 software was used to analyze data. Data are expressed as means ± SD. t-tests were used to compare two groups, and multiple groups were compared with the variance (F) test. Count data are expressed as rates (%), and two groups were compared with χ2 test. Correlations were determined using Pearson’s test. Receiver operating characteristic (ROC) curves were used to analyze the early diagnostic value of EAT, carotid artery elasticity, and CIMT for coronary heart disease. P < 0.05 indicated statistically significant differences.
No significant differences in age, gender, BMI, heart rate, right atrial diameter, history of alcohol consumption, SBP, DBP, TC, TG, HDL-C, and LDL-C indicators were detected between the case and control groups (P > 0.05) (Table 1). β, EP, PWV-β, CIMT, and EAT were significantly higher in the case group compared with the corresponding parameters in the control group (P < 0.001) (Table 2).
| Clinical data | Case group (n = 107) | Control group (n = 41) | t/χ2 value | P value |
| Age (yr) | 59.54 ± 10.62 | 59.28 ± 10.53 | 0.134 | 0.893 |
| Gender | 0.774 | 0.379 | ||
| Male | 62 (57.94) | 27 (65.85) | ||
| Female | 45 (42.06) | 14 (34.15) | ||
| BMI, kg/m2 | 24.18 ± 1.51 | 23.79 ± 1.26 | 1.469 | 0.144 |
| Heart rate, beats/min | 77.43 ± 5.65 | 76.51 ± 5.47 | 0.894 | 0.373 |
| Upper and lower diameter of right atrium, mm | 45.17 ± 5.98 | 45.04 ± 5.85 | 0.119 | 0.905 |
| Drinking history | 31 (28.97) | 8 (19.51) | 1.367 | 0.242 |
| FPG, mmol/L | 5.26 ± 0.35 | 5.18 ± 0.26 | 1.329 | 0.186 |
| SBP, mm Hg | 131.84 ± 15.46 | 127.39 ± 10.57 | 1.696 | 0.092 |
| DBP, mm Hg | 84.33 ± 8.92 | 81.58 ± 8.06 | 1.722 | 0.087 |
| TC, mmol/L | 3.74 ± 0.51 | 3.85 ± 0.62 | 1.104 | 0.271 |
| TG, mmol/L | 1.42 ± 0.26 | 1.34 ± 0.23 | 1.727 | 0.086 |
| HDL-C, mmol/L | 1.08 ± 0.15 | 1.12 ± 0.17 | 1.398 | 0.164 |
| LDL-C, mmol/L | 2.32 ± 0.54 | 2.28 ± 0.51 | 0.409 | 0.683 |
| Ultrasound data | Case group (n = 107) | Control group (n = 41) | t value | P value |
| β | 9.32 ± 2.75 | 4.18 ± 0.93 | 11.690 | < 0.001 |
| EP (kPa) | 117.84 ± 31.49 | 56.92 ± 14.17 | 11.910 | < 0.001 |
| PWV-β (m/s) | 7.81 ± 1.27 | 4.95 ± 0.86 | 13.290 | < 0.001 |
| CIMT (mm) | 0.97 ± 0.32 | 0.63 ± 0.13 | 6.587 | < 0.001 |
| EAT (mm) | 7.48 ± 2.13 | 2.04 ± 0.64 | 16.050 | < 0.001 |
Among the 107 patients with coronary heart disease, 34 patients had 1-vessel disease, 38 patients had 2-vessel disease, and 35 patients had 3-vessel disease. EP, β, PWV-β, CIMT and EAT levels significantly increased as the number of involved vessels increased: 1-vessel disease < 2-vessel disease < 3-vessel disease (all, P < 0.001) (Table 3).
| Ultrasonic data | 1-vessel disease (n = 34) | 2-vessel disease (n = 38) | 3-vessel disease (n = 35) | F value | P value |
| β | 7.48 ± 2.35 | 9.18 ± 2.72a | 10.99 ± 3.16a,b | 13.901 | < 0.001 |
| Ep (kPa) | 89.92 ± 25.06 | 118.24 ± 31.57a | 141.15 ± 35.83a,b | 23.310 | < 0.001 |
| PWV-β (m/s) | 6.56 ± 1.04 | 7.79 ± 1.25a | 8.82 ± 1.47a,b | 27.493 | < 0.001 |
| CIMT (mm) | 0.75 ± 0.23 | 0.96 ± 0.28a | 1.17 ± 0.34a,b | 18.450 | < 0.001 |
| EAT (mm) | 5.19 ± 1.58 | 7.52 ± 2.26a | 9.45 ± 2.87a,b | 29.592 | < 0.001 |
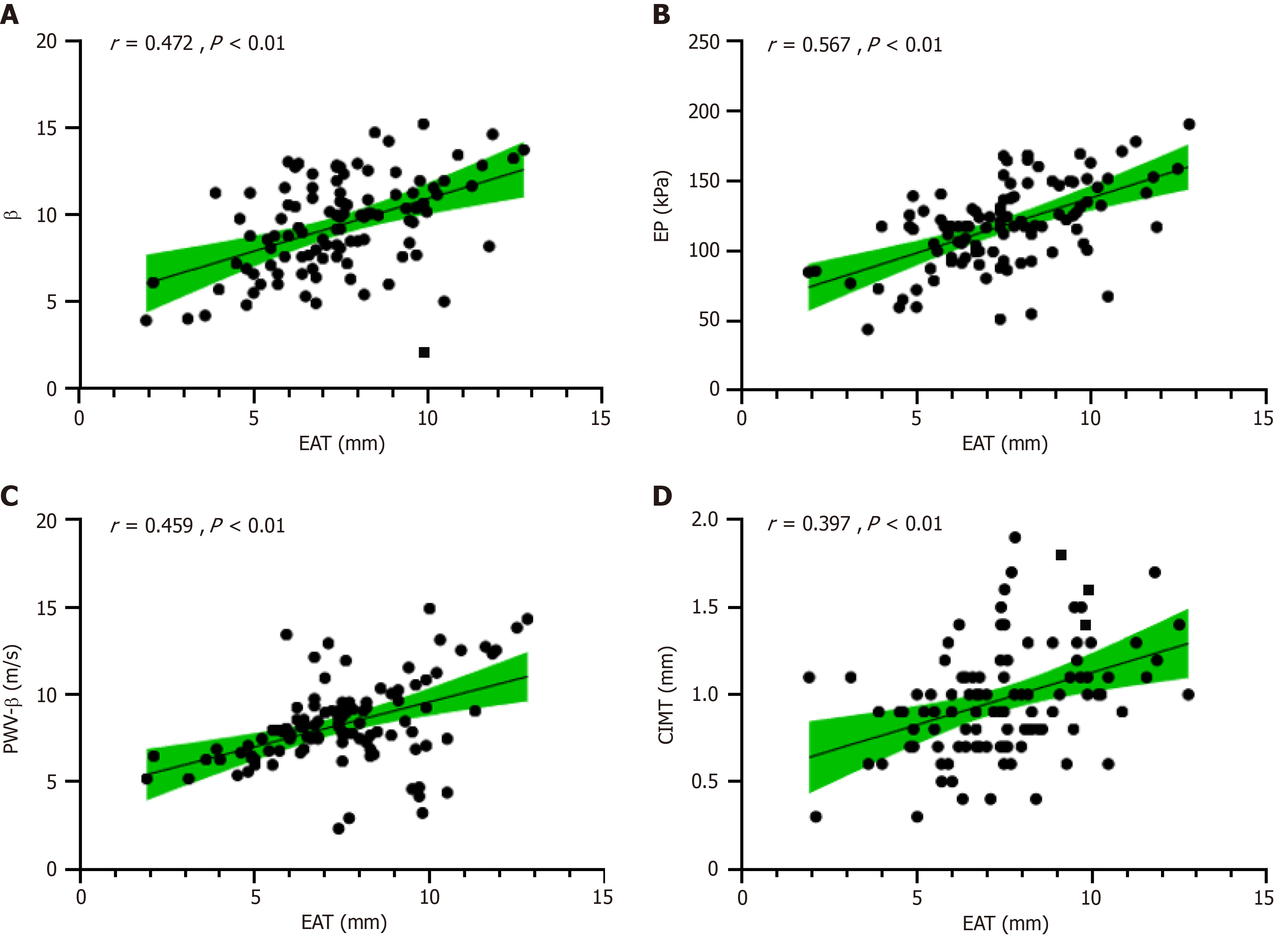
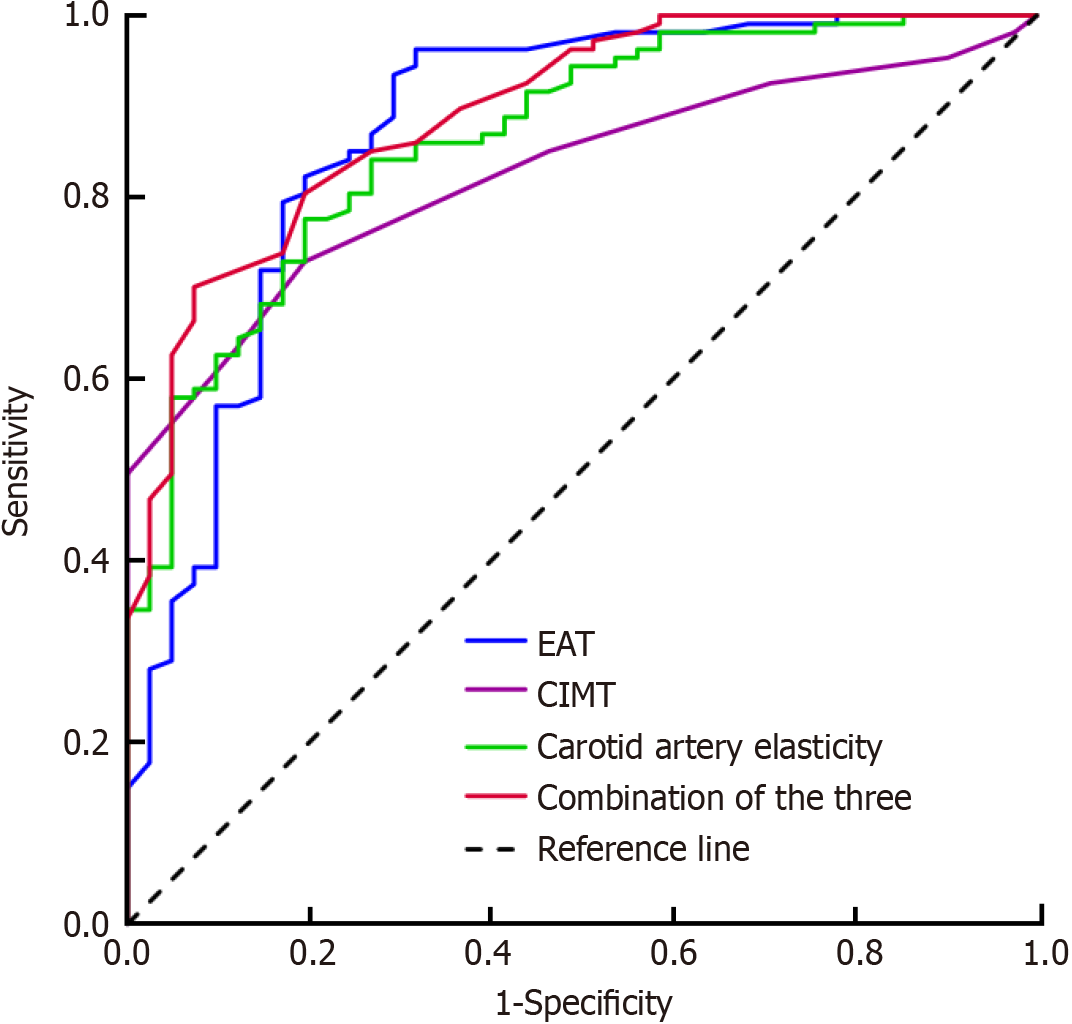
EAT positively correlated with β, EP, PWV-β, and CIMT (r = 0.472, 0.567, 0.459, and 0.397, respectively; all P < 0.01) (Figure 1). The area under the curve (AUC) of EAT combined with CIMT and carotid artery elasticity for predicting coronary heart disease was 0.893, and the sensitivity and specificity were 0.890 and 0.837, respectively, which were superior to EAT alone (AUC, 0.872; sensitivity, 0.863; and specificity, 0.814), CIMT alone (AUC, 0.826; sensitivity, 0.805; and specificity, 0.789), or the elasticity of the common carotid artery alone (AUC, 0.864; sensitivity, 0.854; and specificity, 0.802) (Figure 2 and Table 4).
| Index | AUC | 95%CI | Optimal cutoff value | Sensitivity | Specificity |
| EAT (mm) | 0.872 | 0.801–0.943 | 6.35 | 0.863 | 0.814 |
| CIMT (mm) | 0.826 | 0.760–0.891 | 0.95 | 0.805 | 0.789 |
| Carotid artery elasticity | 0.864 | 0.803–0.926 | 0.854 | 0.802 | |
| Combination of the three | 0.893 | 0.839–0.947 | 0.890 | 0.837 |
CAG has gradually developed into the gold standard for diagnosing coronary heart disease. However, due to the invasiveness, high cost, poor repeatability, and inconsistent tolerance of patients to the contrast media, CAG cannot be performed on all patients. CAG may cause complications, such as vascular spasm, dissection, and local lesion site trauma. Furthermore, the diagnostic value is highly controversial[9]. Therefore, exploring noninvasive diagnostic methods with high predictive accuracy has become a hot topic in clinical research.
EAT is the visceral fat between the myocardium and pericardium, covering 80% of the heart surface[10]. The EAT is supplied by the coronary artery. Therefore, the EAT thickness is closely related to the condition of the myocardium and the coronary artery[11]. The thickness of the EAT can be measured through a simple two-dimensional echocardiography. This procedure is simple, low-cost, noninvasive, and easy to obtain[12]. During the development of atherosclerosis, changes in arterial elasticity occur earlier than changes in atherosclerosis and vascular morphology[13]. Carotid atherosclerosis and coronary atherosclerosis have a common pathological basis and common risk factors[14]. The anatomical position of the carotid artery is relatively shallow; thus, high-quality images can be obtained using ultrasound[15]. This, carotid ultrasound may be ideal for determining the progression of coronary artery disease[16].
The carotid ultrasound parameters β, EP, and PWV-β are sensitive indicators for evaluating arterial elasticity. Increases in β and EP indicate decreased arterial elasticity. PWV-β, the propagation speed of pulse waves in arteries, indicates the degree of vessel hardening, which affects arterial compliance and stiffness. A faster PWV-β indicates higher arterial stiffness and decreased compliance[17]. CIMT reflects diffuse thickening of the carotid artery and is an inflammatory indicator. CIMT is used to identify early subclinical atherosclerosis[18]. In this study, β, EP, PWV-β, CIMT, and EAT levels increased significantly as the number of lesions increased. Consistent with the results of Guo et al[19], EAT thickening decreased carotid artery elasticity and increased CIMT[19]. Based on these results, thicker EAT corresponds with more severe coronary artery disease. The EAT surrounds the main branches of the coronary vessels and can secrete interleukin-6 and tumor necrosis factor α, which induce the release of other proinflammatory mediators. These inflammatory factors can directly mediate damage to the surrounding myocardium and coronary artery and promote plaque formation. At the same time, the proinflammatory factors circulate in the blood to the carotid artery and cause inflammation, dysfunction, and increased thickness of the intima-media layer of the carotid artery, leading to distortion of arterial blood vessels and reduced arterial elasticity.
The correlation analysis revealed that EAT thickening positively correlated with CIMT thickening and decreased carotid artery elasticity. The EAT is close to the myocardium and has special metabolism, heat production, and mechanical functions. The EAT is also an important source of bioactive molecules, such as adiponectin and inflammatory cytokines. Thus, the EAT acts as an endocrine gland organ, regulating heart and blood vessel functions through paracrine and vascular secretion mechanisms[20]. During the synthesis of the EAT, inflammatory cytokines are produced and can be released by endothelial cells. The inflammatory cytokines diffuse through the coronary artery wall and modulate the occurrence and development of atherosclerosis. Adipocytokines and free fatty acids (FFAs) are released from epicardial tissue into blood vessels and are transported to the arterial wall[21]. FFAs can induce the expression of inflammatory factors through phosphatidylinositol-3-kinase/serine-threonine protein kinase and mitogen-activated protein kinase signaling pathways to increase CIMT thickness. Thus, the FFAs are also involved in the development of atherosclerosis. In addition, FFAs reduce the bioavailability of nitric oxide, which regulates the function of vascular endothelial cells, and cause erosion and rupture of atherosclerosis to promote thrombosis. EAT can also promote atherosclerosis through mechanical action. Increased EAT thickness causes arterial distortion, and the resulting shear stress causes turbulence, leading to decreased arterial elasticity[22] and the accelerated development of atherosclerosis.
Early carotid atherosclerosis leads to decreased arterial elasticity in patients with coronary heart disease[23]. Carotid atherosclerosis acts directly on the heart, increasing cardiac systolic resistance, improving myocardial compensation, and inducing ventricular remodeling. Continuous central nervous system[24] excitement causes arterial structural remo
This study has several limitations. The sample size is small. In addition, the correlation between EAT and carotid artery ultrasound parameters and coronary heart disease, and the mechanistic link of these parameters with coronary heart disease require clarification. Further evidence from multicenter large-scale studies is needed to confirm our results.
EAT and changes in carotid artery elasticity and CIMT correlate well in patients with coronary heart disease. The combination of carotid artery ultrasound parameters and EAT detection is expected to become a noninvasive examination method for the early diagnosis of coronary heart disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Wilkes A, United States S-Editor: Liu H L-Editor: A P-Editor: Zhao YQ
| 1. | Song Y, Dang Y, Wang P, Tian G, Ruan L. CHD is Associated With Higher Grades of NAFLD Predicted by Liver Stiffness. J Clin Gastroenterol. 2020;54:271-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Jin C, Sharma AN, Thevakumar B, Majid M, Al Chalaby S, Takahashi N, Tanious A, Arockiam AD, Beri N, Amsterdam EA. Carcinoid Heart Disease: Pathophysiology, Pathology, Clinical Manifestations, and Management. Cardiology. 2021;146:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Gujral DM, Shah BN, Chahal NS, Bhattacharyya S, Hooper J, Senior R, Harrington KJ, Nutting CM. Carotid intima-medial thickness as a marker of radiation-induced carotid atherosclerosis. Radiother Oncol. 2016;118:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Zhang LJ, Tang CX. [Novel study and applications of CT angiography derived fractional flow reserve in China]. Zhonghua Yi Xue Za Zhi. 2022;102:2563-2566. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Kang HJ, Kim MH, Sung J, Kim SH, Kim CH, Park JE, Ge J, Oh BH; IMPACT on IMT investigators. Effect of Probucol and/or Cilostazol on Carotid Intima Media Thickness in Patients with Coronary Heart Disease: A Randomized, Multicenter, Multinational Study. J Atheroscler Thromb. 2021;28:124-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Polyakova EA, Berkovich OA, Baranova EI. Prognostic value of epicardial fat thickness in coronary heart disease patients after myocardial revascularization. Kardiologiia. 2020;60:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Åkra S, Seljeflot I, Braathen B, Bratseth V, Hansen CH, Arnesen H, Tønnessen T, Solheim S. The NLRP3 inflammasome activation in subcutaneous, epicardial and pericardial adipose tissue in patients with coronary heart disease undergoing coronary by-pass surgery. Atheroscler Plus. 2022;48:47-54. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Bytyçi I, Shenouda R, Wester P, Henein MY. Carotid Atherosclerosis in Predicting Coronary Artery Disease: A Systematic Review and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2021;41:e224-e237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Chmiel C, Reich O, Signorell A, Tandjung R, Rosemann T, Senn O. Appropriateness of diagnostic coronary angiography as a measure of cardiac ischemia testing in non-emergency patients - a retrospective cross-sectional analysis. PLoS One. 2015;10:e0117172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Tekaya AB, Mehmli T, Mrad IB, Fendri A, Boukriba S, Bouden S, Rouached L, Tekaya R, Saidane O, Mahmoud I, Abdelmoula L. Increased epicardial adipose tissue thickness correlates with endothelial dysfunction in spondyloarthritis. Clin Rheumatol. 2022;41:3017-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Nyawo TA, Dludla PV, Mazibuko-Mbeje SE, Mthembu SXH, Nyambuya TM, Nkambule BB, Sadie-Van Gijsen H, Strijdom H, Pheiffer C. A systematic review exploring the significance of measuring epicardial fat thickness in correlation to B-type natriuretic peptide levels as prognostic and diagnostic markers in patients with or at risk of heart failure. Heart Fail Rev. 2022;27:665-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Saha S, Singh R, Mir IA, Bansal N, Singh PK, Nadeem M. Epicardial Fat Thickness: A Cardiometabolic Risk Marker in Rheumatoid Arthritis. Cureus. 2022;14:e21397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Demir AM, Kuloğlu Z, Yaman A, Fitöz S, Nergizoğlu G, Kansu A. Carotid intima-media thickness and arterial stiffness as early markers of atherosclerosis in pediatric celiac disease. Turk J Pediatr. 2016;58:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Qin C, Zhang L, Wang X, Duan Y, Ye Z, Xie M. Evaluation of Carotid Plaque Neovascularization in Patients With Coronary Heart Disease on Contrast-Enhanced Ultrasonography. J Ultrasound Med. 2018;37:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Zhang H, Liu M, Ren T, Wang X, Liu D, Xu M, Han L, Wu Z, Li H, Zhu Y, Wen Y, Sun W. Associations between Carotid Artery Plaque Score, Carotid Hemodynamics and Coronary Heart Disease. Int J Environ Res Public Health. 2015;12:14275-14284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Singh TA, Villines TC, Taylor AJ. The triple line pattern on carotid intima media thickness imaging and its relationship to cardiovascular risk factors in patients on lipid lowering therapy. Vasc Health Risk Manag. 2014;10:363-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Gale NS, Albarrati AM, Munnery MM, Mcdonnell BJ, Benson VS, Singer RMT, Cockcroft JR, Shale DJ. Aortic Pulse Wave Velocity as a Measure of Cardiovascular Risk in Chronic Obstructive Pulmonary Disease: Two-Year Follow-Up Data from the ARCADE Study. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Song Y, Yang J, Zhao Q, Bai Y, Ruan L. Remnant Cholesterol and Common Carotid Artery Intima-Media Thickness in Community Population with Normal Low-Density Lipoprotein Cholesterol. Cerebrovasc Dis. 2023;52:487-494. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Guo X, Li JS, Wang X. [Correlation between epicardial adipose layer thickness and carotid artery elasticity evaluated by ultrasound in middle-aged and elderly patients with coronary heart disease]. Zhongguo Dongmai Yinghua Zazhi. 2023;31:785-790. |
| 20. | Elie AG, Jensen PS, Nissen KD, Geraets IM, Xu A, Song E, Hansen ML, Irmukhamedov A, Rasmussen LM, Wang Y, De Mey JG. Adipokine Imbalance in the Pericardial Cavity of Cardiac and Vascular Disease Patients. PLoS One. 2016;11:e0154693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | van Woerden G, van Veldhuisen DJ, Westenbrink BD, de Boer RA, Rienstra M, Gorter TM. Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: mechanisms, management and modern perspectives. Eur J Heart Fail. 2022;24:2238-2250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 22. | Esparham A, Roohi S, Ahmadyar S, Dalili A, Nelson PR, Khorgami Z. Impact of bariatric surgery on carotid intima-media thickness, flow-mediated dilation, and nitrite-mediated dilation: a systematic review and meta-analysis. Surg Obes Relat Dis. 2023;19:1188-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Pluta A, Stróżecki P, Krintus M, Odrowąż-Sypniewska G, Manitius J. Left ventricular remodeling and arterial remodeling in patients with chronic kidney disease stage 1-3. Ren Fail. 2015;37:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Bacharaki D, Petrakis I, Kyriazis P, Markaki A, Pleros C, Tsirpanlis G, Theodoridis M, Balafa O, Georgoulidou A, Drosataki E, Stylianou K. Adherence to the Mediterranean Diet Is Associated with a More Favorable Left Ventricular Geometry in Patients with End-Stage Kidney Disease. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 25. | Hao MJ, Chen WW, Wang D, Huang H, He Y, Liu G. [The relationship between carotid atherosclerosis ultrasound related parameters and the severity of lesions in patients with coronary heart disease]. Xiandai Shengwu Yixue Jinzhan. 2017;17:2275-2278. |