Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2904
Revised: April 8, 2024
Accepted: April 19, 2024
Published online: June 6, 2024
Processing time: 104 Days and 21.1 Hours
Primary intraspinal malignant melanoma is a very rare tumor that most often occurs in the cervical, thoracic, or thoracolumbar segment.
A rare case of primary thoracolumbar malignant melanoma is described. A 45-year-old female patient complained of low back pain with numbness and fatigue in both lower limbs. MR revealed an intradural space-occupying lesion at the thoracic 12 to lumbar 1 level. The tumor was partially excised, and a malignant melanoma was confirmed by histopathology.
Primary intraspinal malignant melanoma has rarely been reported, and surgical resection and related characteristics and diagnoses have been discussed.
Core Tip: Primary intraspinal malignant melanoma is an exceedingly rare tumor, with limited documentation in the existing literature. This manuscript presents a detailed analysis of the case and imaging characteristics of a patient diagnosed with primary thoracolumbar malignant melanoma, who underwent partial tumor resection. The excised tumor was subjected to pathological examination, and the patient was subsequently followed up for one year post-surgery. Furthermore, this study delves into the pertinent characteristics and diagnostic considerations associated with malignant melanoma.
- Citation: Huang JB, Xue HJ, Zhu BY, Lei Y, Pan L. Primary thoracolumbar intraspinal malignant melanoma: A case report. World J Clin Cases 2024; 12(16): 2904-2910
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2904.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2904
The incidence of primary intraspinal melanoma [intraspinal primary melanocyte neoplasm (ISPMN)] is extremely low, and only a small number of cases have been reported in the domestic and international literature. In November 2022, a patient with primary thoracic 12 to lumbar 1 (T12-L1) intraspinal malignant melanoma was admitted to our hospital and treated with surgery. After more than 1 year of follow-up observation, the results are as follows.
A 45-year-old woman who presented with low back pain with numbness and weakness of both lower limbs for two months was admitted to our hospital.
Two months before admission, the patient developed back pain, numbness and fatigue in both lower limbs; obvious symptoms on the left side; no fever or night sweats; and normal urine and feces. She visited the outpatient clinic in our hospital and other hospitals many times. After analgesia, nutrition, and sealing, the symptoms did not resolve, and they became worse.
The patient had no history of acute or chronic infectious diseases, heart disease, hypertension or diabetes.
The patient did not have any relevant family medical history.
There was no systemic skin pigmentation. The patient had a poor gait, no kyphotic spinal scoliosis deformity, no bilateral intervertebral space, no spinous space tenderness, no bilateral hip tenderness, double lower limbs without deformity, and normal joint active flexion and extension activity. Her bilateral hip "4" character was negative, her double lower limb straight leg elevation test (70°) was negative, her double lower limb superficial sensibility decreased, and her left side was even worse. The left iliopsoas and quadriceps muscles were level 3, the right muscles were level 4, and the bilateral anterior tibialis, dorsal extensor hallux, and ankle plantar flexor muscles were level 5. Both patellar tendon reflexes and Achilles tendon reflexes were normal, and no pathological signs were found in either lower limb. The visual analogue scale score for lumbar pain was 6.
No abnormalities were found by blood draw after admission.
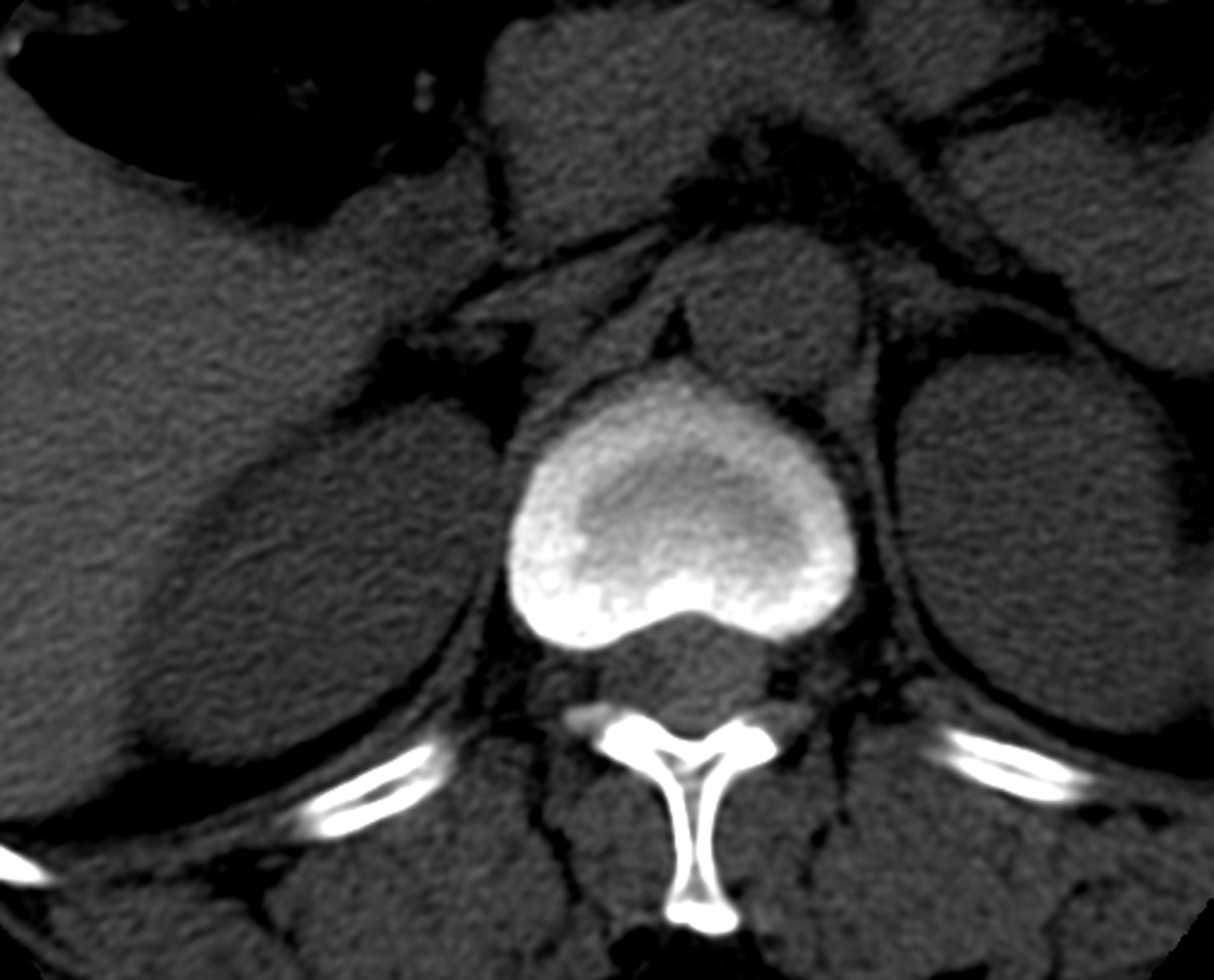
Lumbar computed tomography (CT) revealed that the spinal canal at T12-L1 had a slightly greater nodular density and unclear boundaries, approximately 11 mm × 13 mm × 32 mm. Diagnostic consideration: Considering the extramedullary space-occupying lesion at the T12-L1 level, the possibility of chomenioma should be considered (Figure 1).
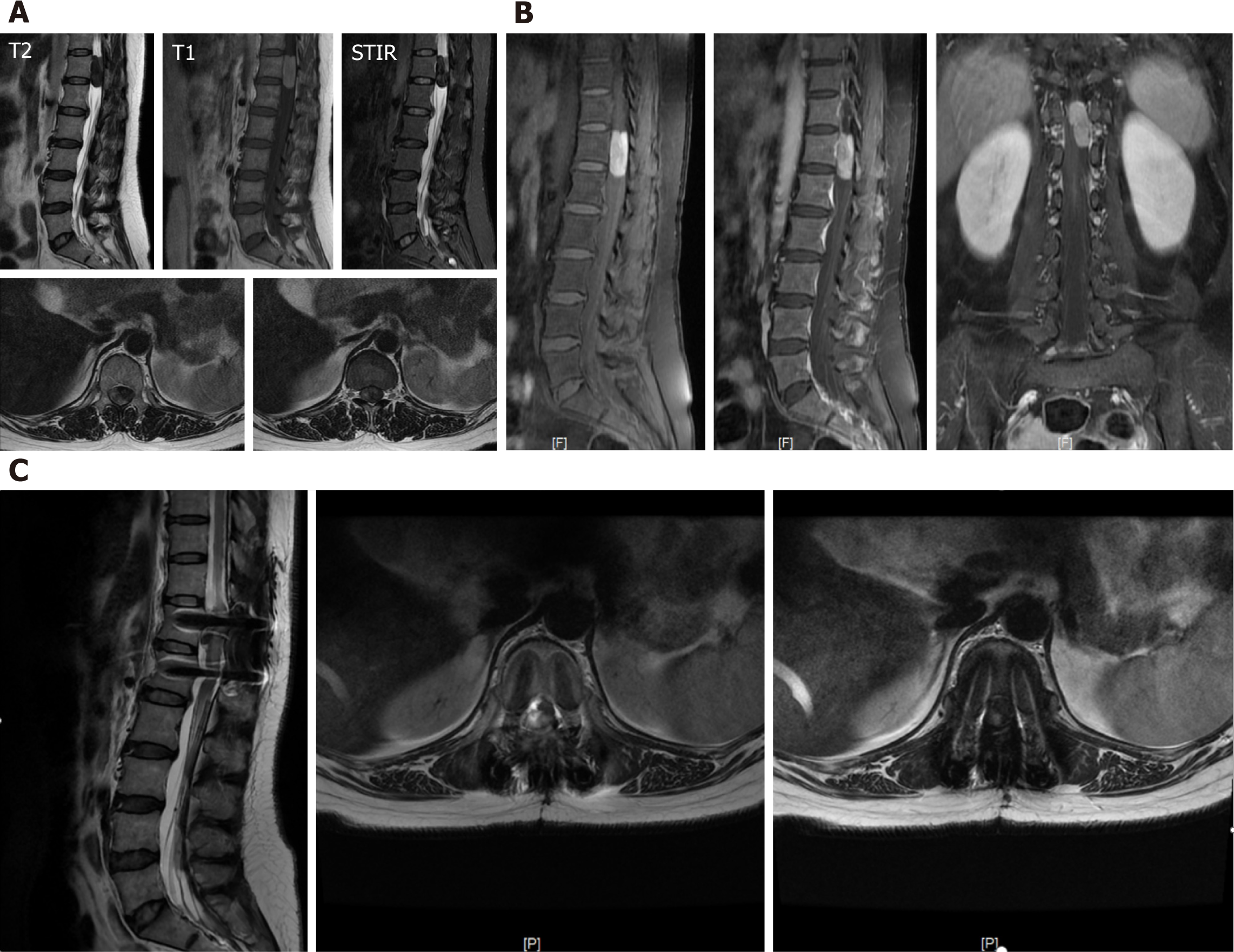
Enhanced magnetic resonance imaging (MRI) revealed spindle swelling in the T12-T1 region (approximately 13 mm × 13 mm × 34 mm). Compared with muscle signals, T1-weighted imaging (T1WI) signals were slightly greater, T2-weighted imaging (T2WI) signals were equal, and there was a clear boundary. The adjacent border of the left intervertebral foramen side was irregular, the lesion was significantly enhanced under the enhanced scan, and the spinal cord was displaced to the right and half-wrapped. The diagnosis was as follows: Extramedullary intradural space-occupying lesion of the T12-L1 lesions, which was considered a benign tumor: Meningioma or a neurogenic tumor (Figure 2A and B).
The patient had no history or clinical manifestations of the primary cutaneous or ocular lesion. Based on the MRI analysis and histological examination, the final diagnosis was primary thoracolumbar intraspinal malignant melanoma.
The patient underwent "posterior open T12 intraspinal mass resection + spinal decompression + internal fixation surgery". During the operation, the dural sac was slightly expanded, and the size of the spinal canal after the dural incision was approximately 25 mm × 10 mm × 10 mm (black brown mass) and clot-shaped. The mass had an unclear boundary and obvious adhesion to the cauda equina nerve. The severe adhesions between the mass and the cauda equina nerve could not be completely removed.
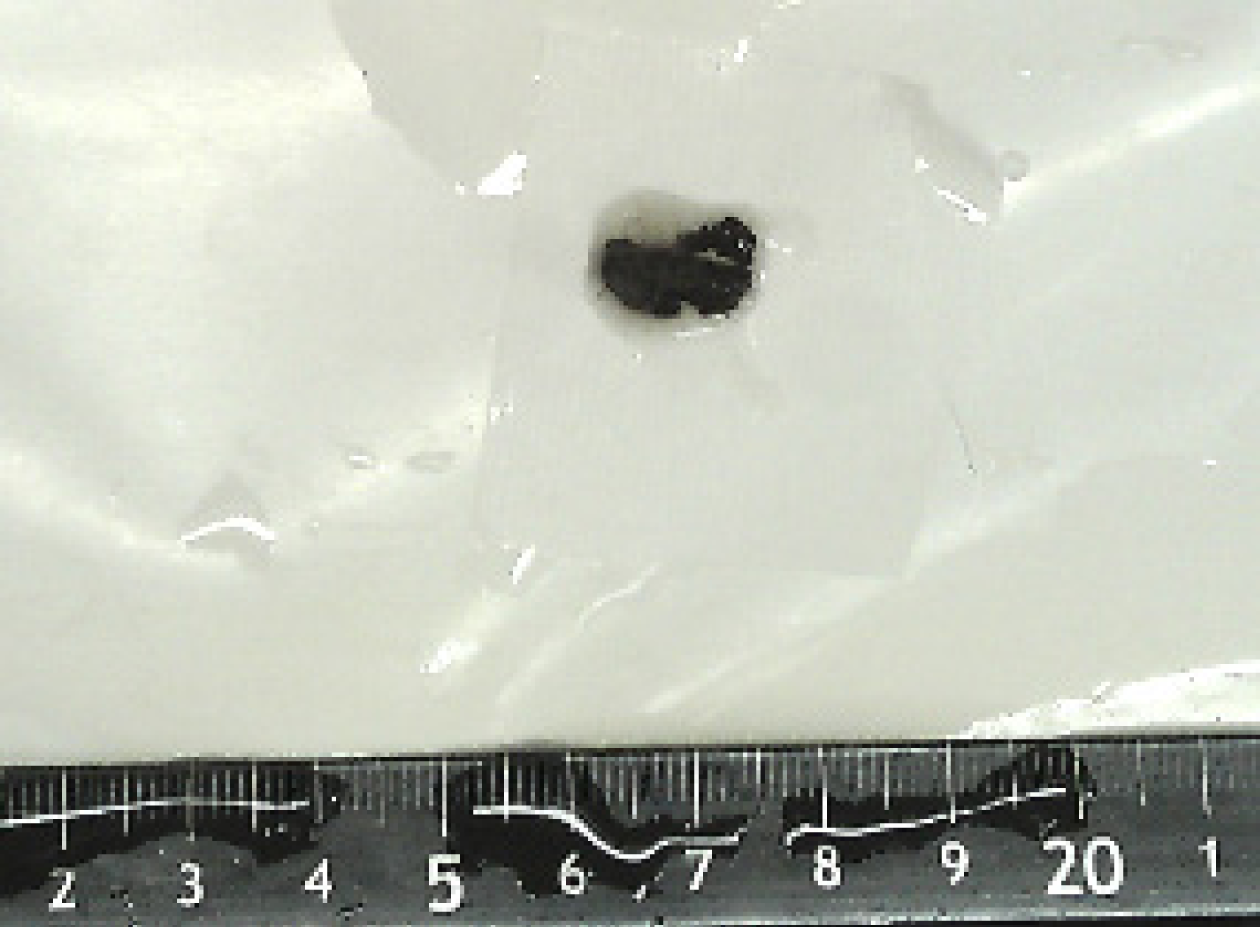
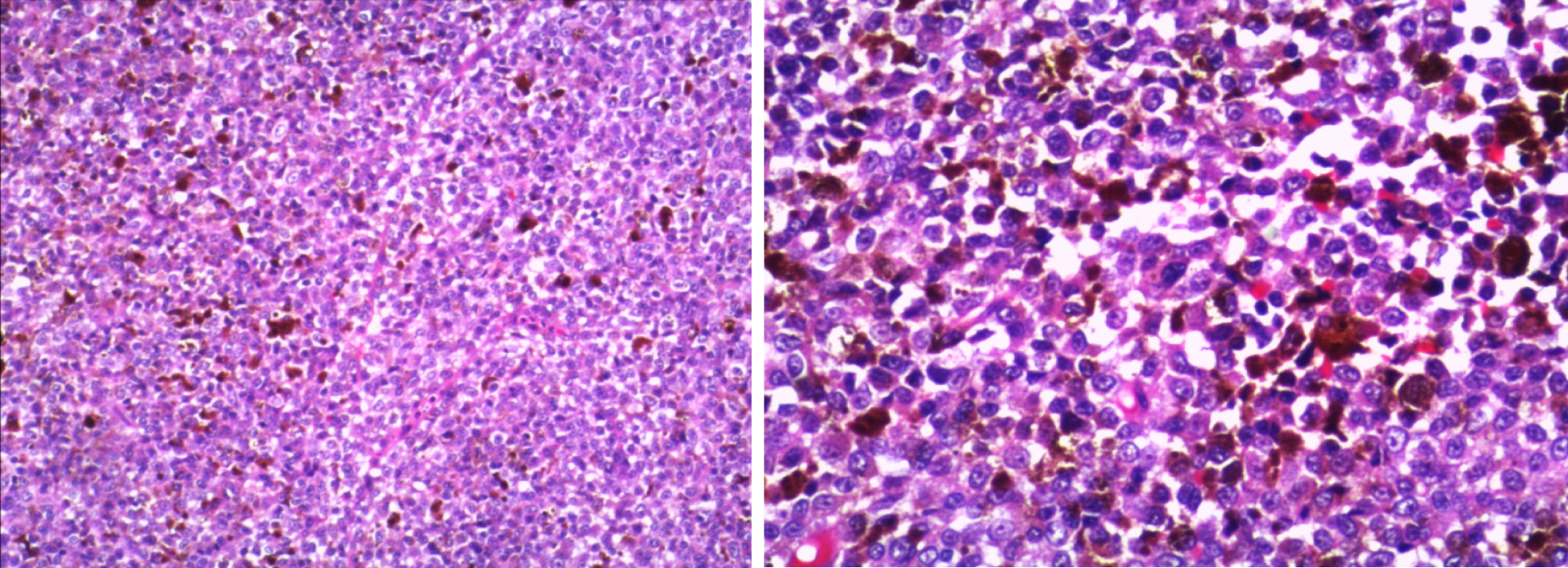
Postoperative pathology revealed that the T12 spinal mass was generally dark brown and clot-shaped (Figure 3), with microscopic tumor cells, rich pigment, heterogeneous tumor cells, and large nuclei.
Immunohistochemistry revealed the following: HMB 45(+), S-100(+), Milan-A(+), SOX-10(+), Bcl-2(+), P16(+), CK (partial +), CyclinD1 (partial +), BRAF (V600E) (partial +), and Ki-67 (hot zone 40% +). The results of immunohistochemistry were consistent with malignant melanoma (Figure 4).
Postoperative examination via brain enhanced MR, chest and whole abdomen enhanced CT revealed no suspicious tumor lesions. After surgery, the patient's lower limb numbness was reduced, and the patient still experienced mild numbness of the left lower limb and fatigue, especially in the left thigh. After discharge, the patient underwent regular outpatient follow-up and experienced numbness in the left thigh, occasionally with needle-like pain. One month, five months and one year after surgery, enhanced MR revealed that the signal was still abnormal in the spinal canal and was approximately 7 mm × 12 mm × 30 mm, indicating residual tumor tissue (Figure 2C).
Intracranial primary melanoma [primary melanocytic neoplasm (PMN)] originates from leptomal melanocytes and is rare in clinical practice, accounting for 0.07%-0.17%[1] of intracranial tumors. Primary intraspinal melanoma is even rarer and is mostly located in the extramullary dural ridge or near the dural spinal nerve roots. Nericular pain often occurs first[2], with the greatest occurrence occurring in individuals approximately 50 years old. Brat et al[3] reported 33 cases of central nervous system (CNS) melanoma, with 52% occurring in the spinal canal and 48% in the intracranial area. Among them, the age of onset of cerebrospinal membrane melanoma was 16-73 years, the mean age was 51 years, the female/male ratio was 1.43:1, while the age of onset of malignant melanoma was 15-71 years, the mean age was 43 years, and the female/male ratio was 1.6:1. Rahimi-Movaghar and Karimi[4] summarized 95 cases of CNS cerebrospinal membrane melanoma (45 intracranial cases, 50 spinal cases); the mean age of intracranial patients was 40 years, the mean age of patients in the spinal canal was 49 years, and 57.9% of the patients were female.
CNS-PMN originates from the leptomial sheath of the vascular plexus or embryonic remnant melanoblasts, with a high degree of aggregation at the craniocervical junction, the ventral medulla, and the pontine cerebellum angle[5]. Although melanocytes are concentrated in the upper cervical segment, tumorigenesis is not limited to the cervical segment, occurs in the lower thoracic segment or thoracolumbar segment, and often originates near the cranial nerve and spinal nerve roots, which may be related to the extreme rarity of tumors, small sample sizes and significant differences[6,7].
Brat et al[3] classified PMNs into benign cerebrospinal membrane melanoma, malignant melanoma and the intervariable intermediate type, accounting for 52%, 39% and 9%, respectively. Benign cerebrospinal membrane melanoma primarily occurs in the spinal canal in 70.6% and 29.4% of malignant melanomas, respectively. Malignant melanoma cells can be shed in the subarachnoid space and spread on the soft brain membrane, where they can form several black nodules of varying sizes, rich tumor blood transport and clear boundaries. Malignant melanoma occurs from the skin and mucosa and is a highly malignant tumor. Microscopy of malignant melanoma revealed that tumor cells are significantly pleo
ISPMN occurs near the spinal nerve root, mostly in extramedullary subdural or dural lesions, and nerve root pain caused by nerve root compression is often the initial manifestation. Compression of tumor development in the spinal cord can lead to sensory, motor or stool and urine dysfunction in the corresponding segments. Stimulation of the meninges by tumor cell metabolites may cause meningitis. Tumors can cause bleeding and recurrent episodes of spontaneous subarachnoid hemorrhage. The diagnostic conditions for spinal canal primary melanoma were as follows: (1) Only spinal canal lesion, no intracranial homologous lesion was found; (2) Except for skin, ocular pigment membrane melanin lesions, and parenchymal viscera melanoma; and (3) The tumor had a clear histopathological diagnosis. Due to the extremely low incidence of ISPMN and the lack of specificity of clinical symptoms and signs, comprehensive imaging, surgical and pathological data are needed to confirm the diagnosis.
MRI of ISPMNs is mainly divided into type 4[8]: (1) Melanin type, in which T1WI has a high signal and T2WI has a low signal; (2) Nonpigmented type, in which the T1WI signal is low or equal, while the T2WI signal is high or equal; (3) Mixed type, in which the signal is different from that of melanin and nonpigmented type; and (4) Blood type, in which the signal is only bleeding. In this case, MRI of an intraspinal malignant melanoma showed a slightly greater signal in T1WI and an equal signal in T2WI. The plain ISPMN CT scan was round or round, with a high-density shadow, and the degree of enhanced bleeding was high. Due to the very low incidence of intraspinal melanoma and the lack of specific clinical symptoms and signs for diagnosis, pathological and immunohistochemical examinations are needed. HMB-45, Melan-A, and S-100 are commonly used for melanoma immunohistochemistry. The S-100 protein is highly expressed in malignant melanoma but is less specific; HMB-45 is highly specific, and combining HMB-45 and Melan-A can prevent missed diagnoses[9-11]. Typical melanin imaging findings can improve the accuracy of preoperative diagnosis.
Because intraspinal malignant melanoma is very rare and its imaging manifestations are variable, it mainly needs to be distinguished[12] from intraspinal schwannoma and meningioma. In terms of location, morphology, and growth mode, ISPMNs are easily confused with meningioma and schwannoma. All three are common in the thoracolumbar intraspinal region. There are multiple predominant extramedullary subdural lesions. Melanomas mostly originate around the nerve root and can grow into the "dumbbell type" of the intervertebral foramen, leading to enlargement of the intervertebral foramen and destruction of bone, which are common imaging features of schwannomas. Some ISPMNs are closely related to the dura, grow by "creeping" along the dura and connecting to the broad base, exhibit an enhanced visible "dura" tail sign and show the characteristics of meningioma. The differences among the three imaging modalities mainly depend on the signal intensity characteristics of the three signals: (1) Meningioma T1WI signal is equal to or slightly greater than the spinal cord signal, reinforcement is obvious and uniform, and tumor calcification can exhibit a low signal; (2) Schwannoma T1WI signal is low, T2WI signal is slightly greater, and tumors are prone to cystic changes and uneven signals and uneven mixed enhancement; and (3) Typical melanin-containing granule tumors exhibit "short T1 high signal and short T2 low signal"[13].
The best treatment may be total surgical resection of the lesion as much as possible, and radiotherapy is usually recommended after surgery[13-16]. Patients with intraspinal melanoma that causes neurological dysfunction should be removed as soon as possible to relieve spinal cord compression. However, the following factors limit the extent of resection: (1) The onset is insidious, and the tumor volume is often large; (2) The tumor is well located in the upper cervical spinal cord, inferior thoracic segment around the nerve root, and closely adhered to important nerve structures; (3) The tumor is highly vascular and prone to bleeding; and (4) Few tumors have focal infiltration, which can invade the medulla. Whether there is radiotherapy for residual tumors has been controversial. With surrounding tissue infiltration in the spinal canal of malignant melanoma, total resection is difficult. Timely adjuvant chemoradiotherapy after surgery is important for prolonging survival and reducing local recurrence and implant metastasis. Metastasis of other extraspinal organs after surgery for intraspinal malignant melanoma is rare.
Finally, primary intraspinal malignant melanoma is extremely rare, with a high degree of malignancy, no clinical specificity, and large differences in imaging manifestations, which ultimately depend on pathological diagnosis[17]. At present, surgery supplemented with postoperative radiotherapy and chemotherapy is the first-line treatment.
| 1. | Sinha R, Rizvi TH, Chakraborti S, Ballal CK, Kumar A. Primary melanoma of the spinal cord: a case report. J Clin Diagn Res. 2013;7:1148-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Rades D, Schild SE. Dose–response relationship for fractionated irradiation in the treatment of spinal meningeal melanocytomas: a review of the literature. J Neurooncol. 2006;77:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol. 1999;23:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 204] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Rahimi-Movaghar V, Karimi M. Meningeal melanocytoma of the brain and oculodermal melanocytosis (nevus of Ota): case report and literature review. Surg Neurol. 2003;59:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Xia J, Wang Y, Li F, Wang J, Mu Y, Mei X, Li X, Zhu W, Jin X, Yu K. Expression of microphthalmia transcription factor, S100 protein, and HMB-45 in malignant melanoma and pigmented nevi. Biomed Rep. 2016;5:327-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Turhan T, Oner K, Yurtseven T, Akalin T, Ovul I. Spinal meningeal melanocytoma. Report of two cases and review of the literature. J Neurosurg. 2004;100:287-290. [PubMed] |
| 7. | Mineo JF, P-Ruchoux MM, Pasquier D, Rigolle H, Assaker R. Tumeur mélanocytaire primitive maligne radiculaire. A propos d'un cas [Primitive malignant melanoma arising in a spinal nerve root. A case report]. Neurochirurgie. 2006;52:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Isiklar I, Leeds NE, Fuller GN, Kumar AJ. Intracranial metastatic melanoma: correlation between MR imaging characteristics and melanin content. AJR Am J Roentgenol. 1995;165:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Iga T, Iwanami A, Funakoshi T, Mikami S, Tsuji O, Nagoshi N, Okada E, Fujita N, Yagi M, Watanabe K, Nakamura M, Matsumoto M. Multifocal primary melanoma of the cervical spinal cord successfully treated by tumorectomy: a case report. Spinal Cord Ser Cases. 2018;4:24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Lee CH, Moon KY, Chung CK, Kim HJ, Chang KH, Park SH, Jahng TA. Primary intradural extramedullary melanoma of the cervical spinal cord: case report. Spine (Phila Pa 1976). 2010;35:E303-E307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Sun LD, Chu X, Xu L, Fan XZ, Qian Y, Zuo DM. Primary intramedullary melanoma of lumbar spinal cord: A case report. World J Clin Cases. 2021;9:2352-2356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Jo KW, Kim SR, Kim SD, Park IS. Primary thoracic epidural melanoma : a case report. Asian Spine J. 2010;4:48-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Farrokh D, Fransen P, Faverly D. MR findings of a primary intramedullary malignant melanoma: case report and literature review. AJNR Am J Neuroradiol. 2001;22:1864-1866. [PubMed] [DOI] [Full Text] |
| 14. | Kolasa M, Jesionek-Kupnicka D, Kordek R, Kolasa P. Primary spinal cord melanoma-a case report. Folia Neuropathol. 2010;48:212-216. [PubMed] |
| 15. | Kounin GK, Romansky KV, Traykov LD, Shotekov PM, Stoilova DZ. Primary spinal melanoma with bilateral papilledema. Clin Neurol Neurosurg. 2005;107:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Beul H, Skomorac R, Jusi A, Meki A, Ali F, Mustadenagi J, Efendi A, Baru N. A rare case of primary extramedullary intradural and extradural malignant melanoma of cervical spine. Medeni Med J. 2015;30:182-185. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Foit NA, Neidert MC, Woernle CM, Rushing EJ, Krayenbühl N. Bifocal extra- and intradural melanocytoma of the spine: case report and literature review. Eur Spine J. 2013;22 Suppl 3:S521-S525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |